Abstract
Wolbachia manipulate insect host biology through a variety of means that result in increased production of infected females, enhancing its own transmission. A Wolbachia strain (wInn) naturally infecting Drosophila innubila induces male killing, while native strains of D. melanogaster and D. simulans usually induce cytoplasmic incompatibility (CI). In this study, we transferred wInn to D. melanogaster and D. simulans by embryonic microinjection, expecting conservation of the male-killing phenotype to the novel hosts, which are more suitable for genetic analysis. In contrast to our expectations, there was no effect on offspring sex ratio. Furthermore, no CI was observed in the transinfected flies. Overall, transinfected D. melanogaster lines displayed lower transmission rate and lower densities of Wolbachia than transinfected D. simulans lines, in which established infections were transmitted with near-perfect fidelity. In D. simulans, strain wInn had no effect on fecundity and egg-to-adult development. Surprisingly, one of the two transinfected lines tested showed increased longevity. We discuss our results in the context of host-symbiont co-evolution and the potential of symbionts to invade novel host species.
Keywords: Wolbachia, symbiosis, male-killing, cytoplasmic incompatibility
Introduction
The occurrence of Wolbachia, a group of maternally transmitted endosymbionts, in two-thirds of all species of insects renders them perhaps the most diverse group ever to inhabit the earth (Hilgenboecker et al., 2008). Equally striking is the almost complete lack of phylogenetic congruence between insect host species and the Wolbachia lineages that infect them (for example, Werren et al., 1995), indicating that colonization of the world's insect species by Wolbachia has occurred largely by lateral transmission from one species to another. The lack of phylogenetic congruence also indicates that these infections are short-lived on a macroevolutionary time scale (Werren et al., 1995; Werren et al., 2008).
Wolbachia spread within host species by increasing the relative fitness of infected cytoplasmic lineages, either by conferring direct fitness benefits (Vavre et al., 1999) or by manipulating host reproduction via cytoplasmic incompatibility (CI), male-killing, feminization of genetic males or parthenogenesis (thelytoky) (Werren et al., 2008; Saridaki and Bourtzis, 2010). The fitness advantage conferred to infected cytoplasmic lineages and the fidelity of maternal transmission jointly determine the dynamics of infection within a host species, including the tendency to increase following Wolbachia's introduction to a new host and the eventual equilibrium prevalence of infection. Thus, the global association between insects and Wolbachia is continually reconfigured by the processes of lateral transmission between species and by the phenotypic effects and maternal transmission fidelity of Wolbachia within infected host species.
The development of a macroevolutionary theory of insect–Wolbachia associations requires understanding how the phenotypic effects and transmission fidelity of Wolbachia depend on host species or genotype, Wolbachia strain, environmental conditions and interactions among these factors. The fate of novel infections will be more predictable if the phenotypic effect of Wolbachia depends solely on host species or Wolbachia strain, rather than on idiosyncratic interactions between these factors. For instance, the 'popcorn' strain of Wolbachia causes CI and reduces adult lifespan both in its native host, Drosophila melanogaster, and in a very distantly related host, Aedes aegypti, to which it has been experimentally transferred (McMeniman et al., 2009). In other cases, however, Wolbachia fail to express the original phenotype and sometimes express entirely novel phenotypes (for example, Grenier et al., 1998; van Meer and Stouthamer, 1999; Sasaki et al., 2002; Sasaki et al., 2005; Jaenike, 2007). From an applied standpoint, predictability of Wolbachia phenotypic effects is highly desirable in programs using these endosymbionts for control or genetic manipulation of insect populations (Cook et al., 2008). Given the growing interest in using Wolbachia for such purposes, it is clearly important to develop an understanding of the degree to which the phenotypic effects of a given Wolbachia strain are conserved across host species.
One way to distinguish the effects of Wolbachia strain from those of the host species involves transfer of a Wolbachia strain that has a particular phenotypic effect (for example, male-killing) in its native host to a novel host whose native Wolbachia has a different phenotypic effect (for example, CI). If the Wolbachia expresses the same phenotype in both hosts, this would indicate that the Wolbachia strain determines the phenotype. In contrast, if the recipient host species expresses the same phenotype with both its native and the introduced Wolbachia strains, this would indicate that Wolbachia phenotype is governed by the host species. Finally, if a novel (or no) phenotype is expressed, this reveals the importance of host species by Wolbachia strain interactions.
In the present study, we transferred Wolbachia from D. innubila to both D. melanogaster and D. simulans. D. innubila is a member of the quinaria group within the subgenus Drosophila, whereas D. melanogaster and D. simulans belong to the melanogaster group within the subgenus Sophophora. These two subgenera are thought to have split ∼60 mya (Tamura et al., 2004). D. innubila is naturally infected with a strain of Wolbachia (wInn) that experiences nearly perfect maternal transmission and causes ∼100% mortality of infected male embryos (Dyer and Jaenike, 2004). D. melanogaster and D. simulans both harbor natural Wolbachia infections that cause CI (Hoffmann et al., 1986; Hoffmann and Turelli, 1988; O'Neill and Karr, 1990; Bourtzis et al., 1996; Zabalou et al., 2008). The expression of CI is particularly strong in D. simulans. Besides testing the relative roles of host species and Wolbachia strain on the expressed phenotype, the transfer of Wolbachia into D. melanogaster could allow in-depth genetic and developmental analyses of Wolbachia–host interactions, specifically, in this case, the mechanism by which Wolbachia brings about embryonic male killing.
A male-killing strain of Wolbachia very closely related to wInn has been discovered in D. borealis (Sheeley and McAllister, 2009). D. innubila and D. borealis belong, respectively, to the quinaria and virilis groups of the subgenus Drosophila, which split over 30 million years ago (O'Grady and DeSalle, 2008; Tamura et al., 2004). Thus, to cause male killing in these distantly related flies, the Wolbachia in D. innubila and D. borealis may target a highly conserved developmental mechanism in early embryonic development. If such a mechanism was conserved across the genus Drosophila, then male killing by these Wolbachia might be expressed in species of the melanogaster group.
The wInn strain found in D. innubila is notable in another respect, as it belongs to the ST-13 strain complex of Wolbachia (Baldo et al., 2006). Recent surveys have found that this strain complex has been extraordinarily successful in the recent colonization of innumerable species of Diptera, including D. simulans and D. melanogaster (Baldo et al., 2006; Stahlhut et al., 2010). This strain complex is also remarkable in the variety of reproductive phenotypes it can cause, including male killing (in D. innubila), parthenogenesis (in Muscidifurax uniraptor) and CI (for example, D. simulans, D. melanogaster and Nasonia longicornis). Thus, the experimental transfer of Wolbachia strain wInn to novel host species may shed light on the mechanisms responsible for the success of this strain complex and the lability of its reproductive phenotypes.
Our results indicate that in the novel hosts D. simulans and D. melanogaster wInn does not express the phenotype manifest in its native host (male killing) nor the phenotype expressed by the Wolbachia normally resident in D. simulans and D. melanogaster (CI). In fact, we found no reproductive manipulation at all. We did find that these novel infections are either benign or perhaps slightly beneficial in the new hosts. These findings have potentially important implications for the evolution of insect—Wolbachia associations.
Materials and methods
Establishment of the transinfected lines
Microinjections were carried out as previously reported (Zabalou et al., 2004). Cytoplasmic donor was D. innubila infected with the wInn male-killing Wolbachia strain (Dyer and Jaenike, 2004). Recipient lines were uninfected lines iso31 for D. melanogaster and STCP for D. simulans that have nearly homozygous genetic backgrounds and have been used repeatedly in our laboratory for Wolbachia transfers (Zabalou et al., 2008).
Presence of Wolbachia in transinfected flies was checked every generation by STE/boiling method for DNA extraction and subsequent PCR using primers for 16S recombinant DNA or wsp genes (O'Neill et al., 1992; Zabalou et al., 2008). The Wolbachia strain(s) of the donor and of the transinfected Drosophila hosts were genotyped using a Multi Locus Sequence Typing system developed in Paraskevopoulos et al. (2006).
Maternal transmission, sex ratio and CI assays
For each generation after injection, females were tested for Wolbachia infection and isofemale lines were set up accordingly. We measured the transmission efficiency by determining the proportion of their offspring that was infected. We simultaneously determined the offspring sex ratio and tested for CI several generations after injection, using previously described methods (Zabalou et al., 2008). In brief, young virgin females and males were used in these experiments, mating was confirmed by visual inspection, eggs were collected for three consecutive days and egg hatch rates were then determined. The statistical analysis for the sex ratio was based on t-test comparisons of the proportion of females in the offspring of each infected female to the expected 50% proportion (one-sample t-test). These comparisons were performed separately for each female type. This kind of analysis was selected because it takes into account the sex ratio variability among infected flies. The statistical analysis for the CI was based on one-way analysis of variance (ANOVA) comparing the embryonic mortality among crosses. Groups of similar crosses were determined by Tukey's Multiple Comparisons Test.
Fitness measurements
Female fecundity, egg-to-adult viability and adult longevity of Wolbachia-infected D. simulans were measured 1 year after establishment of infection in the laboratory. Fecundity was scored for three consecutive days at three different periods during females' life span: early (days 1–3), middle (days 11–13) and late (days 21–23). For egg-to-adult viability, 15 eggs deposited through the ‘early period' by these same females were added to food vials and the number of emerging adults was subsequently counted. Longevity of the flies was assessed by holding groups of 6 to 13 females and 6 to 13 males together in food vials, with 10 to 13 replicate vials per line, and monitoring the number of individuals that died every 5 days. The longevity experiment was repeated twice, at post-infection generation 45 in 2007 and generation 95 in 2009. The longevity data were analyzed with the Kaplan–Meier survival analysis, while the Mantel–Cox (log-rank) test was used for comparisons among vials (Kleinbaum and Klein, 2005). The latter analysis indicated that there were significant among-vial effects on longevity for both sexes of all three strains in both generations. Consequently, for each vial, we used median survival time (that is, when 50% of the flies were dead) of males and females as two data points. We then carried out two-way ANOVAs of these vial-level median survival times for each generation, searching for effects of line (including Wolbachia infection status) and sex.
Immunofluorescence
Eggs, testes and ovaries of Wolbachia-infected D. melanogaster and D. simulans were processed and stained with the anti-WSP antibody and propidium iodide with standard immunofluorescence techniques (Veneti et al., 2004). For D. innubila, fixation and staining procedures were based on Ferree et al. (2005). After dissection, ovaries were fixed and devitelinized in a 1 : 3 mixture of 4% paraformaldehyde in phosphate-buffered saline with 0.5% NP40 detergent and heptane on agitation for 20 min. The samples were then washed three times for 10 min in PBS-T (0.1% Triton) before overnight treatment with 10 mg ml−1 solution of RNase A. After washing 4–5 times over 2 h, samples were incubated in Alexa 488-conjugated phalloidin for 2 h and then washed a further 4–5 times over 2 h before mounting in propidium iodide-containing mounting media (10 μg ml−1 propidium iodide in solution of 70% glycerol in PBS). Samples were observed on a Leica SP5 inverted confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) using a × 63 oil objective.
Results
Establishment of the transinfected lines
A total of 1840 eggs of the uninfected D. melanogaster iso31 line were injected with cytoplasm from D. innubila infected with wInn. Out of 46 fertile females recovered, 16 gave positive PCR signals for infection. Seven of them were used to set up several isofemale lines and three of them transmitted the infection from G0 to the next generation (F1) with the following transmission rates: line 23 (28.5% 4/14 females), line 35 (10% 1/10 females) and line 46 (87.5% 7/8 females). Line 23 lost the infection by generation 11, while the two other infected lines were maintained in the laboratory by selection until about generation 40.
For D. simulans 1600 microinjections were performed, and 10 out of 50 female flies were found to be fertile and positive for the infection. F1 females were used to establish 10 isofemale lines for each initially infected female. Transmission rates for the infection to the next generation (F1) were as follows: line 4 (100%), line 22 (0%), line 24 (40%), line 29 (30%), line 30 (60%), line 42 (0%), line 43 (0%), line 45 (50%), line 46 (0%) and line 48 (0%). From each infected isofemale line, several new lines were started and tested for infection. All isofemale sublines within lines 4, 24, 30 or 45 were positive for infection, while all of the isofemale sublines from line 29 were negative. No further loss of infection was found for lines 4, 24, 30 or 45 in subsequent generations. Multi Locus Sequence Typing analysis clearly indicated that all transinfected lines were infected with the wInn Wolbachia strain that naturally infects D. innubila.
Maternal transmission, sex ratio and CI assays
The average percentage of Wolbachia transmission to the next generation in the transinfected D. melanogaster line is shown in Table 1. PCR tests were carried out for line 23 from generations 2 through 11, at which point the infection was lost, while for lines 35 and 46 the assays were performed until the thirtieth generation. These assays revealed variable levels of maternal transmission with no evidence of fixation (prefect transmission). In D. simulans, the maternal transmission fidelity reached 100% two generations after injection and remained at 100% subsequently, as mentioned above.
Table 1. Transmission rate of D. melanogaster transinfected lines.
|
Offspring | |||
|---|---|---|---|
| Female type | Tested | Infected | Percentage infected (%) |
| Infected23 | 317 | 254 | 80.1 |
| Infected35 | 425 | 304 | 71.5 |
| Infected46 | 476 | 333 | 70.0 |
| Total | 1218 | 891 | 73.2 |
The sex ratio was not significantly distorted from 1:1 in any of the generations or lines tested, indicating that Wolbachia strain wInn does not cause male killing in either D. melanogaster or D. simulans (Tables 2 and 3).
Table 2. Sex ratio of D. melanogaster transinfected flies.
|
Progeny | |||
|---|---|---|---|
| Female type | N | Females (%), mean (s.d.) | t-test, P-values |
| Infected23 | 46 | 50.27 (7.9) | 0.280, NS |
| Infected35 | 160 | 50.42 (7.9) | 0.506, NS |
| Infected46 | 156 | 50.31 (6.2) | 0.529, NS |
The sex ratio was determined in every generation from generation fourth to eleventh for line 23 and from generation fourth to thirtieth for lines 35 and 46.
N, number of infected females whose offspring sex ratios were determined.
Females (%), number of female offspring/total number of offspring.
t-test, one-sample t-test comparison with the assumed 50% proportion of females.
NS, not significant.
Table 3. Sex ratio of D. simulans transinfected flies.
|
Progeny | |||
|---|---|---|---|
| Female type | N | Females (%), mean (s.d.) | t-test, P-values |
| Infected4 | 25 | 48.2 (9.6) | 0.351, NS |
| Infected24 | 25 | 48.6 (6.7) | 0.313, NS |
| Infected30 | 25 | 49.8 (6.9) | 0.861, NS |
| infected45 | 25 | 49.3 (5.5) | 0.518, NS |
The sex ratio was determined in every generation from generation tenth to thirteenth for all four lines.
N, number of infected females whose offspring sex ratios were determined.
Females (%), number of female offspring/total number of offspring.
t-test=one-sample t-test comparison with the assumed 50% proportion of females.
NS, not significant.
Several generations after injection, transinfected lines were tested for CI expression. For D. melanogaster, ANOVA comparison of all crosses was significant (F=2.23, degree of freedom (d.f.)=9, 196, P=0.021) owing to the difference observed in the comparison of the cross (Infected46 × Infected46) with that of [uninfected × uninfected] (Table 4). For D. simulans, ANOVA comparison of all crosses was also significant (F=2.98, d.f.=12, 200, P<0.01) owing to the differences observed in the comparison of the crosses (uninfected × infected4), (infected4 × infected4) and (infected24 × infected24) with that of (uninfected × infected45) (Table 5). These results show that none of seven transinfected lines expressed elevated mortality in crosses between uninfected females and infected males, indicating that Wolbachia strain wInn does not cause detectable levels of CI in either D. melanogaster or D. simulans.
Table 4. Egg mortality of D. melanogaster transinfected flies.
| Cross (female × male) | Eggs | Number of crosses | % Mortality | Tukey groups |
|---|---|---|---|---|
| Uninfected × infected23 | 1562 | 24 | 9.64±1.2 | ab |
| Uninfected × infected35 | 2160 | 27 | 10.59±1.0 | ab |
| Uninfected × infected46 | 2132 | 26 | 10.27±2.0 | ab |
| Infected23 × uninfected | 761 | 9 | 11.94±2.5 | ab |
| Infected35 × uninfected | 1350 | 16 | 11.38±2.8 | ab |
| Infected46 × uninfected | 1139 | 13 | 13.45±2.9 | ab |
| Infected23 × infected23 | 1958 | 21 | 12.10±1.3 | ab |
| Infected35 × infected35 | 1629 | 21 | 15.46±3.9 | ab |
| Infected46 × infected46 | 1928 | 24 | 17.99±2.5 | b |
| Uninfected × uninfected | 2011 | 25 | 7.26±1.2 | a |
Tukey groups, different letters correspond to statistically significant differences at 5%.
The cytoplasmic incompatibility tests were performed in generation third, fifth, tenth and eleventh.
Table 5. Egg mortality of D. simulans transinfected flies.
| Cross (female × male) | Eggs | Number of crosses | % Mortality | Tukey groups |
|---|---|---|---|---|
| Uninfected × infected4 | 875 | 16 | 1.62±0.6 | a |
| Uninfected × infected24 | 881 | 16 | 3.77±0.9 | ab |
| Uninfected × infected30 | 1551 | 18 | 5.25±1.2 | ab |
| Uninfected × infected45 | 1319 | 17 | 6.22±1.9 | b |
| Infected4 × uninfected | 1000 | 16 | 1.84±0.4 | ab |
| Infected24 × uninfected | 1349 | 21 | 2.40±0.6 | ab |
| Infected30 × uninfected | 827 | 13 | 4.00±1.4 | ab |
| Infected45 × uninfected | 1173 | 18 | 3.65±1.1 | ab |
| Infected4 × infected4 | 1214 | 18 | 1.35±0.3 | a |
| Infected24 × infected24 | 1047 | 15 | 1.02±0.2 | a |
| Infected30 × infected30 | 1234 | 14 | 1.86±0.5 | ab |
| Infected45 × infected45 | 1162 | 17 | 4.49±0.8 | ab |
| Uninfected × uninfected | 1313 | 14 | 2.77±0.8 | ab |
Tukey groups, different letters correspond to statistically significant differences at 5%.
The cytoplasmic incompatibility tests were performed in generations eleventh to fourteenth.
Fitness effects of Wolbachia in transinfected D. simulans
No major consistent difference in female fecundity was observed at any age between transinfected and control (uninfected) STCP lines (ANOVA and Tukey test: early F=0.752, P=0.476; middle F=3.672, P=0.034; and late F=0.128, P=0.880). A difference was found between infected lines 24 and 30 for the middle period, but the rebound in fecundity of older line 30 flies suggests that their low fecundity in the middle period may have been anomalous (Table 6). No significant effect of infection upon egg-to-adult viability was found in the two transinfected lines tested (ANOVA, F=0.618, P=0.54; Table 7).
Table 6. Fecundity of D. simulans transinfected flies.
|
Line | |||
|---|---|---|---|
| Female age | Infected24 | Infected30 | STCP (uninfected) |
| Early | 156±34.69 (21) | 160.87±23 (23) | 169.16±40.42 (16) |
| Middle | 91.57±25.36 (14) | 53.94±47.51 (17) | 71.58±34.71 (12) |
| Late | 95.63±53.56 (8) | 83.88±52.3 (8) | 70.13±43.09 (8) |
Entries are the mean number of eggs laid±s.d. (sample size) over a 3-day period. The fecundity was determined in generation fourtieth for both lines.
Table 7. Egg-to-adult viability of D. simulans transinfected flies.
| Line | Infected24 | Infected30 | STCP (uninfected) |
|---|---|---|---|
| 11.9±2.91 (21) | 12.39±2.44 (23) | 12.88±2.53 (16) |
Entries are the mean number of adults emerged from 15 eggs deposited by each of the original flies used for fecundity measures±s.d. (number of laying females). The egg-to-adult viability was determined in generation fourtieth for both lines.
Kaplan–Meier survival analysis performed separately for sex, line and generation revealed significant differences among vials in all possible combinations (Log-rank test—P<0.05, Supplementary Table 1). The median survival time for flies in each vial (days) was determined. These medians were subjected into two-way ANOVA using sex and line as fixed factors for both experiments in generation 45 (in 2007) and 95 (in 2009). In both cases, the interaction between sex and line was not significant (P=0.09 for 2007 and P=0.31 for generation 95). On the other hand, a highly significant difference was found among the lines for both generations (F=6.23, d.f.=2, 71, P=0.003 for generation 45 and F=6.30, d.f.=2, 59, P=0.003 for generation 95). Post-hoc Tukey test revealed that the D. simulans transinfected line 24 exhibited longer median adult survival than the control line in the two independent experiments performed in generations 45 and 90 (Table 8). In addition, males exhibited longer adult survival times than females for all lines. In generation 45, this difference is highly significant (F=23.58, d.f.=1, 71, and P<0.001), while in generation 95, the difference is marginal (F=4.05, d.f.=1, 59, and P=0.049).
Table 8. Survival analysis of D. simulans transinfected flies.
| Line | N | Survival days (mean (s.d.)) | Tukey groups |
|---|---|---|---|
| Generation 45 | |||
| STCP | 26 | 29.23 (12.06) | a |
| Insim30 | 22 | 31.59 (6.97) | a |
| Insim24 | 24 | 37.92 (11.32) | b |
| Generation 95 | |||
| STCP | 26 | 44.50 (8.26) | ab |
| Insim30 | 22 | 39.75 (6.68) | a |
| Insim24 | 24 | 48.00 (7.68) | b |
N, total number of vials per line (males and females).
Tukey, different letters correspond to statistically significant differences at 5%.
Wolbachia density
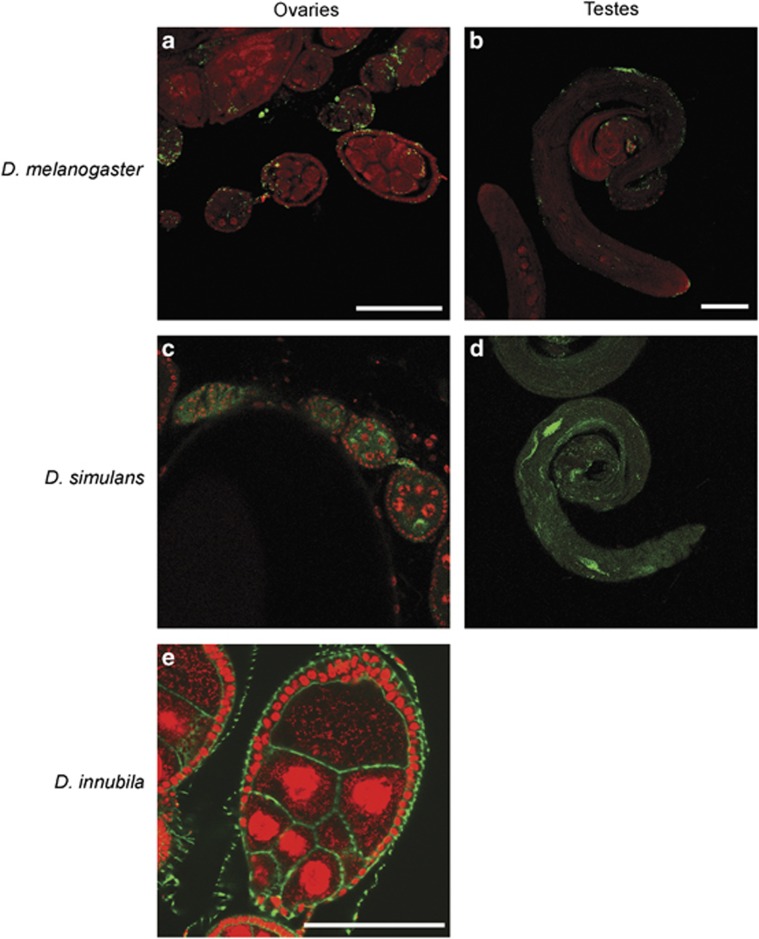
Immunofluorescence data of transinfected eggs, testes and ovaries overall suggested the presence of lower levels of bacteria in D. melanogaster than D. simulans (Figure 1). Bacteria were evenly distributed throughout the embryos of both D. melanogaster and D. simulans transinfected flies with considerable intra-line variation in density and D. simulans having, on average, 10 times more bacteria. The even distribution of Wolbachia throughout the embryos of both species suggested that the bacteria may not exhibit a special affinity for the germ plasm at this stage.
Figure 1.
Representative ovaries and testes of adult transinfected D. melanogaster (a and b) and transinfected D. simulans (c and d), respectively. Wolbachia are stained green and nuclei red (propidium iodide). Representative D. innubila egg chamber (e), the red stain is propidium iodide (which stains host and Wolbachia DNA), and the green is phalloidin (which stains F actin). Scale bars are 100 μm.
Testes of transinfected D. melanogaster flies contained very few bacteria of somatic origin and no infected cysts, while D. simulans testes showed a few heavily infected cysts. Finally, Wolbachia were abundant in the ovaries, especially in the early stages of oogenesis of both D. melanogaster and D. simulans transinfected flies.
Discussion
The first requirement for successful colonization of a new host species is that, following a lateral transfer event, Wolbachia must be transmitted from infected mothers to their offspring. More specifically, the relative selective advantage of infection to a cytoplasmic lineage (s) must, to a first approximation, exceed the proportion of uninfected offspring produced by an infected female (u). We observed only fair transmission of wInn within D. melanogaster, averaging only 73.2% (Table 1). To spread within D. melanogaster, the Wolbachia would have to confer a fitness advantage sufficient to overcome this imperfect transmission, that is, on the order of 30%. This is much greater than the 4–5% selective advantage that has been inferred in the native host, D. innubila (Dyer and Jaenike, 2004).
In contrast, the wInn infection was either quickly lost or quickly established with essentially perfect transmission in D. simulans. Such quick establishment indicates that the selective advantage required for spread would not have to overcome an initially low rate of maternal transmission, and thus that a relatively minor selective benefit would suffice for invasion. D. simulans may well be particularly conducive to Wolbachia transmission, as it has been successfully colonized at least four times by different Wolbachia strains in nature (Ballard, 2004). In D. innubila, wInn cells are dispersed within oocytes, rather than being localized at the posterior pole, as they are in D. simulans. This indicates that Wolbachia strain wInn can experience very high rates of maternal transmission, even when their patterns of localization are very different, as they are in D. innubila and D. simulans.
Studies of Wolbachia localization in late stage (10–14) oocytes have revealed strain-specific differences in localization patterns (Serbus and Sullivan, 2007). Specifically, the wMel strain was shown to localize to the posterior cortex in its native host and after transfer to D. simulans. In contrast, the wRi strain native to D. simulans does not exhibit localization to the posterior cortex in its native host. It is plausible that cortical localization is a feature important for efficient transmission of Wolbachia in D. melanogaster, owing to some unknown feature of the biology of this species. As wInn does not localize to the cortex of stage 10–14 oocytes in D. innubila, the low transmission efficiency of wInn in D. melanogaster relative to D. simulans could be linked to the absence of cortical localization.
Although wInn experiences moderate to high transmission in both D. melanogaster and D. simulans, this Wolbachia strain did not cause male killing in either of these novel host species (Tables 2 and 3). Given that wInn causes nearly 100% male killing in its native host, D. innubila, it is clear that the expression of male killing is highly dependent on host genetic background. The nearly equal offspring sex ratios in D. melanogaster and D. simulans also indicate that these Wolbachia do not cause parthenogenesis or feminization in either of these species.
We do not know why wInn does not cause embryonic male killing in the melanogaster group, nor why it does cause male killing in D. innubila. Wolbachia may target the dosage compensation system of D. innubila, as male-killing Spiroplasma have been shown to do in D. melanogaster (Veneti et al., 2005). As Drosophila males are XY, failure to upregulate transcription of their single X chromosome results in male lethality. Thus, interfering with the dosage compensation complex (DDC), which is responsible for such X chromosome upregulation in males, could bring about embryonic male killing. It is therefore notable that most or all of the genes encoding the proteins of the DDC have undergone rapid, positive selection in the melanogaster group (Levine et al., 2007; Rodriguez et al., 2007). Therefore, if wInn targets a certain component of the DDC of D. innubila, it is possible that this component is sufficiently different in D. melanogaster and D. simulans to be unrecognizable. In fact, Rodriguez et al. (2007) postulate that rapid evolution of DDC genes in D. melanogaster may result from an arms race with the male-killing Spiroplasma that targets that complex. Thus, even though D. melanogaster is currently susceptible to Spiroplasma-induced male killing, this arms race may have rendered melanogaster group species resistant to the male-killing effects of Wolbachia strain wInn.
Alternatively, perhaps male-killing Wolbachia attack males even earlier in the process of sexual differentiation although it should be noted that male killing does not necessarily target early processes, as shown in Hypolimnas bolina (Charlat et al., 2007). Although the basic molecular mechanisms involved in sex determination are conserved across the genus Drosophila, there are some variations on the basic theme (Marín and Baker, 1998). The master gene at the top of the regulatory hierarchy, Sxl, is expressed only in females in species of the subgenus Sophophora (including D. melanogaster and D. simulans), whereas it is expressed in both males and females in species of the virilis group, including D. borealis, which belongs to the subgenus Drosophila (Bopp et al., 1996). Intriguingly, the male-specific Sxl protein in the virilis group is somewhat smaller than the female-specific protein. Thus, D. borealis (and perhaps D. innubila) produce a male-specific protein in the sex determination pathway that is not produced by species of the melanogaster group. Perhaps wInn and the closely related Wolbachia strain in D. borealis target this protein or something downstream to cause male killing. In any case, our study demonstrates that male killing, although perhaps less complex mechanistically than CI, is not a default phenotype for Wolbachia strain wInn. Instead, the phenotypic expression of male killing by wInn exhibits a higher level of host specificity than the potential host range of this strain.
We also found no evidence that wInn causes CI in either D. simulans or D. melanogaster (Tables 4 and 5). The lack of CI in D. melanogaster may result from the failure of strain wInn to colonize the testes of this species (Figure 1), presumably a necessary requirement for Wolbachia-mediated modification of sperm that underlies the expression of CI (Veneti et al., 2003). The lack of CI in D. simulans is especially noteworthy, as several other strains of Wolbachia, including those that naturally infect this species, as well as some transinfected into D. simulans (Braig et al., 1994; Poinsot et al., 1998), do cause CI (all but wAu; (Hoffmann et al., 1996)). Thus, although D. simulans appears to be particularly susceptible to CI, strain wInn is unable to cause such effects in this species, even though these Wolbachia clearly colonize the testes (Figure 1). That strain wInn does colonize and proliferate within the testes of D. simulans is particularly interesting. As wInn is a male killer in D. innubila, this strain of Wolbachia very rarely occurs in males in its native host. Furthermore, because this strain has probably been a male killer for at least 15 000 years (Jaenike and Dyer, 2008), this suggests a very slow decay in the ability of Wolbachia to colonize the testes of host Drosophila.
Thus, the results of our experiments indicate that the strain of Wolbachia that causes male killing in D. innubila exhibits no reproductive manipulation in either D. simulans or D. melanogaster. In contrast, it is interesting to note that the male-killing strain of Wolbachia that infects the butterfly H. bolina expresses CI upon the evolution of resistance to male killing in the butterflies (Hornett et al., 2006). Whether this occurs within D. innubila is not known, as this species does not appear to have evolved any resistance to male killing (Jaenike and Dyer, 2008).
Our assays of D. simulans fitness revealed that wInn had no significant effect on either egg-to-adult viability or lifetime female fecundity. Relative to an uninfected line, adult survival was unaffected in one line of infected flies, and slightly, but significantly and consistently greater in another. Although the two recipient lines of D. simulans were derived from the same strain, it is possible that they were genetically slightly different, having been derived from different transinfected flies, or that they harbored different microbial gut communities (including, possibly, pathogenic microbial species), owing to the ecological independence of these lines during many generations of laboratory culture. Thus, the difference in survival between the lines might be owing to direct effects of differences between their gut microbiotas or genetic makeup or to an interaction between Wolbachia infection and either host genotype or gut microbiota. If the increased longevity is indeed owing to Wolbachia, the present findings have important implications for understanding Wolbachia dynamics. First, if this survival advantage occurs across multiple genotypes of D. simulans, then wInn could spread deterministically as a mutualist, independently of any reproductive manipulation of its new host species. Furthermore, it has recently been found that wInn has antiviral protective effects in D. innubila (Unckless and Jaenike, 2011). If such effects were manifest in another host species, such as D. simulans, this could provide the necessary selective advantage to spread in natural populations.
Second, suppose the dynamics of infection in a new host species were governed by a balance between weak beneficial fitness effects and imperfect maternal transmission. This could result in a low, but stable equilibrium, prevalence of infection. This might enable the Wolbachia population in this host species to persist in sufficient numbers and for sufficient time to accumulate mutations enabling it to become a reproductive parasite and achieve a higher prevalence of infection. Thus, the association could conceivably evolve from mutualism to parasitism.
Finally, consider the fate of a new Wolbachia strain that causes CI in D. simulans. If this strain has an adverse effect on female fitness, then the prevalence of Wolbachia infection must exceed a particular threshold frequency in order to spread (Caspari and Watson, 1959; Turelli and Barton, 1994). The lack of any adverse effects of wInn infection on D. simulans means that, if it could cause CI, this infection could spread from an arbitrarily low initial frequency. Perhaps the success of the ST-13 strain complex results in part from the relatively benign effects of these Wolbachia on female hosts.
Data archiving
Data have been deposited at Dryad; doi:10.5061/dryad.m78fk.
Acknowledgments
We thank the editor and two anonymous reviewers for helpful comments on the manuscript. This research was supported in part by the European Union grants CSA-SA_REGPOT-2007-1 (MICROBEGR—under grant agreement no. 203590) and CSA-SA REGPOT-2008-2 (BIODESERT—under grant agreement no. 245746) to KB, and NSF grants DEB 0542094 and 0918872 to JJ. KB also acknowledges support through the European Union COST Action FA0701: ‘Arthropod Symbiosis: From Fundamental Studies to Pest and Disease Management'.
Footnotes
Supplementary Information accompanies the paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Baldo L, Dunning Hotopp JC, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO. Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Mol Biol Evol. 2004;21:428–442. doi: 10.1093/molbev/msh028. [DOI] [PubMed] [Google Scholar]
- Bopp D, Calhoun G, Horabin JI, Samuels M, Schedl P. Sex-specific control of Sex-lethal is a conserved mechanism for sex determination in the genus Drosophila. Development (Cambridge, England) 1996;122:971–982. doi: 10.1242/dev.122.3.971. [DOI] [PubMed] [Google Scholar]
- Bourtzis K, Nirgianaki A, Markakis G, Savakis C. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics. 1996;144:1063–1073. doi: 10.1093/genetics/144.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig HR, Guzman H, Tesh RB, O'Neill SL. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature. 1994;367:453–455. doi: 10.1038/367453a0. [DOI] [PubMed] [Google Scholar]
- Caspari E, Watson GS. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution. 1959;13:568–570. [Google Scholar]
- Charlat S, Davies N, Roderick GK, Hurst GD. Disrupting the timing of Wolbachia-induced male-killing. Biol Lett. 2007;3:154–156. doi: 10.1098/rsbl.2006.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PE, McMeniman CJ, O'Neill SL. Modifying insect population age structure to control vector-borne disease. Adv Exp Med Biol. 2008;627:126–140. doi: 10.1007/978-0-387-78225-6_11. [DOI] [PubMed] [Google Scholar]
- Dyer KA, Jaenike J. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genomes. Genetics. 2004;168:1443–1455. doi: 10.1534/genetics.104.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree PM, Frydman HM, Li JM, Cao J, Wieschaus E, Sullivan W. Wolbachia utilizes host microtubules and Dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 2005;1:e14. doi: 10.1371/journal.ppat.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier S, Pintureau B, Heddi A, Lassabliere F, Jager C, Louis C, et al. Successful horizontal transfer of Wolbachia symbionts between Trichogramma wasps. Proc R Soc Lond B. 1998;265:1441–1445. [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia?--A statistical analysis of current data. FEMS Microbiol Lett. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Turelli M. Unidirectional incompatibility in Drosophila simulans: inheritance, geographic variation and fitness effects. Genetics. 1988;119:435–444. doi: 10.1093/genetics/119.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Turelli M, Simmons GM. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Hornett EA, Charlat S, Duplouy AMR, Davies N, Roderick GK, Wedell N, et al. Evolution of male-killer suppression in a natural population. PLoS Biol. 2006;4:e283. doi: 10.1371/journal.pbio.0040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61:2244–2252. doi: 10.1111/j.1558-5646.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer KA. No resistance to male-killing Wolbachia after thousands of years of infection. J Evol Biol. 2008;21:1570–1577. doi: 10.1111/j.1420-9101.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M.2005Survival Analysis: A Self-Learning Text2nd edn.Springer; Science+Business Media, inc., New York, NY, USA. p.590 [Google Scholar]
- Levine MT, Holloway AK, Arshad U, Begun DJ. Pervasive and largely lineage-specific adaptive protein evolution in the dosage compensation complex of Drosophila melanogaster. Genetics. 2007;177:1959–1962. doi: 10.1534/genetics.107.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín I, Baker BS. The evolutionary dynamics of sex determination. Science (New York, NY) 1998;281:1990–1994. doi: 10.1126/science.281.5385.1990. [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang Y-F, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- O'Grady P, DeSalle R. Out of Hawaii: the origin and biogeography of the genus Scaptomyza (Diptera: Drosophilidae) Biol Lett. 2008;4:195–199. doi: 10.1098/rsbl.2007.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SL, Giordano R, Colbert AM, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill SL, Karr TL. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature. 1990;348:178–180. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulos C, Bordenstein SR, Wernegreen JJ, Werren JH, Bourtzis K. Toward a Wolbachia multilocus sequence typing system: discrimination of Wolbachia strains present in Drosophila species. Curr Microbiol. 2006;53:388–395. doi: 10.1007/s00284-006-0054-1. [DOI] [PubMed] [Google Scholar]
- Poinsot D, Bourtzis K, Markakis G, Savakis C, Merçot H. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics. 1998;150:227–237. doi: 10.1093/genetics/150.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, Vermaak D, Bayes JJ, Malik HS. Species-specific positive selection of the male-specific lethal complex that participates in dosage compensation in Drosophila. Proc Natl Acad Sci USA. 2007;104:15412–15417. doi: 10.1073/pnas.0707445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridaki A, Bourtzis K. Wolbachia: more than just a bug in insects genitals. Curr Opin Microbiol. 2010;13:62–72. doi: 10.1016/j.mib.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kubo T, Ishikawa H. Interspecific transfer of Wolbachia between two lepidopteran insects expressing cytoplasmic incompatibility: a Wolbachia variant naturally infecting Cadra cautella causes male killing in Ephestia kuehniella. Genetics. 2002;162:1313–1319. doi: 10.1093/genetics/162.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Massaki N, Kubo T. Wolbachia variant that induces two distinct reproductive phenotypes in different hosts. Heredity. 2005;95:389–393. doi: 10.1038/sj.hdy.6800737. [DOI] [PubMed] [Google Scholar]
- Serbus L, Sullivan W. A cellular basis for Wolbachia recruitment to the host germline. PLoS Pathog. 2007;3:e190. doi: 10.1371/journal.ppat.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeley SL, McAllister BF. Mobile male-killer: similar Wolbachia strains kill males of divergent Drosophila hosts. Heredity. 2009;102:286–292. doi: 10.1038/hdy.2008.126. [DOI] [PubMed] [Google Scholar]
- Stahlhut JK, Desjardins CA, Clark ME, Baldo L, Russell JA, Werren JH, et al. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Mol Ecol. 2010;19:1940–1952. doi: 10.1111/j.1365-294X.2010.04572.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Subramanian S, Kumar S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol. 2004;21:36–44. doi: 10.1093/molbev/msg236. [DOI] [PubMed] [Google Scholar]
- Turelli M, Barton NH. Genetic and statistical analyses of strong selection on polygenic traits: what, me normal. Genetics. 1994;138:913–941. doi: 10.1093/genetics/138.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Jaenike J. Maintenance of a male-killing Wolbachia in Drosophila Innubile by male-killing dependent and male killing independent mechanisms. Evolution. 2011;66:678–689. doi: 10.1111/j.1558-5646.2011.01485.x. [DOI] [PubMed] [Google Scholar]
- van Meer MM, Stouthamer R. Cross-order transfer of Wolbachia from Muscidifurax uniraptor (Hymenoptera: Pteromalidae) to Drosophila simulans (Diptera: Drosophilidae) Heredity. 1999;82:163–169. doi: 10.1038/sj.hdy.6884610. [DOI] [PubMed] [Google Scholar]
- Vavre F, Girin C, Bouletreau M. Phylogenetic status of a fecundity-enhancing Wolbachia that does not induce thelytoky in Trichogramma. Insect Mol Biol. 1999;8:67–72. doi: 10.1046/j.1365-2583.1999.810067.x. [DOI] [PubMed] [Google Scholar]
- Veneti Z, Bentley JK, Koana T, Braig HR, Hurst GD. A functional dosage compensation complex required for male killing in Drosophila. Science. 2005;307:1461–1463. doi: 10.1126/science.1107182. [DOI] [PubMed] [Google Scholar]
- Veneti Z, Clark ME, Karr TL, Savakis C, Bourtzis K. Heads or tails: host-parasite interactions in the Drosophila-Wolbachia system. Appl Environ Microbiol. 2004;70:5366–5372. doi: 10.1128/AEM.70.9.5366-5372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneti Z, Clark ME, Zabalou S, Karr TL, Savakis C, Bourtzis K. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics. 2003;164:545–552. doi: 10.1093/genetics/164.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc Biol Sci. 1995;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I, et al. Multiple rescue factors within a Wolbachia strain. Genetics. 2008;178:2145–2160. doi: 10.1534/genetics.107.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Charlat S, Nirgianaki A, Lachaise D, Merçot H, Bourtzis K. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics. 2004;167:827–834. doi: 10.1534/genetics.103.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.