Abstract
Study Design: A control group study with repeated measures. Objective: To compare trunk repositioning parameters in chronic low back pain (LBP) and healthy subjects. Summary and background data: Recent evidence suggests that chronic LBP patients exhibit deficits in trunk proprioception and motor control. Trunk repositioning and the various spatio-temporal parameters related to it can be used to evaluate sensori-motor control and movement strategies. Methods: Fifteen control subjects and 16 chronic LBP subjects participated in this study. Subjects were required to reproduce different trunk position in flexion (15°, 30° and 60°) and extension (15°). In the learning phase preceding each condition, visual feedback was provided. Following these learning trials, subjects were asked to perform ten consecutive trials without any feedback. Movement time, movement time variability and peak velocity were obtained and a temporal symmetry ratio was calculated. Peak angular position variability and absolute error in peak angular position were also calculated to evaluate spatial accuracy. Results: Two subgroups of LBP patients were identified. One subgroup of LBP subjects demonstrated longer movement time and smaller peak velocities and symmetry ratios than normal subjects. No group difference was observed for peak angular position variability and absolute error in peak angular position. Conclusion: Chronic LBP patients, when given a sufficient learning period, were able to reproduce trunk position with a spatial accuracy similar to control subjects. Some LBP subjects, however, showed modifications of movement time, peak velocity and acceleration parameters. We propose that the presence of persistent chronic pain could induce an alteration or an adaptation in the motor responses of chronic LBP subjects.
Keywords: Trunk positioning, Low back pain, Motor control, Sensori-motor deficits
Introduction
Low back pain (LBP) is a frequent condition found in all categories of adult population. Epidemiological studies have shown that 50%–80% of the population is affected by LBP, at least once in a lifetime [7]. Over the past decades, researchers and clinicians have failed to identify the mechanisms responsible for chronic back conditions. The presence of sensori-motor deficits in the LBP population is one of the current hypotheses that could explain the high prevalence of LBP conditions. Many authors have demonstrated that sensori-motor deficits are present in LBP patients [2, 3, 7, 13, 14, 17, 18]. These deficits can affect and compromise segmental spinal stability and eventually lead to articular damage and subsequent chronic pain [4, 19]. Amongst the numerous aspects of trunk sensori-motor control, trunk and lumbar positioning have been frequently studied in the normal population and in LBP patients [2, 3, 7, 13, 14, 17, 18]. Such a task (trunk positioning) and the various parameters related to it can be used to evaluate sensori-motor control and movement strategies.
Brumagne and colleagues [2, 3] investigated position sense in control and LBP subjects during a lumbar repositioning task. They reported that the repositioning accuracy of LBP subjects was significantly lower than that of healthy subjects. Parkhurst and Burnett [18] studied the relationships between the history of LBP injuries and the proprioception of the lumbar spine. No significant correlation was found between low back injuries and repositioning errors. Newcomer et al. [13, 14] conducted two different studies in which they compared repositioning accuracy of healthy and LBP subjects. In their first study, the authors observed no trunk repositioning differences between healthy subjects and chronic LBP subjects. In their second study, the subjects stood with their legs and pelvis stabilised to limit proprioceptive information from the lower limbs. LBP subjects demonstrated significantly higher repositioning error in flexion and significantly lower repositioning error in extension. Finally, Gill and Callaghan [7] compared the repositioning accuracy of healthy and LBP subjects in two different tasks: (1) trunk repositioning in flexion and (2) lumbar repositioning in a four-point kneeling position. Chronic LBP patients showed greater absolute errors for both conditions. This diversity of results probably can be explained by the different protocols used in each experiment. Indeed, factors such as repositioning amplitudes, starting positions, between trial intervals and task learning differed across studies yielding several hypotheses for explaining the observed results.
One of the most important factors affecting motor learning and performance is practice. Clearly, practice improves performance in a pointing or positioning task [11]. If an immediate feedback (knowledge of result or knowledge of performance) is available after a practice trial, it will have a positive effect on task accomplishment [15, 16]. For example, in a trunk positioning task, knowledge that an error is made in a particular direction gives a strong indication of the ways in which the trunk positioning should be modified in the next trial [20]. For LBP subjects, practice trials (with or without feedback) could be even more critical for motor performance. Our study included a learning period in order to minimise the pain-related fear and its associated avoidance behaviour in the group of LBP subjects. We believe that a stabilisation of the motor response will help to identify motor control alterations associated with chronic LBP rather than pain-related fear or simple learning processes.
In a previous study in which learning trials and visual feedback were provided to subjects, Descarreaux et al. [5] observed that LBP subjects were able to produce isometric trunk forces as accurately as healthy subjects. Two different control strategies, however, were used by chronic LBP subjects to produce accurate trunk isometric forces. Our conclusion was that some LBP subjects changed their motor strategy, by using a more “close loop” control, to perform tasks as accurately as the healthy subjects. For the present study, trunk repositioning parameters were measured by following a learning period during which visual feedbacks were provided to all subjects. The aim was to test the trunk repositioning accuracy of healthy and LBP subjects after a learning period. A second objective was to evaluate whether different trunk motor control strategies could be observed between healthy and LBP subjects. If motor planning is affected by chronic lumbar pain, a different strategy of movement production between chronic LBP subjects and healthy subjects should be observed for the LBP subjects.
Methods
Trunk repositioning parameters were measured in 16 subjects with chronic non-specific LBP and in 15 control subjects. Every subject gave their informed written consent and the study was approved by the Université Laval (Canada) Ethics Committee. All subjects were recruited through local advertising. The experimental group (LBP) included 16 subjects (11 men, 5 women, age: 41.1 years) who had a history of chronic recurrent LBP that lasted for at least 6 months. Exclusion criteria for both groups were: spondylolisthesis or spondylolysis, ankylosing spondylitis, moderate to severe spinal osteoarthritis, inflammatory arthritis, nerve root compression, trunk neuromuscular disease, scoliosis (15° or more), previous spinal surgery, malignant tumour, hypertension, pregnancy and breastfeeding. Lateral and antero-posterior X-ray films of the lumbar spine (including pelvis) were taken to rule out the possibility of congenital, degenerative or inflammatory diseases of the lumbar spine. A total 28 LBP subjects contacted us and 12 were rejected on the basis of our exclusion criteria. Pain levels at the beginning and the end of the experiment were assessed by using a standard 100-mm visual analogue pain scale (VAS). Each chronic LBP subject filled out the modified Oswerstry questionnaire before the experiment. The control group consisted of 15 healthy subjects (9 men, 6 women, age: 38.2 years). Before the testing began, all subjects performed maximal trunk flexion and extension to determine whether they were able to perform the experimental task without any additional pain. The trunk movements of subjects (in a standing position) were recorded by using a rehabilitation device (Loredan Biomedical, West Sacramento, USA). Testing was performed in a neutral standing posture (0° of flexion or extension) with the pelvis and legs immobilised to minimise proprioceptive information from the lower limbs. Subjects were asked to reproduce three different trunk positions in flexion (15°, 30° and 60°) and one position in extension (15°). Conditions were presented by blocks, with the order of presentation being randomised across subjects. Subjects were encouraged to produce the angular movement without correction of the motor response once it was initiated. For each condition, a learning phase was provided. During this phase and after each trial, subjects were given visual accuracy feedback through an oscilloscope located in front of them. Subjects were informed of their spatial accuracy and could then evaluate the amplitude and the direction of their error. Subjects were specifically asked to flex or extend the trunk to the predetermined position within 10% of the goal target (i.e. ±1.5°, 3.0° and 6.0°). This learning phase stopped when five consecutive trunk positionings were within the 10% margin. Following these learning trials, subjects performed 10 consecutive trials without any visual feedback. All dependent variables were derived from the behaviour observed for these 10 trials.
For every trial, trunk position data were recorded at a sampling rate of 500 Hz and digitally filtered with a second-order Butterworth filter (5 Hz low-pass cut-off frequency). Each trial lasted 5 s. Onset of movement and peak angular position were determined in every trial for each subject. By using this information, movement time, movement time variability and peak velocity (obtained from the first derivative of the movement signal) were obtained. A temporal symmetry ratio was also calculated by dividing acceleration time by deceleration time [12]. The temporal symmetry ratio of an ideally symmetric movement is 1. Movements performed at moderate speed demonstrate a symmetrical velocity profile. Faster and slower movements, however, do not follow this rule [12]. Peak angular position variability and absolute error in peak angular position were calculated for each condition to evaluate the precision of trunk repositioning. The absolute error in peak angular position represents the positive difference between the reached angular position and the target for a particular trial, whereas the peak angular position variability is the standard deviation of 10 pointing trials without feedback. Finally, the total number of practice trials (across all four experimental conditions) was determined for each subject.
A preliminary analysis revealed that differences were present between two subgroups of chronic LBP subjects and that these subgroups were the same subgroups identified in a previous study of isometric force reproduction in chronic LBP subjects [5]. LBP and control subjects participated in both studies and we decided to use the same subgroup to establish whether similar motor control adaptations were present for kinematic variables. Data for both isometric force production and trunk repositioning protocols were collected the same day and one half of the subjects were initially tested for the isometric force protocol, whereas the other half began with the repositioning protocol. In the present experiment, movement time was significantly longer in one subgroup of subjects and the data analyses were completed by using one control group and two chronic LBP subgroups. Table 1 shows the characteristics of the control group and both LBP subgroups. Hereafter, the two subgroups of LBP subjects are named LBPlong and LBPshort.
Table 1.
Characteristics of the control group and both LBP subgroups (VAS visual analogue pain scale). Values are given as means (SD)
| Subjects | LBPlong, n=9 (6 men, 3 women) | LBPshort, n=7 (5 men, 2 women) | Control, n=15(9 men, 6 women) |
|---|---|---|---|
| Age (years) | 42.1 (10.1) | 39.7 (12.7) | 38.2 (10.7) |
| Height (cm) | 169.4 (7.9) | 173.6 (7.4) | 172.8 (6.4) |
| Weight (kg) | 75.4 (13.9) | 68.1 (15.2) | 74.1 (15.2) |
| Duration of LBP (months) | 45.5(17.4) | 52.4 (12.8) | – |
| Oswerstry index (%) | 27.4 (10.8) | 26.0 (9.3) | – |
| VAS (mm) | |||
| Before testing* | 14 (8) | 43 (9) | – |
| After testing* | 17 (9) | 48 (10) | |
*Significant difference (P<0.001)
The total number of practice trials was submitted to a one way ANOVA (Group factor). All other dependant variables were submitted to a Group × Trunk position ANOVA with repeated measures on the last factor. When a main effect of Group or an interaction of Group × Trunk position was observed, post hoc comparisons were performed by Tukey tests. For all analyses, statistical significance was set at P<0.05.
Results
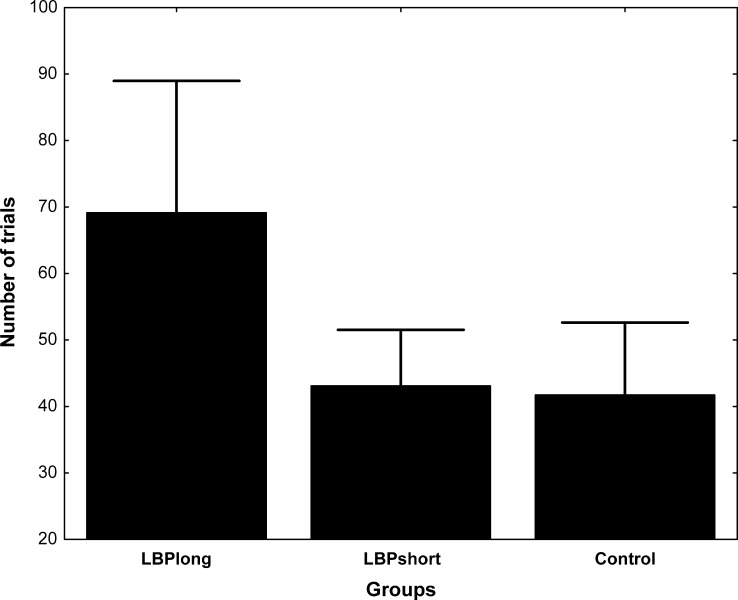
As reported in Table 1, pain levels of the LBP subjects, measured with the VAS, did not vary significantly during the course of the experiment (P>0.05). A significant Group difference was observed for the total number of practice trials. Figure 1 illustrates these differences. On average, the healthy subjects and subjects from the LBPshort subgroup needed, respectively, 41.7 (95% CI=35.0–48.5) and 43.1 (95% CI=34.3–51.8) practice trials. The LBPlong subjects needed 69.4 (95% CI=59.2–79.0) practice trials. Figure 2 illustrates the movement time, peak velocity and temporal symmetry ratio for the control group and both LBP subgroups. The ANOVA yielded a significant main effect of Group for the movement time [F (2, 28)=5.04, P=0.013] and the temporal symmetry ratio [F (2, 28)=3.55, P=0.042], whereas peak velocity approached but did not reach statistical significance. Post-hoc analyses revealed a significant difference between the two LBP subgroups for movement time. The LBPlong subgroup showed a significantly longer movement time (mean 1.77 s, 95% CI=1.60–1.94 s) than the LBPshort subgroup (mean 1.49 s, 95% CI=1.34–1.64 s). Interestingly, the LBPshort subgroup and control group had similar movement times. Post-hoc analyses for the temporal symmetry ratio revealed a significant difference between the two LBP subgroups. The LBPlong subgroup showed a significantly smaller temporal symmetry ratio (mean 0.38, 95% CI=0.11–0.65) than the LBPshort subgroup (mean 0.84, 95% CI=0.60 – 1.08). The effect was a consequence of an increased deceleration time for the LBPlong subgroup (LBPlong=1.3 s, LBPshort=0.91 s, control=0.96 s). Again, the LBPshort subgroup and control group had a similar temporal symmetry ratio. No Group effect was noted for the time to peak variability (P>0.05).
Fig. 1.
Mean (bars SD) total number of practice trials needed for each group of participants
Fig. 2.
Mean (CI 95%) movement time, peak velocity and temporal symmetry ratio for each group of participants
No group difference was observed for the two spatial accuracy variables, viz. peak angular position variability and absolute error in peak angular position (P>0.05).
Significant trunk position effects were noted for movement time [F (3, 84)=37.156, P<0.0001], temporal symmetry ratio [F (3, 84)=4.7155, P=0.004], absolute error in peak angular position [F (3, 84)=18.418, P<0.0001] and peak angular position variability [F (3, 84)=7.1772, P=0.0002]. Table 2 shows the different trunk position effects. Movement time, absolute error in peak angular position and peak angular variability were similar for 15° of flexion and extension but increased for 30° and 60° of flexion. The temporal symmetry ratio significantly decreased at 60° of flexion. Trunk peak velocity was similar throughout the four positions (P>0.05). No interaction of Group × Trunk position was noted for any of the variables (P>0.05).
Table 2.
Trunk position effects for the control group and both LBP subgroups. Values are given as means (SD)
| Parameter | 15° flexion | 30° flexion | 60° flexion | 15° extension |
|---|---|---|---|---|
| Movement time (s)* | 1.35 (0.05) | 1.62 (0.06) | 1.88 (0.05) | 1.44 (0.05) |
| Temporal symmetry ratio* | 0.75 (0.10) | 0.62 (0.09) | 0.46 (0.04) | 0.70 (0.09) |
| Absolute error in peak angular position (°)* | 2.13 (0.23) | 2.82 (0.27) | 5.20 (0.62) | 1.74 (0.23) |
| Peak angular position variability (°)* | 1.73 (0.16) | 1.87 (0.13) | 2.12 (0.15) | 1.29 (0.13) |
*Significant position effect
Discussion
The objectives of this study were to test the trunk repositioning accuracy of healthy and LBP subjects after a learning period and to evaluate whether different trunk motor control strategies could be observed between healthy and LBP subjects. Our results revealed that, when given a sufficient learning period, LBP subjects were able to reproduce different trunk positioning as accurately as the healthy subjects. To achieve this accuracy, however, some LBP subjects used a different control strategy. More specifically, they increased their movement time by increasing the duration of the deceleration phase (on average, LBPlong=1.3 s, LBPshort=0.91 s, control=0.96 s) and consequently decreased the temporal symmetry ratio.
Repositioning accuracy
Our results show that, when given a sufficient number of practice trials, repositioning accuracy is similar in LBP and healthy subjects. These results contrast with those of various authors who have investigated repositioning accuracy in LBP subjects and reported greater repositioning errors in this population [7, 13, 14, 18]. Similar protocols were used for all these studies. Participants were positioned at the desired target trunk position for 2 – 5 s and were asked to remember this position. They were then brought to another position (full flexion or neutral posture) for a few seconds before attempting to reproduce the initial target position. Most of these studies did not include a learning period and no feedback was given before or during testing. Differences between our study protocol and the previously mentioned studies could explain the contrasts between the different results reported in the literature. For example, our results did not confirm those of Newcomer et al. [14] who reported that LBP subjects repositioning error in flexion positions was significantly higher than that of control subjects but was significantly lower than that of control subjects in extension positions. Only the study conducted by Gill and Callaghan [7] included 10 practice trials with visual feedbacks but the number of learning trials were predetermined and similar for the subjects. Our study protocol included a practice session during which each subject had to complete five consecutive trunk positionings within a ±10% margin of the predetermined target position. Sherwood [21] showed that giving a “bandwidth knowledge of results” type of feedback about a relatively large bandwidth (i.e. ±10%) enhanced movement consistency in a pointing task. The methodological differences between our study and the various studies previously mentioned are important. In our study, some LBP subjects took more than 100 practice trials to stabilise their motor response across the four trunk positions tested. Our results clearly demonstrate that when given a sufficient number of learning trials (based on a constant criterion of consecutive successful trials), LBP subjects can reproduce a trunk position with the precision and the variability observed in normal healthy subjects. Such a learning period helps in the stabilisation of the motor responses. The LBPlong subgroup of LBP subjects, however, needed significantly more practice trials than the LBPshort subgroup and the healthy subjects. Two different hypotheses could be responsible for these changes. (1) Modifications to sensori-motor deficits caused by chronic pain [2, 3, 7, 13, 14, 17, 18] and fear-avoidance behaviour could explain the higher number of practice trials observed in one subgroup of LBP subjects (LBPlong subgroup). (2) Fear-avoidance behaviour could have been responsible for the larger number of practice trials needed to stabilise the motor response. Al-Obaidi and colleagues [1] have proposed that spinal physical capacity in chronic LBP patients is not explained solely by the sensory perception of pain. They have shown that isometric trunk strength can be greatly influenced by the anticipation of pain and fear-avoidance belief. Interestingly, the LBPlong subgroup exhibited a lower pain score at the time of testing and one could argue that a different motor control strategy has led this subgroup to a better adaptation to chronic LBP. In order to understand the different factors involved in the development of new motor control strategies in LBP subjects, psychological evaluation and a validated fear-of-pain questionnaire should be included in future studies.
The subjects (LBPlong) who needed more practice trials to stabilise their motor performance also used a different control strategy than that used by the other LBPshort subgroup and control subjects to complete the trunk positioning task. Presumably, these changes could be associated with a strategy allowing them to perform lumbar tasks with less pain.
Motor control strategies
The isometric trunk force data obtained on the same day yielded similar results to those above. Indeed, the two subgroups of LBP subjects (the same subgroup as that used in the present study) were identified on the basis of their force production parameters and the strategy of control used throughout the experiment [5]. Interestingly, LBP subjects were differentiated by using similar parameters to those adopted in the present experiment (longer time to peak force and, hence, duration of contraction for a subgroup of LBP subjects showing less pain at the time of testing). In the present experiment, one subgroup of LBP subjects (LBPlong) had a longer movement time, a reduced peak velocity and a lower temporal symmetry ratio compared with the second LBP subgroup (LBPshort) and the control group. Therefore, the LBPlong subgroup was not only slower but also used a longer deceleration phase. Although trunk pointing clearly involves a different and a more complex muscular and skeletal organisation than mono-articular upper limb pointing, data obtained from the latter type of movements could provide some hints for the interpretation of our results. For instance, Jaric et al. [12] have proposed that the agonist–antagonist burst characteristics could explain variation in the temporal symmetry ratio. They have shown that slower movements are characterised by a peak velocity occurring sooner than for faster movements. These changes yield a decreased temporal symmetry ratio (as observed in our experiment). Jaric et al. [12] suggest that, in faster movement, muscle viscosity in the agonist muscle would increase the duration and amplitude of the agonist burst, whereas the increased viscosity would assist the antagonist muscle and delay the deceleration phase. In the present experiment, the slower movement time and reduced temporal symmetry ratio (increased deceleration time) of one subgroup of LBP subjects could allow a more efficient utilisation of proprioceptive information and a decrease of the muscular stiffness and compressive forces produced by the erector spinae. It will be interesting to evaluate whether this control strategy is developed in order to reduce pain associated with movement production and to avoid further injuries.
These adaptations can be quantified in some chronic LBP subjects in whom movement time, peak velocity and temporal symmetry ratio are different from those of control subjects. Indahl et al. [8–10] have proposed that motion and stabilisation of the spine are based on a complex reflex activation system that can be activated by various vertebral proprioceptive signals and modulated by interneurons and central commands. Holm et al. [8] have suggested that lesions of vertebral articular structures, such as intervertebral discs, ligaments or zygapophysial joints, could lead to an increased activation of paraspinal muscles. Our results and the apparent sensori-motor deficits described above could either be explained by changes in the central motor command (adaptation to pain) or modifications in the local lumbar reflex system caused by articular pain and degeneration. Electromyographic studies of trunk muscles in repositioning tasks might be helpful in the identification of neurophysiological adaptations and should be included in future investigations.
Influence of position
Testing was performed under four different repositioning conditions for all subjects: flexion 15°, flexion 30°, flexion 60° and extension 15°. Peak velocity was constant throughout the four experimental conditions but movement time, absolute error in peak angular position and peak angular variability increased with an increased angular movement. These observations are normal features of fast movements and have been frequently studied and described in the past [20].
Conclusion
The present data confirm the presence of different motor control strategies in the presence of chronic LBP. Use of a different control strategy could help some chronic LBP subjects to perform various trunk positioning with a precision and variability similar to those of normal healthy subjects. These subjects, however, needed nearly twice as many trials to stabilise their performance than did control subjects. Interestingly, chronic LBP subjects who adopted a similar control strategy to that of controls were also those reporting more pain at the time of testing. The identification and clinical evaluation of sensori-motor modifications could eventually help to develop new diagnostic tools and better rehabilitation programs for acute and chronic LBP patients.
Acknowledgements
The authors thank Marcel Kaszap for technical support. This study was supported by FCQ, CIHR-FCQ, NSERC-Canada and FCAR-Québec.
References
- 1.Al-Obaidi SM, Nelson RM, Al-Awadhi S, et al. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic LBP. Spine. 2000;25:1126–1131. doi: 10.1097/00007632-200005010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Brumagne S, Cordo P, Lysens R, et al. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without LBP. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Brumagne S, Lysens R, Swinnen S, et al. Effect of paraspinal muscle vibration on position sense of the lumbosacral spine. Spine. 1999;24:1328–1331. doi: 10.1097/00007632-199907010-00010. [DOI] [PubMed] [Google Scholar]
- 4.Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic LBP. Clin Biomech (Bristol, Avon) 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 5.Descarreaux M, Blouin JS, Teasdale N. Force production parameters in patients with LBP and healthy control study participants. Spine. 2004;29:311–317. doi: 10.1097/01.BRS.0000105983.19980.A8. [DOI] [PubMed] [Google Scholar]
- 6.Frymoyer JW, Ducker TB. The adult spine: principles and practice. 2. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 7.Gill KP, Callaghan MJ. The measurement of lumbar proprioception in individuals with and without LBP. Spine. 1998;23:371–377. doi: 10.1097/00007632-199802010-00017. [DOI] [PubMed] [Google Scholar]
- 8.Holm S, Indahl A, Solomonow M. Sensorimotor control of the spine. J Electromyogr Kinesiol. 2002;12:219–234. doi: 10.1016/S1050-6411(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 9.Indahl A, Kaigle A, Reikeras O, et al. Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine. 1995;20:2652–2658. doi: 10.1097/00007632-199512150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Indahl A, Kaigle AM, Reikeras O, et al. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine. 1997;22:2834–2840. doi: 10.1097/00007632-199712150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Jaric S, Corcos DM, Latash ML. Effects of practice on final position reproduction. Exp Brain Res. 1992;91:129–134. doi: 10.1007/BF00230021. [DOI] [PubMed] [Google Scholar]
- 12.Jaric S, Gottlieb GL, Latash ML, et al. Changes in the symmetry of rapid movements. Effects of velocity and viscosity. Exp Brain Res. 1998;120:52–60. doi: 10.1007/s002210050377. [DOI] [PubMed] [Google Scholar]
- 13.Newcomer K, Laskowski ER, Yu B, et al. Repositioning error in LBP. Comparing trunk repositioning error in subjects with chronic LBP and control subjects. Spine. 2000;25:245–250. doi: 10.1097/00007632-200001150-00017. [DOI] [PubMed] [Google Scholar]
- 14.Newcomer KL, Laskowski ER, Yu B, et al. Differences in repositioning error among patients with LBP compared with control subjects. Spine. 2000;25:2488–2493. doi: 10.1097/00007632-200010010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Newell KM. Knowledge of results and motor learning. Exerc Sport Sci Rev. 1976;4:195–228. [PubMed] [Google Scholar]
- 16.Newell KM, Walter CB. Kinematic and kinetic parameters as information feedback in motor skill acquisition. J Human Movements Stud. 1981;7:235–254. [Google Scholar]
- 17.O’Sullivan PB, Burnett A, Floyd AN, et al. Lumbar repositioning deficit in a specific LBP population. Spine. 2003;28:1074–1079. doi: 10.1097/00007632-200305150-00022. [DOI] [PubMed] [Google Scholar]
- 18.Parkhurst TM, Burnett CN. Injury and proprioception in the lower back. J Orthop Sports Phys Ther. 1994;19:282–295. doi: 10.2519/jospt.1994.19.5.282. [DOI] [PubMed] [Google Scholar]
- 19.Radebold A, Cholewicki J, Polzhofer GK, et al. Impaired postural control of the lumbar spine is associated with delayed muscle response times in patients with chronic idiopathic LBP. Spine. 2001;26:724–730. doi: 10.1097/00007632-200104010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt RA, Lee TD. Motor control and learning: a behavioral emphasis. 3. Champaign, Ill., USA: Human Kinetics; 1999. [Google Scholar]
- 21.Sherwood DE. Effect of bandwidth knowledge of results on movement consistency. Percept Mot Skills. 1988;66:535–542. doi: 10.2466/pms.1988.66.2.535. [DOI] [PubMed] [Google Scholar]
- 22.Waddell G. Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine. 1987;12:632–644. doi: 10.1097/00007632-198709000-00002. [DOI] [PubMed] [Google Scholar]