Abstract
We studied the distribution of fibronectin (a marker for “active” reparative connective tissue processes) and TGF-β1 (a cytokine controlling the connective tissue metabolism) in intervertebral disc tissue from individuals of different age and various histomorphological evidence for tissue degeneration. The protein deposition was determined by immunohistochemistry on 30 complete cross-sections of lumbar spine obtained at autopsy (0–86 years) and 12 surgically removed disc samples. The mRNA expression was detected by non-radioactive in situ hybridization in the surgical material. All control experiments (blank and isotype controls in immunohistochemistry/sense controls in in situ hybridization) were negative. Immunohistochemically, we detected enhanced staining for fibronectin in both nuclear and anular tissues in areas with histological signs of mild-to-severe tissue degeneration (e.g., cleft formation and cell clustering) beginning with 16 years of age. Anular tissue showed less fibronectin staining than did nuclear areas. Fibronectin mRNA was detected mainly in nuclear cells by in situ hybridization corresponding to the protein staining indicating de novo synthesis. In parallel, TGF-β1 was expressed by nuclear and occasional anular cells spatially associated with the fibronectin synthesizing cells. This was seen by both immunohistochemistry and in situ hybridization. This preliminary study provides evidence for a significant ongoing rearrangement of the extracellular matrix during disc degeneration, as monitored by enhanced fibronectin deposition that is produced by local disc cells. These cells also synthesize TGF-β1, as shown by protein and mRNA expression. Since it is known that TGF-β1 induces matrix alterations (by auto and paracrine stimulation of matrix synthesis), these observations suggest that the recently described disturbance of the matrix during disc degeneration may be induced by TGF-β. This may offer new approaches to interfere with disc matrix alterations.
Keywords: Intervertebral disc, In situ hybridization, Fibronectin, Cytokine
Introduction
The human lumbar intervertebral disc is a highly specialized structure composed of a complex system of various connective tissues. These are mainly made up of collagen fibrils providing either mechanical stability and/or flexibility. For the maintenance of the structure and, thus, function, it is essential that the collagenous matrix be properly organized. It is well established that normal intervertebral discs contain several different collagen types, the proportion of which is essential for the disc structure [17]. The composition of these fibrils is controlled by local cells that are involved directly or indirectly in their production by controlling the matrix synthesis through cytokine action [3, 29].
Ageing and degeneration of intervertebral discs are closely associated with a disarrangement of the matrix. Recent histomorphological analyses have shown that disc degeneration coincides with a significant disruption and remodeling of the matrix along with cell proliferation and decay [7, 10, 11, 16, 24, 23]. This is paralleled by the change in the collagen type distribution, such as the occurrence of collagen III and enhanced deposition of the microfibrillar collagen VI in discs with minor degenerative alterations [17, 25]. In advanced stages of disc alteration, the appearance of collagen I in the nucleus pulposus [17, 25] and the expression of collagen X in late stage degeneration [1, 6] are important features. In parallel, the production of major matrix degrading enzymes, the matrix metalloproteinases (MMPs), is significantly increased confirming the notion of enhanced disc matrix metabolism [26, 30].
These matrix alterations are a predominant feature of disc degeneration. However, “early” and “active” matrix lesions are still poorly defined and only rare data are as yet available on the occurrence and distribution of other matrix proteins, such as fibronectin [3, 20, 28, 29]. Since this protein is observed to be enhanced in the very early steps of various reparative processes, such as wound healing [4], the localization of fibronectin may indicate an “active” attempt of tissue remodeling. In addition, there is good evidence that fibronectin plays an important role in the mediation of reparative processes, since it is a potent chemoattractant for fibroblasts [2]. These features render the analysis of fibronectin attractive also in defining the onset of matrix disarrangement during disc degeneration.
Besides the investigation of the onset of the “degeneration-associated” matrix remodeling, the analysis of regulation factors for this process are of utmost importance. One of the most significant factors is the “transforming growth factor-β” (TGF-β), a family of cytokines that induces the synthesis of matrix constituents [14]. As yet, no study has been performed on disc material of various ages and degrees of degeneration—except for herniated material [20]. This, however, may not be representative for intradiscal alterations. Since both the occurrence and the cell type involved in the TGF-β production remain unclear, we examined the occurrence and distribution of TGF-β1 in intervertebral discs of defined disc pathology.
Material and methods
Study populations and tissue preparation
Two different study populations were included in this study, the autopsy group and the surgical group. The first group consisted of 30 intervertebral disc specimens covering the whole age range (fetal to 86 years) obtained from 20 cadavers (nine female, 11 male). None of the included individuals died of a consuming illness (e.g., tumor, infection) or had a known back problem. Complete lumbar motion segments were harvested at routine autopsy, as described in previous studies [6, 7, 17, 18, 19, 30]. Midsagittal and parasagittal sections through the motion segment were prepared for subsequent histology and immunohistochemistry. This allowed a correlation of the observations with the respective anatomic sub-setting. For the present analysis, these cases were selected to be representative with respect to age, sex, and histomorphological features.
Thin sagittal slices (5 mm) of the complete motion segments were fixed in 4–6% buffered formaldehyde, pH 7.4, subsequently decalcified (0.1 M ethylenediaminetetraacetic acid (EDTA), pH 7.4) for a period of 1–4 months and, after completion of decalcification, embedded into paraffin wax as routinely performed and as described previously [6, 7, 17, 18, 19, 30].
The surgical group encompassed 12 lumbar specimens obtained during surgical procedures (i.e., discectomies, anterior lumbar interbody fusion and scoliosis surgery) from 12 individuals (age range 31–76 years, five females and seven males). All patients underwent surgery for disc herniation or degenerative disc disease. The distribution of the samples across the disc levels was as follows: L1/2 (n=1), L2/3 (n=2), L4/5 (n=5), L5/S1 (n=4). Clinical data on low back pain history, related disability and imaging findings (radiographs, MRI) were available for all cases.
The material was immediately after removal (i.e., within few seconds) dissected into several small (0.5 cm×0.5 cm) pieces and immersed in buffered 4–6% formaldehyde in order to ensure conservation of mRNA. Thus, a rapid fixation of the material, i.e., fixation within less than 30 min after removal, was ensured. All manipulations of this material were carried out under RNase-free conditions (gloves, RNase-free reagents, etc.). The formalin-fixed tissue samples were treated as in group 1, i.e., cases with obvious calcifications or residual bone material had to be smoothly decalcified before embedding, which was performed in a significantly shorter time period than for group 1. All specimens were cut in slices (2–4 µm) and placed on silanized glass slides for routine and histochemical stainings (H&E, Masson-Goldner or Elastica-van Gieson’s connective-tissue stain, Alcian blue-PAS) as performed previously [7] and immunohistochemistry, respectively.
This second study population was necessary to obtain fresh tissue samples, because autopsy material is unsuitable for in situ hybridization analysis due to the well-known rapid postmortem degradation of the mRNA.
Immunohistochemistry
The immunostainings were essentially performed as described previously in detail [6, 7, 17, 18, 19]. For the localization of fibronectin, we used a commercially available antibody (DAKO, Hamburg, Germany), and for the immunostaining of TGF-β1 we applied a specific antibody purchased from Santa Cruz Inc. (Santa Cruz, CA, USA). Briefly, de-paraffinized appropriate slides were incubated with the specific primary antibody. Following washing steps, a secondary antibody system coupled with the avidin-biotin complex (Vector, Burlingame, USA) was used in order to localize the antigen. As a chromogenic substrate, we used diaminobenzidine (DAKO).
Control sections using either nonspecific immunoglobulins of comparable concentration (“isotype control”) or after omission of the specific primary antibody (“blank control”) were run in parallel.
Preparation of riboprobes for the non-radioactive in situ hybridization
For the preparation of riboprobes for in situ hybridization, we used the following specific cDNA clones: a 1365 bp clone for fibronectin was purchased from Biomol, Hamburg, Germany and a 360 bp clone for TGF-β1 came from Biermann Diagn., Bad Nauheim, Germany. The specificity of all probes was assessed by Northern blot analysis showing specific bands.
All probes had been subcloned into a pGEM3Z plasmid (Promega, Madison, MI, USA). After linearization of the plasmid, single-stranded RNA probes complementary (antisense) or anticomplementary (sense probe, negative control) to cellular RNA were obtained by run-off transcription using T7 or SP6 polymerase. The probes were labeled either with digoxigenin, or they were biotinylated (both, Boehringer, Mannheim, Germany) using labeled uridine triphosphate. This procedure was performed using labeling kits (Boehringer, Mannheim, Germany) according to the manufacturer’s instructions.
In situ hybridization
The rationale for in situ-hybridization investigations was to provide additional evidence for the synthesis of the fibronectin/TGF-β1 within resident disc cells that have been localized in the respective disc tissue by immunohistochemistry.
Appropriate paraffin sections from the 4% buffered paraformaldehyde/PBS-fixed material were de-paraffinized, rehydrated and subjected to proteinase K digest (0.4%; Sigma Chemical, Munich, Germany). In situ hybridization was performed overnight, essentially as described previously in detail [8, 30]. Therefore, the predigested slides were incubated with the digoxigenin-labeled probes overnight, the reaction products were detected by a monoclonal anti-digoxigenin antibody (Boehringer, Mannheim, Germany) and localized using the alkaline phosphatase-anti-alkaline phosphatase (APAAP) system [9] (Dianova; fast red, Sigma Chemical).
Evaluation of stainings
All slides were first subjected to a qualitative examination for the immunostaining/ in situ hybridization results. The evaluation of the slides was performed by two independent observers (A.G.N., N.B.). Divergent results were resolved in conference.
The observations were related to the underlying routine morphological alterations that were classified as following: clustering of chondrocytes, tear and cleft formation, granular matrix changes and fibrillar demasking of collagen fibrils, as well as cellular decay, were regarded as morphological signs for disc degeneration [7]. The immunostainings and in situ hybridization data were related to those tissue changes and their staining intensity (immunohistochemistry for fibronectin) and/or amount of labeled cells (immunohistochemistry for TGF-β1 and in situ hybridizations) were estimated.
In addition, we conducted a semi-quantitative estimation of the amount of positively labeled cells. This was applied to the expression levels of protein and mRNA for TGF-β1 (which is localized only within the cells) and for the mRNA expression for fibronectin. (The immunohistochemical staining for fibronectin protein is present only in the extracellular matrix). In order to make the results comparable between different regions within the nucleus pulposus or annulus fibrosus and to keep the intra-individual variability low, the observations were ranked into five grades: 0, no staining; 1, 1–5% of all cells are labeled; 2, >5–10% of all cells are stained; 3, >10–25% of all cells are labeled; 4, >25% are stained. These rankings were done in ten random areas of both the nuclear and anular areas of the specimen. The predominant ranking for each specimen of both study groups is indicated in Tables 1 and 2.
Table 1.
Localization of fibronectin (FN) and TGF β1 protein by immunohistochemistry (IHC) in nucleus pulposus (NP) and anulus fibrosus (AF) material of autopsy specimens
| Specimen | Age/sex | HDS* | IHC-FN (+/+++)1 | IHC-TGFβ1 2 (% of cells) |
|---|---|---|---|---|
| 1 | 35 weeks/f | 0 | NP: + | NP: 0 |
| AF: 0 | AF: 0 | |||
| 2 | 1 day/m | 2 | NP: 0 | NP: 1 |
| AF: 0 | AF: 0 | |||
| 3 | 2 months/f | 2 | NP: + | NP: 0 |
| AF: 0 | AF: 0 | |||
| 4 | 24 months/m | 2 | NP: ++ | NP: 1 |
| AF: + | AF: 1 | |||
| 5 | 28 months/f | 3 | NP: + | NP: 0 |
| AF: 0 | AF: 0 | |||
| 6 | 8 years/m | 3 | NP: + | NP: 0 |
| AF: 0 | AF: 0 | |||
| 7 | 13 years/m | 3 | NP: + | NP: 2 |
| AF: + | AF: 1 | |||
| 8 | 16 years/f | 10 | NP: ++ | NP: 1 |
| AF: 0 | AF: 1 | |||
| 9 | 18 years/m | 11 | NP: +++ | NP: 3 |
| AF: + | AF: 1 | |||
| 10 | 20 years/m | 13 | NP: +++ | NP: 3 |
| AF: + | AF: 1 | |||
| 11 | 22 years/m | 15 | NP: ++ | NP: 2 |
| AF: 0 | AF: 0 | |||
| 12 | 22 years/f | 12 | NP: ++ | NP: 3 |
| AF: + | AF: 1 | |||
| 13 | 24 years/m | 11 | NP:+++ | NP:3 |
| AF:+ | AF:1 | |||
| 14 | 24 years/m | 14 | NP:+++ | NP:4 |
| AF:++ | AF:2 | |||
| 15 | 25 years/m | 13 | NP:++ | NP:2 |
| AF:+ | AF:1 | |||
| 16 | 25 years/m | 10 | NP:+++ | NP:4 |
| AF:+++ | AF:3 | |||
| 17 | 25 years/m | 13 | NP:+++ | NP:2 |
| AF:++ | AF:2 | |||
| 18 | 31 years/m | 15 | NP:++ | NP:3 |
| AF:+++ | AF:3 | |||
| 19 | 47 years/f | 16 | NP:+++ | NP:4 |
| AF:++ | AF:1 | |||
| 20 | 47 years/m | 15 | NP:++ | NP:2 |
| AF:+ | AF:1 | |||
| 21 | 58 years/m | 16 | NP:++ | NP:3 |
| AF:++ | AF:2 | |||
| 22 | 66 years/m | 14 | NP:++ | NP:1 |
| AF:++ | AF:1 | |||
| 23 | 66 years/m | 17 | NP:+++ | NP:3 |
| AF:+ | AF:2 | |||
| 24 | 74 years/f | 16 | NP:+ | NP:1 |
| AF:+ | AF:1 | |||
| 25 | 77 years/m | 14 | NP:+ | NP:1 |
| AF:0 | AF:0 | |||
| 26 | 77 years/f | 15 | NP:++ | NP:2 |
| AF:++ | AF:1 | |||
| 27 | 77 years/f | 15 | NP:+ | NP:1 |
| AF:+ | AF:1 | |||
| 28 | 81 years/f | 12 | NP:0 | NP:1 |
| AF:+ | AF:1 | |||
| 29 | 85 years/f | 14 | NP:+ | NP:0 |
| AF:0 | AF:0 | |||
| 30 | 86 years/f | 13 | NP:0 | NP:1 |
| AF:+ | AF:0 |
*Histodegeneration score (HDS): 0–18 points (NP) [7]
1Extent of stained matrix area with 0=no staining; + = minor, focal stained area; ++ = moderate stained area; +++ = extensive area with positive staining
2Scoring of immunohistochemical TGF-β1 labeling: 0, no labeled cells; 1, 1–5% labeled cells; 2, >5–10% labeled cells; 3, >10–25% labeled cells; 4, >25% labeled cells
Table 2.
Localization of fibronectin (FN) and TGF β1 protein by immunohistochemistry (IHC) and mRNA by in situ hybridization (ISH) in nucleus pulposus (NP) and anulus fibrosus (AF) material of surgical specimens
| No. | Age/sex | Clinical diagnosis | Surgical procedure | HDS-m* | IHC-FN (+/+++)1 | ISH-FN2 | IHC-TGFβ12 | ISH-TGFβ12 |
|---|---|---|---|---|---|---|---|---|
| 1 | 27/m | Protrusion | Discectomy | 11 | NP: ++ | NP: 3 | NP: 2 | NP: 2 |
| AF: + | AF: 1 | AF: 1 | AF: 1 | |||||
| 2 | 34/m | Degeneration | Fusion | 7 | NP: + | NP: 1 | NP: 2 | NP: 2 |
| AF: n.p.3 | AF: n.p.3 | AF: n.p.3 | AF: n.p.3 | |||||
| 3 | 35/f | Extrusion | Discectomy | 8 | NP: + | NP: 2 | NP: 1 | NP: 0 |
| AF: 0 | AF: 1 | AF: 1 | AF: 0 | |||||
| 4 | 36/m | Protrusion | Discectomy | 11 | NP: +++ | NP: 3 | NP: 2 | NP: 2 |
| AF: 0 | AF: 0 | AF: 1 | AF: 1 | |||||
| 5 | 39/f | Extrusion | Discectomy | 12 | NP: +++ | NP: 4 | NP: 1 | NP: 2 |
| AF: + | AF: 1 | AF: 0 | AF: 0 | |||||
| 6 | 41/f | Degeneration | Fusion | 9 | NP: + | NP: 2 | NP: 1 | NP: 1 |
| AF: n.p.3 | AF: n.p.3 | AF: n.p.3 | AF: n.p.3 | |||||
| 7 | 41/f | Degeneration | Fusion | 10 | NP: ++ | NP: 2 | NP: 2 | NP: 2 |
| AF: + | AF: 1 | AF: 1 | AF: 1 | |||||
| 8 | 47/m | Protrusion | Fusion | 10 | NP: ++ | NP: 1 | NP: 1 | NP: 2 |
| AF: + | AF: 1 | AF: 1 | AF: 1 | |||||
| 9 | 48/m | Protrusion | Fusion | 11 | NP: ++ | NP: 3 | NP: 2 | NP: 2 |
| AF: 0 | AF: 0 | AF: 0 | AF: 0 | |||||
| 10 | 52/m | Extrusion | Discectomy | 12 | NP: ++ | NP: 2 | NP: 2 | NP: 1 |
| AF: + | AF: 1 | AF: 1 | AF: 1 | |||||
| 11 | 58/m | Degeneration | Fusion | 9 | NP: ++ | NP: 2 | NP: 1 | NP: 1 |
| AF: n.p3 | AF: n.p.3 | AF: n.p.3 | AF: n.p.3 | |||||
| 12 | 62/f | Degeneration | Fusion | 11 | NP: +++ | NP: 4 | NP: 1 | NP: 1 |
| AF: ++ | AF: 3 | AF: 1 | AF: 0 |
*HDS-m=modified histodegeneration score (NP) for surgical specimens: 0–14 points [7]
1Extent of stained matrix area with 0=no staining; + = minor, focal stained area; ++ = moderate stained area; +++ = extensive area with positive staining
2Scoring of immunohistochemical/ in situ hybridization labeling: 0=no labeled cells; 1, 1–5% labeled cells; 2, >5–10% labeled cells; 3, >10–25% labeled cells; 4,–>25% labeled cells
3Structure not present (n.p.)
Results
Control experiments
The reaction specificity of the antibodies used in this study were previously assured by the manufacturer, by Western blots. The reaction specificity of the riboprobes for the in situ hybridization was certified by Northern blot analysis, which provided correct blotting results, as expected.
Control experiments during the immunostaining experiments using blank and isotype controls for both antibodies revealed negative results. All sense control experiments in the in situ hybridizations revealed constantly negative results (data not shown).
Immunohistochemistry
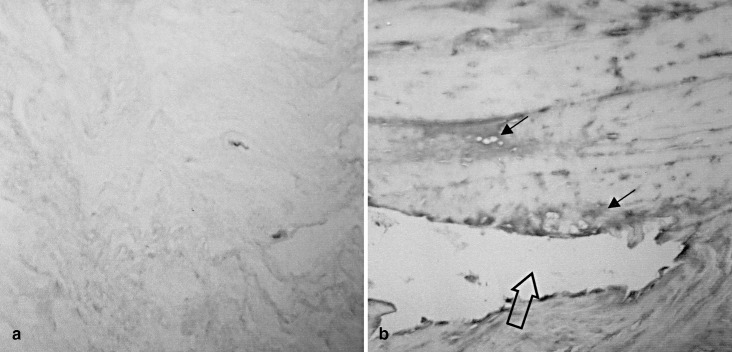
Immunohistochemically, in areas of nucleus pulposus and anulus fibrosus without morphological signs of degeneration, a diffuse, very faint staining was seen for fibronectin (Fig. 1a). This holds particularly true for nuclear and anular disc tissue of individuals younger than 16 years, where only occasional histological signs of tissue degeneration parallel a minor diffuse staining. In discs of individuals between 16 years and 40 years, a significantly enhanced, pericellular staining for fibronectin was seen in nuclear areas, but this was also accentuated in the inter-territorial matrix present close to tissue disruptions, such as clefts (Fig. 1b). Anular disc tissue was significantly less severely altered and less intensely stained. Discs of individuals older than 50 years frequently revealed a minor fibronectin staining. In this group, no close association was seen with any sign for disc degeneration, such as tissue disruption, cell proliferation or scar-like matrix transformation.
Fig. 1.
Immunolocalization of fibronectin in intervertebral disc tissue. a Morphologically unaltered disc areas show a very faint, diffuse interstitial staining for fibronectin. The pericellular matrix remains free of major staining. The section was counterstained with hemalum. NP, case AG No. 7, age 13 years); b disc samples of a young adult with signs of tissue disruption (cleft formation) with a significantly enhanced staining for fibronectin. Note the increased staining close to the cleft (hollow arrow) and around chondrocytes (small arrow) (NP, case AG No. 13, age 24 years). Original magnification: a x400; b: x250 (NP nucleus pulposus, AG autopsy group)
In the surgical specimens, similar findings were seen. Here again, the young-to-middle-age adult group showed a focally enhanced fibronectin deposition with a pericellularly accentuated staining of the territorial matrix. This was particularly seen in those areas with chondrocyte clustering and in the zone adjacent to both nuclear and anular clefts. Again, the anular tissue was less extensively stained for fibronectin.
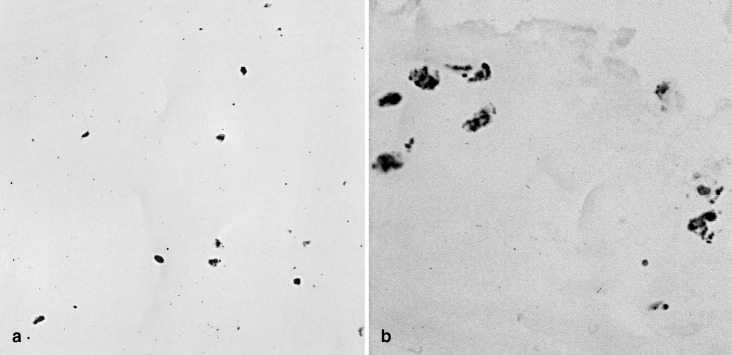
TGF-β1 was seen immunohistochemically exclusively within the cellular cytoplasm, but not in the extracellular matrix. Semi-serial sections from the paraffin blocks—i.e., sections prepared serially for immunostainings, including controls, and then for the corresponding in situ hybridizations—first used for the fibronectin immunohistochemistry revealed the expression of TGF-β1 in the areas with enhanced fibronectin staining. Thus, almost no positively labeled cells were seen in structurally normal areas of both the nucleus pulposus (Fig. 2a) and anulus fibrosus (not shown). In the degenerative tissue areas, such as in zones with disc cell proliferation, an apparently enhanced amount of labeled cells was seen (Fig. 2b). The amount of labeled cells was highest close to tissue clefts.
Fig. 2.
Immunolocalization of TGF-ß1 in intervertebral disc tissue. a Normal disc tissue contains no positively labeled cells for TGF-β1 (same case as Fig. 1a; b in discs with significant degenerative changes, numerous disc cells reveal a positive labeling for TGF-β1 protein (brown pigment) (same case as Fig. 1b). Original magnification: a, b x400
Non-radioactive in situ hybridization
This technique was applied to the freshly fixed surgical material. In morphologically normal structured areas, almost no cells were labeled in the nucleus pulposus and anulus fibrosus using the fibronectin antisense probe (Fig. 3a). In the altered areas, by contrast, fibronectin was mainly synthesized by cells in the nuclear regions (Fig. 3b), with enhanced labeling of cells from cell clusters or cell groups close to tissue clefts. This localization was identical to the positively stained tissue areas in corresponding immunohistochemical stainings (cf., Fig. 1b).
Fig. 3.
Localization of fibronectin mRNA in intervertebral disc tissue. a Normally appearing areas of surgical disc specimens show no specific cellular labeling for the fibronectin antisense probe (case surgical group (SG) No. 6); b by contrast, the disc material with significant cell proliferation reveals numerous cells with typical cytoplasmic positive labeling for the fibronectin antisense probe (arrow) (case SG No. 8) (original magnification: a, b: x600)
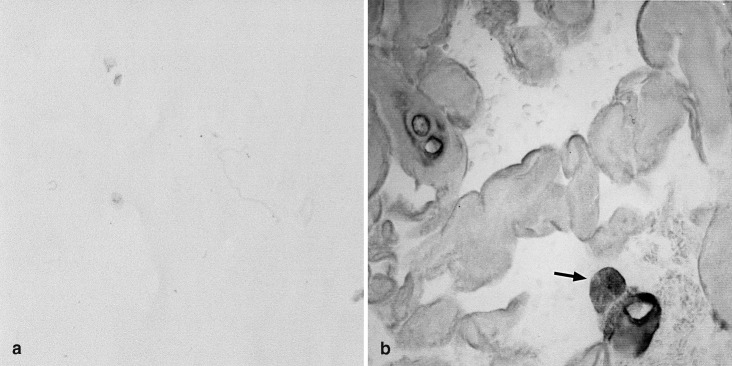
Normal nuclear and anular regions contained only very few TGF-β1 mRNA synthesizing cells (Fig. 4a). In both nuclear and anular areas with evidence of enhanced tissue degeneration a considerable number of cells were positive for TGF-β1 (Fig. 4b). The TGF-β1 mRNA pattern closely resembled that of fibronectin mRNA.
Fig. 4.
In-situ localization of TGF-β1 mRNA in intervertebral disc material. a Disc areas without evidence of tissue alteration did not show significant positive labeling for TGF-β1 mRNA (case SG No. 3); b in disc regions with cleft formation and cell proliferation, a considerable number of positively labeled disc cells indicating TGF-β1 synthesis is seen (arrow) (case SG No. 10). Original magnification: a, b: x600
A comparison between the number of cells positively labeled for TGF-β1 protein and those for TGF-β1 mRNA provided almost the same amount of stained cells (Table 1 and 2) with only minor differences between both techniques.
Discussion
In addition to the localization of fibronectin and the growth factor TGF-β1, we are the first to localize the cells actively synthesizing fibronectin and TGF-β1 in normal and degenerative human intervertebral discs and to relate our findings to surgical material. Moreover, this analysis is the first that uses both immunohistochemistry for the localization of the respective protein and non-radioactive in situ hybridization for the determination of the cells that are involved in neosynthesis. Thus, occurrence and source of pathogenetically relevant proteins were monitored.
Despite its extensive clinical significance, the pathogenesis of disc degeneration is still poorly understood. Previous studies indicated that disc degeneration is associated with changes in both the cellular phenotype and the composition of the extracellular matrix [17, 23, 25]. Both changes are closely related to each other. Likewise, in a recent study, we investigated the occurrence of phagocytic cells within disc tissue, providing clear evidence that part of the local disc cells undergo a phenotypic switch to phagocytic phenotype during degeneration [19]. In parallel, further studies showed that degenerating discs contained significantly up-regulated amounts and activities of major matrix-degrading enzymes, the matrix metalloproteinases [26, 30]. Further investigations analyzed the collagenous disc matrix and its changes during ageing and disc degeneration. Thus, we have shown that the collagens III, V and VI occur in enhanced amounts in those areas that show evidence of minor tissue degeneration, while advanced stages of disc degeneration are associated with the occurrence of fibrillar collagen I in the nucleus pulposus and hypertrophic cartilage collagen X [6, 17, 25]. These studies indicate that disc degeneration is associated with tremendous changes in cellular phenotype and extracellular matrix composition, ultimately leading to the biomechanical impairment of the disc.
Despite these increasing data on the pathogenesis of disc degeneration, very little is as yet known on any putative marker that identifies the early and “active” stages of disc matrix alterations. The aim of the present study was to investigate two potential markers, one a matrix protein that is well-known as a marker for immediate active matrix rearrangement and a second one that represents the most significant regulator for the synthesis of matrix molecules. Thus, we analyzed the age-related distribution of fibronectin and TGF-β1 in disc tissue. Previous studies demonstrated that, in particular, collagens III, V and VI occur in several types of reparative processes, such as wound healing [4, 5]. They appear at early stages. These observations suggest that degenerative disc lesions may represent some kind of reparative process in an effort to restore the proper disc function.
Fibronectin is a protein that is present in the human body in two forms: it occurs as a constituent of the extracellular matrix, and also as a serum protein. Both forms show high structural homology and are assumed to have identical biological functions [22]. Fibronectin is involved in a variety of physiological processes, such as cell adhesion and chemoattraction [2, 13]. Due to these functions, fibronectin plays an important role during various reparative processes, e.g., wound healing, where it occurs at very early stages [4]. Likewise, it is found during wound healing in already initially high amounts, and it is assumed that the initially occurring fibronectin comes from serum insudation, while in later stages this protein is synthesized by fibroblasts and histiocytes. The role of this multifunctional protein during reparative and degenerative processes of the intervertebral disc has been analyzed only very rarely. The few existing studies [20, 28, 29] used either biochemical methods that did not allow the identification of fibronectin localization or they used animal models. Only the study by Specchia et al. [29] used immunohistochemical techniques on disc herniation material and described enhanced staining for fibronectin in diseased tissue. However, no data are available regarding the age-related distribution of fibronectin in discs. In summary, all studies agree well with our present analysis suggesting that fibronectin is present in enhanced amounts during ageing and in degenerative disc tissue. In this respect, it seems interesting that the most significant rise in fibronectin expression is seen in the age group between 20 years and 40 years, while thereafter the high level remains almost constant. This is in parallel to the histodegeneration score (HDS), which shows the most significant increases in the young-to-mature adult groups and remains constantly at high levels in old age [7].
In our present analysis, we provide circumstantial evidence that degenerative lesions contain enhanced amounts of fibronectin and that this is produced—at least in large part—by local stromal cells with the morphology of resident chondrocytic disc cells. This is well in line with the presence of fibronectin in the territorial matrix of articular chondrocytes and in osteoarthrosis of load-bearing joints. A focally enhanced deposition of fibronectin has been described as well [21, 27]. Due to its function, we assume that the enhanced expression of fibronectin in altered disc specimens may represent an attempt to promote reparative processes. Ongoing alterations of the discs, however, may overcome this repair process.
Although there is a good correlation between the appearance of fibronectin and its producing cells, the expected potential value as a marker for the very early matrix disarrangement has not been fulfilled. This is due to the basal presence of fibronectin, even in juvenile discs, which prevents the use of fibronectin as a marker for particular events during disc degeneration. In summary, fibronectin undergoes quantitative, but no qualitative changes during disc degeneration.
Well in line with the occurrence of enhanced amounts of fibronectin in the altered disc samples, we observed an increased expression of TGF-β1. This cytokine is a potent stimulator for the synthesis of various matrix constituents [14]. It is frequently bound to specific extracellular matrix constituents and can be liberated upon matrix cleavage [14]. This may mimic TGF-β induction by matrix molecules. Thus, it is much more likely that the up-regulation of matrix proteins may be the consequence of increased TGF-βaction, rather than that an up-regulation of fibronectin leads to enhanced production of TGF-β1. The previously described alteration of the collagen composition and herewith associated de novo synthesis may also be due to the enhanced TGF-βproduction. As with fibronectin, only very limited knowledge exists about the in situ localization of TGF-β1 in normal and diseased disc tissue. Our observation correlates well with a previous report that describes a good responsiveness of human in vitro disc cells from the annulus to TGF-β1 [12]. Similarly, the only study on the localization of TGF-β1 protein in herniated disc material provides clear evidence for increased expression of this growth factor and, therefore, is well in line with our analysis. Since, however, only diseased tissue had been studied by Specchia et al. [29], no information was as yet available regarding age-related changes in occurrence and distribution of this cytokine.
In contrast to the agreement between our data and some previous reports, recently Matsunaga and co-workers [15] surprisingly described in an animal model of senescence-accelerated mice a decrease in the nuclear and anular expression of TGF-β1, TGF-β2 and TGF-β3 proteins (as detected by immunohistochemistry) with increasing age. However, significant differences in the disc morphology, such as the persistence of notochordal cells until “high” age [15], between the animals and human discs make a direct comparison of the animal model data and our human system very difficult. Furthermore, at present it is unclear which mechanisms in this model are involved in the senescence acceleration, so that possibly a direct influence on the TGF-β system and the activation of alternative cytokine pathways may be seen. A simple transfer of those data to the human system seems, therefore, inappropriate and deserves further analysis. In this respect it may well be noted that other regulating mechanisms and growth factors may also be involved in disc degeneration, such as seen in a variety of different cell types and organs. Nevertheless, our data and those from other cell systems indicate that the TGF-β system is very important for the control and regulation of the cell-matrix interaction and particularly the matrix remodeling.
Besides the evident association between enhanced TGF-βexpression and disc degeneration in human discs, we have a positive correlation between the expression of TGF-β and major matrix proteins, such as fibronectin, as shown in this study. Furthermore, there is a good correlation between TGF-βexpression and the remodeling of other matrix constituents, such as collagens III, V and VI [17, 18]. Therefore, our present study strongly supports the notion that this cytokine is closely associated with the changes of the extracellular matrix. It is therefore likely that the up-regulation of TGF-β1 expression is responsible for significant alterations of the matrix structures, which, in turn, is important for the biomechanical properties of disc tissue.
In summary, this—although still preliminary—study on a restricted patient group provides evidence that the tissue disarrangement during disc degeneration may be monitored by enhanced staining for fibronectin, which is obviously produced by local cells. The concomitant localization of TGF-β1- and fibronectin-synthesizing cells suggests matrix production stimulation of those local cells by the cytokine.
Acknowledgements
This study was supported by a grant from the AO/ASIF Foundation, Switzerland (grant No. 00-B72)
References
- 1.Aigner Calcif Tissue Int. 1998;63:263. doi: 10.1007/s002239900524. [DOI] [PubMed] [Google Scholar]
- 2.Albini Coll Relat Res. 1988;8:23. doi: 10.1016/s0174-173x(88)80033-5. [DOI] [PubMed] [Google Scholar]
- 3.AndersonSpine 200227111805625 [Google Scholar]
- 4.Betz Int J Legal Med. 1992;105:21. doi: 10.1007/BF01371232. [DOI] [PubMed] [Google Scholar]
- 5.Betz Int J Legal Med. 1993;105:329. doi: 10.1007/BF01222117. [DOI] [PubMed] [Google Scholar]
- 6.Boos Histochem Cell Biol. 1998;108:471. doi: 10.1007/s004180050187. [DOI] [PubMed] [Google Scholar]
- 7.Boos Spine. 2002;27:2. doi: 10.1097/00007632-200212010-00002. [DOI] [Google Scholar]
- 8.Ceol Lab Invest. 1996;74:484. [PubMed] [Google Scholar]
- 9.Cordell J Histochem Cytochem. 1984;32:219. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 10.Gries Eur Spine J. 2000;9:23. doi: 10.1007/s005860050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruber Spine. 1998;23:751. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Gruber Exp Cell Res. 1997;235:13. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 13.Homandberg Front Biosci. 1999;4:D713. doi: 10.2741/homandberg. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence Eur Cytokine Netw. 1996;7:363. [PubMed] [Google Scholar]
- 15.Matsunaga S, Nagano S, Onishi T, Morimoto N, Suzuki S, Komiya S (2003) Age-related changes in expression of transforming growth factor-b and receptors in cells of intervertebral discs. J Neurosurg (Spine 1) 98:63–67 [DOI] [PubMed]
- 16.Moore Spine. 1996;21:2. doi: 10.1097/00007632-199611010-00012. [DOI] [Google Scholar]
- 17.Nerlich Spine. 1997;22:2. doi: 10.1097/00007632-199712150-00001. [DOI] [Google Scholar]
- 18.Nerlich Virchows Arch Pathol Anat. 1998;432:67. doi: 10.1007/s004280050136. [DOI] [PubMed] [Google Scholar]
- 19.Nerlich Spine. 2002;27:2. doi: 10.1097/00007632-200212010-00002. [DOI] [Google Scholar]
- 20.Oegema Spine. 2000;25:2. [Google Scholar]
- 21.Pfander J Rheumatol. 1999;26:386. [PubMed] [Google Scholar]
- 22.Potts Matrix Biol. 1996;15:313. doi: 10.1016/S0945-053X(96)90133-X. [DOI] [PubMed] [Google Scholar]
- 23.Roberts Biochem Soc Trans. 2001;30:864. doi: 10.1042/BST0300864. [DOI] [PubMed] [Google Scholar]
- 24.Roberts Spine. 1999;24:500. doi: 10.1097/00007632-199903010-00026. [DOI] [PubMed] [Google Scholar]
- 25.RobertsSpine 19911611825891 [Google Scholar]
- 26.Roberts Spine. 2000;25:3. doi: 10.1097/00007632-200012010-00007. [DOI] [Google Scholar]
- 27.Salter J Histochem Cytochem. 1995;43:447. doi: 10.1177/43.4.7897185. [DOI] [PubMed] [Google Scholar]
- 28.Silberberg Exp Cell Biol. 1989;57:233. doi: 10.1159/000163532. [DOI] [PubMed] [Google Scholar]
- 29.Specchia Eur Spine J. 2002;11:145. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiler Eur Spine J. 2002;11:308. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]