Abstract
The objective of this cohort study—conducted at a regional trauma unit in southern Ontario, Canada—was to review the imaging history of open-section, iliac-wing bone graft donor sites in lumbar fusion patients. Intervention entailed review of available X-ray and CT scan images for all patients undergoing lumbar fusion with iliac autograft in the senior author’s practice over a 4-year period. Outcome was radiographic confirmation of the absence of bony reconstitution at the iliac harvest site. Of 239 primary fusions performed, 209 complete imaging records were available for review. The images of a further 20 patients who had surgery with the senior author prior to the study period and who presented at the office in the first half of 2000 were also assessed. All cases showed persistence of the iliac donor harvest site defect. Only minimal marginal sclerosis to suggest attempted remodeling was observed. We conclude that iliac-wing bone graft donor sites do not remodel. Given that iliac harvesting is known to increase strain in the pelvis, and that lumbosacral stabilization increases stress in the pelvis, permanent deficiency of iliac bone stock at donor harvest site may be a factor in both primary donor site pain and the observed high frequency of this problem in lumbosacral fusion patients.
Keywords: Iliac wing, bone graft, morbidity.
Introduction
Lumbar spinal fusion surgery for the relief of low back pain is an increasingly common procedure, with instrumented cases alone accounting for over 200,000 surgeries annually in the United States alone [15]. Autogenous iliac bone graft is recognized as the “gold standard” bone grafting material for this and other procedures.
Donor site morbidity may relate to a number of potential complications, ranging in severity and acuity from bleeding, wound infection and neurovascular injury to the contents of the sciatic notch to pain, scar tenderness and cluneal neuroma through fracture and sacroiliac dislocation. Chronic and otherwise nonspecific local pain is the most common of these [1, 2, 3, 4, 6, 7, 8, 9, 11, 16, 18].
Bone graft operations are most frequently (21–63% in the lumbosacral fusion population) complicated by chronic iliac-wing donor site pain at the graft-harvesting site [6, 7]. Donor site pain typically presents as a dull ache deep to the medial buttock directly over the harvest bed. The exact etiology of this pain to date remains obscure.
There appears to be a disproportionate frequency of donor site pain complaints presenting in those patients undergoing otherwise pain-relieving lumbar reconstructive procedures, as compared with patients harvested for spinal deformity correction or other non-spinal indications. Frymoyer found that, at 10 years after surgery, 63% of lumbar fusion patients had donor site complaints as opposed to 37% of those harvested for other reasons [6]. Goulet found, at 2 years after surgery, that 21% of lumbar fusion patients had donor site pain compared to only 6% of non-fusion patients [7]. This increased frequency of donor site pain in lumbar fusion patients is commonly attributed to behavioral issues in the subject back-pain population.
Even among spine fusion patients, the frequency of donor site complaints is statistically (P<0.05) shown to be greater in lumbosacral reconstruction fusing the spine to the pelvis than it is in cervical or thoracolumbar fusion cases [18].
The late fate of the bone stock deficiency at the harvested iliac wing is not well investigated. Medige and colleagues investigated the fate of experimental open-section metaphyseal bone defects in the canine distal femur [14]. Their model incorporated a silicon plug inserted within the harvest defect to prevent simple healing of the defects. They found that extensive bony remodeling with marginal cortication of the defects and metaphyseal widening returned the torsional strength of their specimens to 90% of normal in 48 weeks.
The senior author (D.A.B.) has had an established practice in adult spine reconstruction. The observed [1] frequency of chronic iliac-wing donor site pain in this practice, at 25–33% of cases, is consistent with the similar published observations of Frymoyer [6], Goulet [7] and Robertson [18], who found that 63%, 21% and 45%, respectively, of their lumbar fusion patients had late donor site pain.
Late persistence of bone defects at the iliac harvest site has been a frequent observation in this practice, much as has been incidentally noted by the observations of Ebraheim et al. [3] in patients with late sacroiliac pain. These persisting donor site defects typically develop a characteristic and easily recognizable sclerotic margin, seen medially in the AP radiographic view (Fig. 1). The author has encountered several cases of photopenia at the donor site (or “increased uptake” contralaterally) reported in bone scans of these patients as performed for late or persisting pain.
Fig. 1.
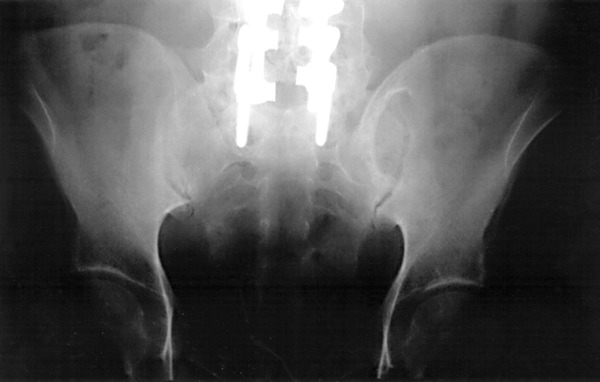
Anteroposterior radiograph of the pelvis of a fusion patient showing the typical sclerotic medial margin of the iliac wing donor site at 38 months
On one occasion, a former fusion patient presenting with acute low back pain in another city was misdiagnosed with a “lytic lesion of the ilium,” as his donor site defect was misinterpreted, and he in fact underwent an unwarranted cancer workup.
We have reviewed the imaging evolution of iliac-wing bone graft harvesting sites in the senior author’s practice and suggest that reconstitution or “healing in” of these defects, if any, is very incomplete as late as decades after the harvesting.
Such incomplete reconstitution of bone stock in the pelvis may have important implications to the etiology of late donor site pain and its increased frequency in the lumbar fusion population as compared to those harvested for other indications.
Materials and methods
Practice records for 1994 through 1997 were reviewed and 241 cases of lumbar fusion identified. Two were patients undergoing repeat surgery, leaving 239 primary procedures for review. Postoperative imaging was available in the hospital imaging files of 209 cases (85%).
The technique of bone graft harvesting from the posterior ilium in this practice is standardized, as reported in an earlier investigation of donor site morbidity [2]. A separate reversed-obliquity iliac incision is made, followed by harvest of the outer table and cancellous core of the iliac wing down to the level of the inner table. As determined by the size of the pelvis, three to five cortical strips measuring 10—15 mm wide by 40—60 mm deep are taken. Every attempt is made to preserve the deep cortex of the ilium intact. Millimetric perforations are occasionally incurred, and harvesting is discontinued when they occur. Harvest is followed by closure over a Gelfoam pledget. This technique creates an open-section defect in the posterior ilium in direct continuity with the overlying abductor musculature.
The patients were routinely seen in follow-up, and X-rays of the lumbar spine and adjacent pelvis were obtained at 6 weeks and 12 weeks, then again at 6 months, 12 months,18 months and 24 months, and thereafter once a year. CT scans were obtained as indicated by persistent or new-onset pain.
All these images were retrieved and independently reviewed for the purposes of this study by a senior orthopedic resident (W.A.T.) for qualitative evidence of a sclerotically marginated, permanent bone-stock deficiency at the ilium. Where the imaging file contained a postoperative or late CT scan of the lumbar spine or pelvis, these scans were similarly reviewed and the persistence or absence of a donor site defect (Fig. 2) was recorded. Defect size was not measured.
Fig. 2.
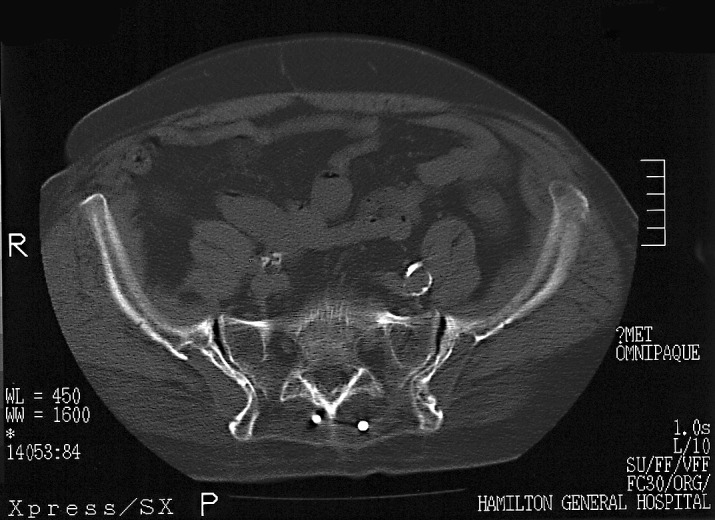
Typical defects seen on the pelvic CT scan of a patient who had two different operations with the senior author. Right-side defect is at 65 months after harvesting, left-side defect is at 50 months
Concomitantly, some 20 former patients who had more remotely undergone surgery with this technique and who randomly re-presented to the senior author’s practice in the first half of 2000 were imaged and their radiographs similarly reviewed by the senior author for persistence of the donor site defect.
Results
Persisting defects of bone stock at the harvesting site were consistently observed in all cases (100%), both radiographically and in CT scans. There were no cases of osteomyelitis, fracture or sacroiliac arthritis identified in this series.
Fully 88 cases (42% of the independent radiographic follow-up group) had a minimum radiographic follow-up of 24 months (mean, 51 months; range, 24–108 months). This group included 42 males of mean age 54 years (range, 38—90 years) and 46 females of mean age 52 years (range, 21—78 years) at surgery. In this group we include the incidental observation of a typical sclerotic donor site defect in a woman who had lumbosacral fusion in 1976, well before the senior author returned her to the operating room for reconstruction of adjacent segment disease proximal to the original procedure. At 27 months, the more recent donor site defect was not distinguishable from the older one (Fig. 3). In all cases (100%), the donor site defect could be visualized.
Fig. 3.
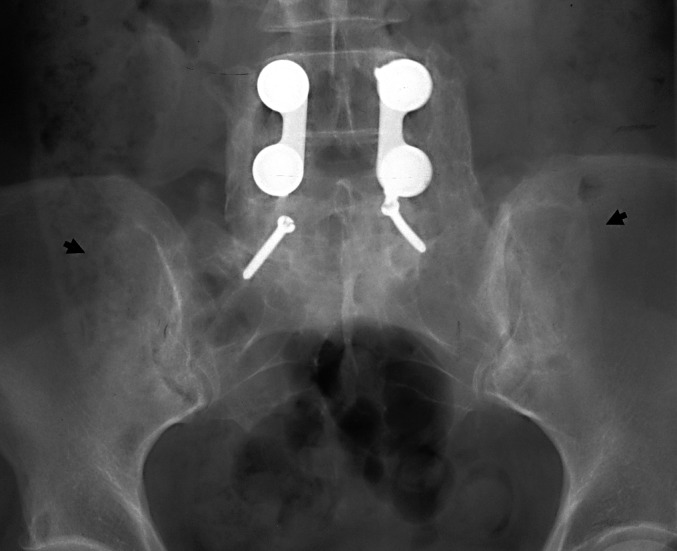
Anteroposterior radiograph shows bilateral donor site defects. Left-side defect is from the author’s operation at 27 months; right-side from an earlier surgery at 24 years
The balance of 121 cases (58% of the independently evaluated group) had X-ray follow-up of less than 24 months (mean, 11 months; range, 1–23 months). This group included 64 males of mean age 51 years (range, 36–78 years) and 57 females of mean age 54 years (range, 34–72 years) at surgery. Again, in all cases (100%) the harvesting defect was obvious.
CT scans were available for review in 46 cases (22%). In all instances, a persisting corticated defect at the ilium was observed. Some 32 cases were scanned at a minimum of 24 months (mean, 51 months; range, 24–87 months) and 14 at less than 24 months (mean, 12 months; range, 2–23 months). In none was there evidence of a major violation of the sacroiliac joint, as has been recently described by Epstein [3].
Twenty former patients re-presented to the senior author’s practice in the first half of the year 2000. These include six males of mean age 53 years (range, 47–67 years) and 14 females, also of mean age 53 years (range, 21–76 years) at follow-up.
Imaged at a mean of 78 months after surgery, in every case the iliac donor site defect was obvious.
Discussion
Failure of reconstitution at open-section, iliac-wing bone graft harvesting sites has obvious implications for the availability of bone stock at repeat surgery and might support the pursuit of several alternative practice options. Harvesting techniques that create a closed-section defect have been proposed [16]. The use of an interposition barrier to close over the donor site after harvest is presented by industry [21]. Local bone as harvested from the spine in performing decompression might be used for fusion [20]. Synthetic bone graft substitutes and bone morphogenetic proteins (BMPs) may be indicated [5].
The biomechanical implications of a permanent defect in the posterior ilium may be a factor in the observed high frequency of donor site complaints in the lumbosacral fusion population. Structural investigations have shown that the removal and subsequent deficiency of bone at the posterior ilium can lead to significantly increased strain in the pelvis [19].
Biomechanical studies of lumbosacral instrumentation have suggested not only increased stress in the lumbar motion segments above a lumbosacral fusion [17], but also increased stress in the pelvis below [17, 22]. These stresses may be responsible for both accelerated degeneration above lumbosacral fusions [11, 12] and even stress fracture of the intact pelvis below [23].
Consider the case of the low-back fusion patient after iliac-wing harvest. The unique combination of increased stress imposed by lumbosacral stabilization on the pelvis below the fusion and increased strain at the incompletely reconstituted iliac-wing donor site may not only be a factor in late donor site pain but may also explain the repeatedly observed high frequency of chronic donor site pain complaints in these patients [6, 7, 18]. This is as compared with those harvested for other indications where pelvic strain would be increased but for which lumbosacral stresses are normal. Possible etiologies for this pain include chronic microfractures within the posterior ilium and altered load transfer across the sacroiliac joint.
Conclusion
An imaging review of 209 lumbar fusion cases with follow-up as late as 24 years suggests that open-section iliac-wing bone graft harvesting sites are not reconstituted. This observation may have significant clinical, biomechanical and treatment implications for the management of diseases of the lumbosacral spine.
References
- 1.Colterjohn J Bone Joint Surg Am. 1998;79:756. doi: 10.2106/00004623-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Coventry J Bone Joint Surg Am. 1972;54:83. [PubMed] [Google Scholar]
- 3.Ebraheim Computed tomographic findings in patients with persistent sacroiliac pain after posterior iliac graft harvesting Spine. 2000;25:2047. doi: 10.1097/00007632-200008150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Fernando Spine. 1999;24:2101. doi: 10.1097/00007632-199910150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Finkemeier J Bone Joint Surg Am. 2002;84:454. doi: 10.2106/00004623-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer Clin Orthop. 1978;134:196. [PubMed] [Google Scholar]
- 7.GouletClin Orthop 1997339769186204 [Google Scholar]
- 8.Guha Br J Plast Surg. 1983;36:305. doi: 10.1016/s0007-1226(83)90048-6. [DOI] [PubMed] [Google Scholar]
- 9.HuClin Orthop 19943092087994963 [Google Scholar]
- 10.KuhnClin Orthop 19862092243731601 [Google Scholar]
- 11.Lee Spine. 1988;13:375. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Lehman Spine. 1997;12:97. [Google Scholar]
- 13.Lichtblau J Bone Joint Surg Am. 1962;44:193. [Google Scholar]
- 14.Medige Clin Orthop. 1982;169:275. [PubMed] [Google Scholar]
- 15.Mendenhall S (1999) Spinal surgery update. Orthopedic Network News (Mendenhall Associates, 1500 Cedar Bend Drive, Ann Arbor, MI 48105, USA) 10(4):1
- 16.Mirovsky Spine. 2000;25:1722. doi: 10.1097/00007632-200007010-00018. [DOI] [PubMed] [Google Scholar]
- 17.Rhanjee R, Wood KB, Wentorf FA (1998) Effect of lumbar and lumbosacral instrumentation on strains on the pelvis. In: Proceedings of the North American Spine Society, 13th Annual Meeting, San Francisco. North American Spine Society, pp 206–207
- 18.Robertson Spine. 2001;26:1473. doi: 10.1097/00007632-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 19.Varga Spine. 1996;21:1494. doi: 10.1097/00007632-199607010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Violas Spine. 2004;29:189. doi: 10.1097/01.BRS.0000105536.65164.B1. [DOI] [PubMed] [Google Scholar]
- 21.WangNeurosurgery 200251413. 10.1097/00006123-200208000-0002012182779 [DOI] [Google Scholar]
- 22.Wood Spine. 1996;21:1185. doi: 10.1097/00007632-199605150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Wood Spine. 1996;21:1357. doi: 10.1097/00007632-199606010-00016. [DOI] [PubMed] [Google Scholar]


