Abstract
Treatment of soybean (Glycine max L. cv Williams 82) cell-suspension cultures with Pseudomonas syringae pv glycinea (Psg) harboring an avirulence gene (avrA) or with yeast elicitor resulted in an oxidative burst characterized by the accumulation of H2O2. This burst, and the resultant induction of glutathione S-transferase transcripts, occurred more rapidly and was more prolonged if cells were simultaneously treated with serine protease inhibitors such as phenylmethylsulfonyl fluoride (PMSF) or diisopropylfluorophosphate. PMSF and diisopropylfluorophosphate potentiate a large oxidative burst in cells exposed to Psg harboring the avrC avirulence gene, which is not recognized by the soybean cultivar used in this study. The potentiated burst was inhibited by diphenylene iodonium, an inhibitor of NADPH oxidase, and by the protein kinase inhibitor K252a. PMSF treatment of elicited cells or cells exposed to Psg:avrA caused a large increase in the accumulation of the isoflavonoid phytoalexin glyceollin; however, this was not associated with increased levels of transcripts encoding key phytoalexin biosynthetic enzymes. Glyceollin accumulation was inhibited by diphenylene iodonium; however, the oxidative burst in cells treated with Psg:avrC and PMSF was not followed by phytoalexin accumulation. We conclude that active oxygen species from the oxidative burst are necessary but not sufficient for inducing isoflavonoid phytoalexin accumulation in soybean cells.
Avirulent pathogens trigger a hypersensitive response characterized by the rapid production of active oxygen species, usually measured as H2O2 (Sutherland, 1991; Doke et al., 1996; Mehdy et al., 1996; Lamb and Dixon, 1997). This response, known as the oxidative burst, can also be mimicked in suspension-cultured cells exposed to elicitors of microbial origin (Apostol et al., 1989; Levine et al., 1994). There is currently intense interest concerning the role of the oxidative burst as a generator of signals for cell death (Levine et al., 1994), activation of antioxidant defenses (Levine et al., 1994), induction of phytoalexin biosynthesis (Apostol et al., 1989; Devlin and Gustine, 1992; Park et al., 1995), and establishment of systemic acquired resistance (Alvarez et al., 1998).
The oxidative burst in plants is believed to share mechanistic similarities to that exhibited by mammalian neutrophils. It involves activation of a plasma membrane-bound NADPH oxidase, and components of the plant oxidase complex have recently been characterized at the biochemical (Van Gestelen et al., 1997) and molecular (Groom et al., 1996; Keller et al., 1998) levels. A significant body of pharmacological evidence points to the involvement of a protein kinase cascade as part of the signal transduction pathway between molecular recognition of elicitors or avirulent bacteria and activation of the oxidase (Schwacke and Hager, 1992; Chandra and Low, 1995; Shirasu et al., 1997). SA, a key chemical regulator of local and systemic resistance in plants (Klessig and Malamy, 1994; Ryals et al., 1995), acts as an agonist-dependent gain control for activation of the oxidative burst via its regulatory kinase cascade (Shirasu et al., 1997).
Reports linking the oxidative burst to phytoalexin biosynthesis have often been contradictory, even with respect to experiments performed on the same plant species. For example, treatment of soybean (Glycine max) cell cultures with catalase immediately before elicitation was shown to inhibit phytoalexin accumulation (Apostol et al., 1989), an observation interpreted as indicating a causal involvement of H2O2 as an inducer of the phytoalexin response. That this might be an oversimplification was apparent from a subsequent observation that certain biotic elicitors induce an oxidative burst in soybean cells, with no resultant phytoalexin production (Davis et al., 1993), and that catalase does not inhibit phytoalexin production in white clover cells (Devlin and Gustine, 1992).
H2O2 is a rapid, direct primary signal for the induction of transcripts encoding the antioxidant enzyme GST, whereas it is only a weak inducer of transcripts encoding the phytoalexin biosynthetic enzymes PAL and chalcone synthase in soybean cell cultures (Levine et al., 1994). This was supported by a recent demonstration that DPI, a suicide inhibitor of NADPH oxidase (O'Donnell et al., 1993), inhibits the oxidative burst in soybean cells exposed to a fungal glucan elicitor without inhibiting elicitor-induced phytoalexin accumulation (Mithofer et al., 1997). It remains to be resolved whether H2O2 is itself the endogenous signal for activation of the phytoalexin response after pathogen recognition.
While attempting to bioassay a proteinaceous elicitor from plant cells, we observed that inclusion of Ser protease inhibitors in the culture medium greatly and synergistically stimulated the elicitor-mediated oxidative burst in cultured soybean cells. Such reagents represent novel tools for the pharmacological dissection of the events controlling the oxidative burst and related processes. We describe the effects of protease inhibitors on the oxidative burst induced by biotic elicitors and virulent or avirulent bacteria, and the consequent effects on phytoalexin biosynthesis and accumulation. Our results reveal the involvement of a protease-inhibitor-sensitive, negative regulatory step in the signal transduction pathway(s) leading to both the oxidative burst and the accumulation of phytoalexins. Furthermore, potentiation of the oxidative burst by protease inhibitors has allowed us to define the relationship between the production of active oxygen species and the phytoalexin response.
MATERIALS AND METHODS
Plant Materials
Cell-suspension cultures of soybean (Glycine max L. cv Williams 82) were grown at 25°C in the dark in Murashige and Skoog medium as described previously (Guo et al., 1997). Cells were transferred to fresh medium every 7 d and were used 3 to 4 d after transfer.
Chemicals
Antipain, DFP, and DPI were from ICN. Pyranine was purchased from Molecular Probes (Eugene, OR). Other chemicals were from Sigma.
Elicitors and Bacteria
Yeast elicitor was isolated according to published procedures (Schumacher et al., 1987) and was dialyzed exhaustively (Spectra/por; cutoff, 3.5 kD; Spectrum, Houston, TX) against distilled water. Its final concentration in the plant-cell culture medium is given in micrograms of Glc equivalents per milliliter.
Pseudomonas syringae pv glycinea (Psg) race 4 containing plasmids with either the avrA or avrC avirulence genes (Keen and Buzzell, 1991) was grown in King's B medium (20 g of protease peptone, 10 mL of glycerol, 2.25 g of K2HPO4, and 1.5 g MySO47H2O L−1, pH 7.2) overnight with streptomycin or kanamycin. The bacteria were collected by centrifugation and resuspended in sterile water for inoculation of cultured cells.
Measurement of the Oxidative Burst
The oxidative burst was determined by measuring H2O2 production by quenching of pyranine fluorescence at 512 nm with excitation at 405 nm (Apostol et al., 1989) using a luminescence spectrophotometer (model LS50B, Perkin-Elmer). One-milliliter batches of cells in 12-well microtiter plates were exposed to proteinase inhibitors 30 min before the addition of yeast extract or bacteria. For proteinase inhibitors that required DMSO for solubilization, an equivalent amount of DMSO (0.1%, v/v) was added to control cultures. This concentration of DMSO did not induce the oxidative burst. For K252a- or DPI-inhibition experiments, 10 μm K252a or 1 μm DPI, respectively, was added immediately before the addition of proteinase inhibitors.
Inhibition of Soybean Ser Protease in Vitro
Soybean Ser protease was purified to homogeneity and assayed in the presence of various protease inhibitors, as described previously (Guo et al., 1998).
Determination of Isoflavonoids
Treated soybean cells were harvested by vacuum filtration and the cells and culture medium were collected separately. Cells were extracted with acetone, the acetone was removed under a stream of nitrogen, and the dried residue was dissolved in a small volume of methanol before reverse-phase HPLC analysis on a C18 column using a gradient of CH3CN (A) and water containing 1% H3PO4 (B). The gradient was 12% to 30% A for 35 min and 30% to 65% A for 20 min, and absorbance of the eluent was monitored by a diode array detector at 287 nm. For determination of glyceollin in the culture medium, the medium was partitioned twice against 2 volumes of ethyl acetate, dried under nitrogen, and the residue was analyzed as described above.
Northern-Blot Analysis
Total RNA was isolated and hybridized in northern blots according to standard procedures (Sambrook et al., 1989). cDNA hybridization probes were soybean PAL (Frank and Vodkin, 1991), soybean GST (Czarnecka et al., 1988), Arabidopsis rRNA (Pepper et al., 1994), and alfalfa IFR (Paiva et al., 1991).
RESULTS
Ser Protease Inhibitors Potentiate the Oxidative Burst in Soybean Cells in Response to Elicitors or Avirulent or Virulent Bacteria
Exposure of suspension-cultured soybean cells to Psg harboring the avrA avirulence gene resulted in an oxidative burst characterized by extracellular accumulation of H2O2 and increased cell death (Levine et al., 1994; Shirasu et al., 1997). In contrast, soybean cv Williams 82 does not contain a resistance gene for recognition of the avrC avirulence gene, and Psg harboring avrC did not induce a sustained oxidative burst, although a small burst is often seen very soon after exposure to the bacteria (Shirasu et al., 1997).
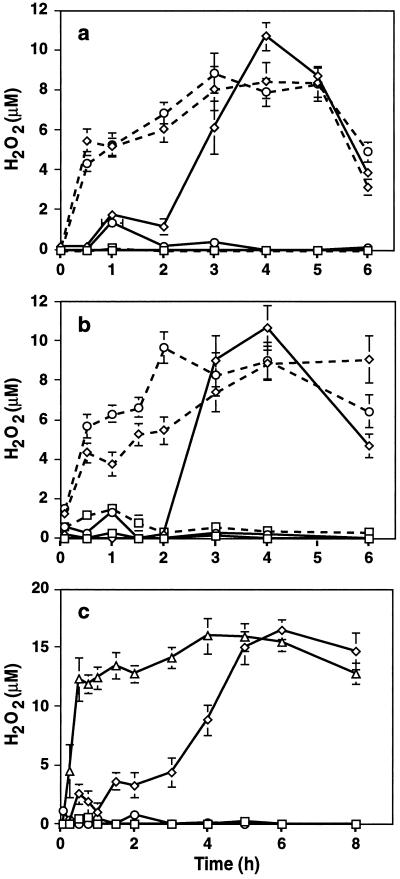
Treatment of soybean cells with Psg:avrA in the presence of 0.5 mm PMSF, a strong inhibitor of soybean Ser protease (Guo et al., 1998), resulted in striking changes in the kinetics of the oxidative burst (Fig. 1a). Rather than a small burst after approximately 1 h followed by a strong burst peaking at 4 h, a strong burst was apparent by 30 min, and only declined after 5 h. Strikingly, an almost identical burst was seen in cells exposed to Psg:avrC in the presence of PMSF, indicating that the burst potentiated by the protease inhibitor in response to Psg:avrA was the early, nonspecific burst, rather than the effect being a more rapid expression of the later, avirulence-gene-specific burst. PMSF itself (or DMSO in which PMSF was dissolved) did not induce H2O2 production in the absence of bacteria. Bacteria that had been pretreated with PMSF and then washed to remove the protease inhibitor induced the same level of H2O2 as untreated bacteria, whereas washing PMSF-treated soybean cells immediately before the addition of bacteria did not prevent potentiation of the burst (data not shown). These results indicate that the effect of PMSF is on the plant cells and not on the inducing bacteria.
Figure 1.
Ser protease inhibitors potentiate the oxidative burst in soybean suspension-cultured cells in response to elicitor and virulent or avirulent bacteria. a, Kinetics of the effects of PMSF (0.5 mm) on H2O2 generation in soybean cells after exposure to Pseudomonas syringae pv syringae (3 × 107 colony-forming units mL−1) harboring the avrA gene (incompatible interaction) or the avrC gene (compatible interaction), or in untreated controls. All cells were treated with DMSO (final concentration, 0.1% [v/v]; continuous lines) or PMSF dissolved in DMSO (dashed lines). □, No bacteria; ⋄, plus Psg:avrA; ○, plus Psg:avrC. b, Same conditions as in a, but using DFP (0.5 mm) in place of PMSF. Symbols are as in a, but DFP replaces PMSF. c, Effects of DFP on H2O2 generation in response to yeast elicitor (50 μg Glc equivalents mL−1). □, DMSO alone; ○, DMSO plus DFP; ⋄, DMSO plus yeast elicitor; ▵, DMSO plus yeast elicitor plus DFP. Results represent the means ± sd of three independent experiments.
An identical potentiation of the oxidative burst in response to avirulent or virulent bacteria was observed with the Ser protease inhibitor DFP (Fig. 1b), although this inhibitor alone produced a small early burst of similar magnitude to that obtained with Psg:avrC. Similar results were obtained with 0.5 mm 3,4-dichloroisocoumarin, although this inhibitor alone induced a larger oxidative burst than DFP (data not shown).
Exposure of soybean cells to yeast elicitor resulted in an oxidative burst with kinetics similar to those induced by Psg:avrA, and this burst was also potentiated by DFP (Fig. 1c). Neither the aminopeptidase inhibitor bestatin (0.1 mm) nor the acidic aspartyl protease inhibitor pepstatin A (0.1 mm) had any effect on the oxidative burst, either alone or in combination with bacteria or elicitor (data not shown).
We recently purified the major Ser protease from suspension-cultured soybean cells (Guo et al., 1998). To determine whether this protease might be the site of action of the various Ser protease inhibitors as potentiators of the oxidative burst, we compared the effects of a panel of protease inhibitors on the burst in vivo and on the activity of the purified protease in vitro. In this and subsequent experiments, the burst was measured at 2 h after elicitation, at which time H2O2 production was still low in the absence of the potentiator. The results shown in Table I indicate that in most cases compounds that inhibited the protease in vitro also potentiated the oxidative burst in response to yeast elicitor. Nearly identical results were obtained when measuring the effects of the inhibitors on H2O2 production after exposure of cells to Psg:avrA or Psg:avrC (data not shown). However, there was no direct correlation between the effectiveness of the compounds to inhibit the protease in vitro and their ability to induce the oxidative burst in vivo. For example, leupeptin was one of the most potent inhibitors of the soybean protease, but it only weakly potentiated the burst at 2 h after elicitation. This result could be explained by poor uptake of the inhibitor into the cells, but PMSF, a strong potentiator of the burst, only weakly inhibited the protease in vitro, suggesting that the Ser protease in the soybean cell culture was not the target (or at least was not the only target) through which Ser protease inhibitors potentiate the oxidative burst.
Table I.
Comparison of the effects of a range of protease inhibitors on the activity of purified soybean Ser protease in vitro and on the oxidative burst in vivo
| Inhibitor | Protease
Activity
|
Oxidative Burst
|
||
|---|---|---|---|---|
| Inhibitor concentration | Protease activity | Control | Yeast elicitor | |
| μm | % | μm H2O2 | ||
| Control (DMSO) | 0 | 100 | 0.00 ± 0.10 | 2.10 ± 0.44 |
| Antipain | 20 | 0 | 4.55 ± 0.43 | 13.2 ± 1.17 |
| DCIa | 1000 | 26 | 1.03 ± 0.27 | 12.4 ± 1.49 |
| DFP | 1000 | 2 | 1.34 ± 0.17 | 9.46 ± 1.03 |
| Leupeptin | 15 | 0 | 0.13 ± 0.11 | 5.90 ± 0.34 |
| PMSF | 1000 | 81 | 0.11 ± 0.06 | 12.4 ± 1.41 |
| TLCKb | 1000 | 61 | 0.10 ± 0.13 | 3.52 ± 0.22 |
| TPCKc | 1000 | 47 | 5.86 ± 0.60 | 8.90 ± 1.07 |
Concentrations of inhibitors used in vitro are given. Inhibitors were applied to cells at a final concentration of 0.5 mm (0.2 mm for antipain), and H2O2 was measured 2 h after exposure to water (control) or yeast elicitor (200 μg Glc equivalents mL−1). Results show the average ± sd from three independent experiments.
DCI, 3,4-dichloroisocoumarin.
TLCK, Nα-p-tosyl-l-lysine chloromethyl ketone.
TPCK, n-tosyl-l-phenylalanine chloromethyl ketone.
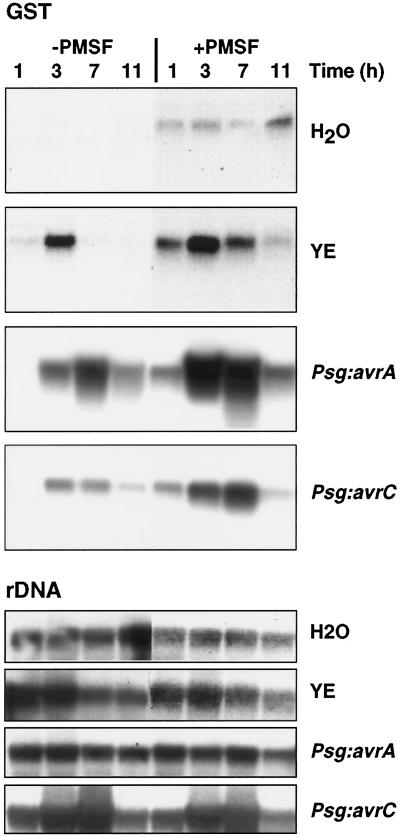
H2O2 generated through the oxidative burst is an inductive signal for expression of antioxidant defenses, including the accumulation of GST transcripts (Levine et al., 1994). GST transcripts were weakly induced after exposure of soybean cells to PMSF alone; however, transcript levels induced in response to yeast elicitor, Psg:avrA, or Psg:avrC increased earlier and were maintained longer in cells exposed at the same time to PMSF (Fig. 2). Therefore, Ser protease inhibitors potentiate both the oxidative burst and a downstream gene-induction response.
Figure 2.
GST transcript levels in soybean suspension-cultured cells exposed to yeast elicitor (YE; 200 μg Glc equivalents mL−1) or Psg harboring the avrA or avrC avirulence genes (3 × 107 colony-forming units mL−1) in the presence or absence of PMSF (0.5 mm in DMSO). Controls were treated with DMSO alone. Samples were harvested at the times shown, and RNA was extracted and subjected to northern-blot analysis using a soybean GST cDNA (Czarnecka et al., 1988) as a probe. Loading and transfer efficiency were checked by reprobing the blots with a rDNA probe.
Oxidative Burst Potentiated by Ser Protease Inhibitors Is Blocked by Inhibitors of NADPH Oxidase and Protein Ser/Thr Kinase
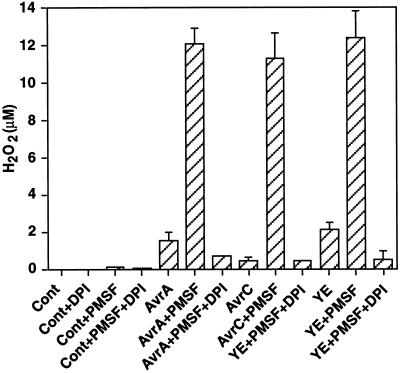
The signal transduction pathway for H2O2 generation in soybean and other plant cells in response to avirulent pathogens or elicitors involves a protein Ser/Thr kinase cascade that leads to activation of the plasma membrane-associated NADPH oxidase complex (Levine et al., 1994; Chandra and Low, 1995; Shirasu et al., 1997; Xing et al., 1997). Treatment of soybean cells with DPI strongly inhibited the accumulation of H2O2 in response to PMSF added along with Psg:avrA, Psg:avrC, or yeast elicitor (Fig. 3). Therefore, the H2O2 produced by potentiation of the early, nonavirulence-gene-specific oxidative burst is generated by the NADPH oxidase complex previously implicated in the specific avirulence-gene-mediated response.
Figure 3.
The oxidative burst in response to elicitor or virulent/avirulent bacteria potentiated by PMSF is blocked by the NADPH oxidase inhibitor DPI. Soybean suspension-cultured cells exposed to yeast elicitor (YE; 50 μg Glc equivalents mL−1), Psg:avrA (AvrA), or Psg:avrC (AvrC) (3 × 107 colony-forming units mL−1) were treated with PMSF (0.5 mm in DMSO) or DMSO alone (Cont). Production of H2O2 at 2 h after elicitation was measured in the presence or absence of DPI (10 μm). Results represent the means ± sd of three independent experiments.
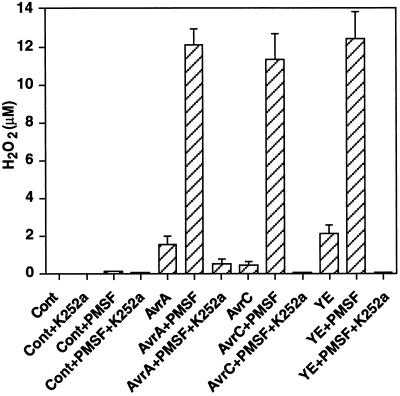
Treatment of soybean cell suspensions with the protein Ser/Thr kinase inhibitor K252a almost completely inhibited the oxidative burst in response to Psg:avrA, Psg:avrC, or yeast elicitor in the presence of PMSF (Fig. 4). The fact that the inhibition was well below the value in the presence of bacteria or elicitors alone suggests that all of the H2O2 produced during the potentiated early oxidative burst arose from the protein-kinase-dependent pathway.
Figure 4.
The oxidative burst in response to elicitor or virulent/avirulent bacteria potentiated by PMSF is blocked by the protein kinase inhibitor K252a. Soybean suspension-cultured cells exposed to yeast elicitor (YE), Psg:avrA (AvrA), or Psg:avrC (AvrC) were treated with PMSF (0.5 mm in DMSO) or DMSO alone (Cont). Production of H2O2 at 2 h after elicitation was measured in the presence or absence of K252a (1 μm). Results represent the means ± sd of three independent experiments.
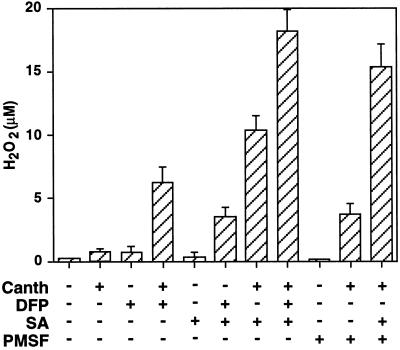
The protein-phosphatase inhibitor cantharidin can induce the oxidative burst at high concentrations (10 μm), and can act synergistically with the potentiator SA at concentrations that alone do not induce H2O2 accumulation (Shirasu et al., 1997). When applied to soybean cell-suspension cultures at a noninductive concentration of 2 μm, cantharidin acted synergistically with DFP or PMSF to induce the oxidative burst (Fig. 5), confirming the operation of a protein kinase cascade for the potentiated burst.
Figure 5.
Ser protease inhibitors act synergistically with the Ser phosphatase inhibitor cantharidin (Canth) to induce the oxidative burst. Cells were treated with cantharidin (2 μm) and DFP or PMSF (0.5 mm in DMSO) singly or in combination, and H2O2 release was measured 60 min later. Effects of the addition of SA (50 μm) alone or in combination with cantharidin, protease inhibitors, or both were also determined. Results represent the average ± sd of two independent experiments.
In agreement with previous results (Shirasu et al., 1997), SA by itself did not induce the oxidative burst, but strongly potentiated the burst in cantharidin-treated cells, in a manner similar to DFP (Fig. 5). No oxidative burst was observed in cells exposed to PMSF and SA in the absence of cantharidin (data not shown). The effect of adding protease inhibitors and SA to cantharidin-treated cells was additive.
Role of Active Oxygen Species in Phytoalexin Induction
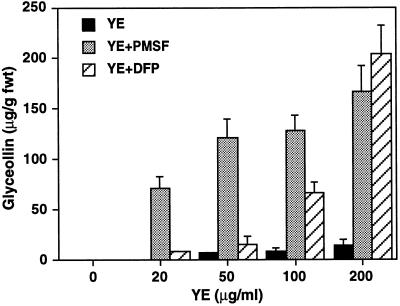
Soybean cell-suspension cultures produce low amounts of the isoflavonoid phytoalexin glyceollin after exposure to yeast elicitor (Guo et al., 1997) (Fig. 6). The addition of PMSF or DFP alone to cell cultures did not induce glyceollin (data not shown); however, both PMSF and DFP had strong synergistic effects with yeast elicitor on glyceollin accumulation. Treatment with 0.5 mm DFP and 200 μg Glc equivalents mL−1 yeast elicitor led to the accumulation of 10 times more glyceollin than accumulated in response to yeast elicitor alone (Fig. 6). Treatment of cells with 0.5 mm PMSF plus 20 μg mL−1 yeast elicitor, which individually induced no glyceollin, resulted in the accumulation of nearly 100 μg glyceollin g−1 fresh weight of cells.
Figure 6.
Ser protease inhibitors potentiate the accumulation of the phytoalexin glyceollin in response to yeast elicitor (YE). Cells were treated with the concentrations of yeast elicitor shown in the presence or absence of PMSF or DFP (0.5 mm in DMSO), and cells were harvested for analysis of glyceollin levels after 24 h. Results represent the means ± sd of three independent experiments. fwt, Fresh weight.
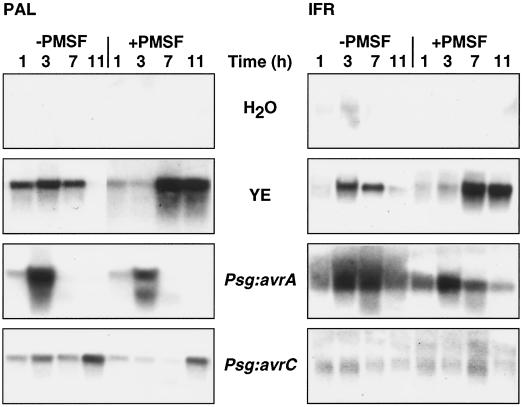
The effects of PMSF on glyceollin induction by yeast elicitor were associated with a delay in the appearance of maximal levels of transcripts encoding PAL and IFR, two key enzymes of isoflavonoid biosynthesis (Fig. 7). Treatment with PMSF inhibited the limited appearance of PAL and IFR transcripts in cells exposed to Psg:avrC, whereas the timing of PAL and IFR transcript appearance in cells exposed to Psg:avrA was unaffected. PMSF reduced the level of IFR transcripts in cells exposed to Psg:avrA. These data indicate that the striking increase in glyceollin accumulation in elicited cells exhibiting a rapid and prolonged oxidative burst after exposure to PMSF was not the result of increased induction of these phytoalexin biosynthetic enzymes.
Figure 7.
Levels of transcripts encoding PAL and IFR in soybean suspension-cultured cells treated with water (control), yeast elicitor (YE; 200 μg Glc equivalents mL−1), or Psg:avrA or Psg:avrC (3 × 107 colony-forming units mL−1) in the presence or absence of PMSF (0.5 mm in DMSO). Controls contained DMSO (final concentration, 0.1% [v/v]) alone. The blot shown in Figure 2 was stripped and reprobed with soybean PAL (Frank and Vodkin, 1991) and alfalfa IFR (Paiva et al., 1991) cDNAs. The relevant RNA-loading control is shown in Figure 2.
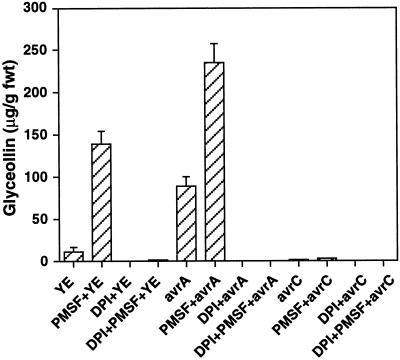
Glyceollin accumulation in response to yeast elicitor or Psg:avrA and the corresponding responses potentiated by PMSF were strongly inhibited by DPI, suggesting that active oxygen species produced by the oxidative burst are necessary for phytoalexin accumulation (Fig. 8). However, although PMSF treatment led to nearly identical production of H2O2 in soybean cells exposed to either Psg:avrA or Psg:avrC (Fig. 1a), significant phytoalexin accumulation was observed only in response to Psg:avrA (Fig. 8). Therefore, the use of protease inhibitors reveals that H2O2 is necessary but not sufficient for glyceollin induction.
Figure 8.
Relationship between glyceollin induction and release of active oxygen species. Data show the effects of DPI (10 μm) on the production of glyceollin in soybean suspension-cultured cells in response to yeast elicitor (YE; 200 μg Glc equivalents mL−1), Psg:avrA, or Psg:avrC in the presence or absence of PMSF (0.5 mm). Cells and culture media were harvested 24 h after elicitation, and glyceollin levels were analyzed by HPLC. In cells treated with yeast elicitor, all of the glyceollin accumulated intracellularly, whereas glyceollin was found in both cells and culture medium in cells treated with Psg:avrA. The glyceollin in the culture medium is included in the values given. Results represent the means ± sd of three independent experiments. fwt, Fresh weight.
DISCUSSION
Source of H2O2 from the Oxidative Burst Potentiated by Ser Protease Inhibitors
Although there is considerable evidence that the H2O2 produced in the oxidative burst arises via a plasma membrane NADPH oxidase (Groom et al., 1996; Keller et al., 1997; Mithofer et al., 1997; Van Gestelen et al., 1997), alternative sources such as extracellular peroxidases and amine oxidases have also been implicated under certain conditions (Bolwell, 1996; Allan and Fluhr, 1997). The fact that the potentiation of the oxidative burst by protease inhibitors is completely inhibited by DPI (Fig. 3), a suicide inhibitor of the NADPH oxidase and other flavoproteins in mammalian cells (O'Donnell et al., 1993), strongly suggests that protease inhibitors act upstream in the signal transduction pathway leading to activation of the oxidase, and are unlikely to affect processes such as the turnover of a peroxidase or amine oxidase. Such a conclusion is also consistent with the inhibition of Ser protease-mediated potentiation of the oxidative burst by K252a (Fig. 4) and with the ability of Ser proteases to act synergistically with cantharidin (Fig. 5), suggesting the involvement of a protein kinase cascade in the Ser protease inhibitor-mediated potentiation of H2O2 accumulation. These observations parallel a significant body of evidence implicating a protein Ser/Thr kinase cascade in the signal transduction pathway leading to activation of the NADPH oxidase (Schwacke and Hager, 1992; Chandra and Low, 1995; Shirasu et al., 1997).
Site of Action of Ser Protease Inhibitors
The observation that several chemically distinct Ser protease inhibitors (Fig. 1; Table I) but not the aminopeptidase inhibitor bestatin or the aspartyl protease inhibitor pepstatin A act to potentiate the burst indicates that the most likely site of action is an endogenous Ser protease. However, this is unlikely to be the Ser protease recently purified from soybean cell cultures (Guo et al., 1998) because PMSF, a potent potentiator of the burst, failed to inhibit this enzyme significantly.
Bestatin is itself a powerful inducer of defense genes for wound responses in tomato leaves (Schaller et al., 1995), and it has been suggested that it might act to inhibit a regulatory protease that might control the levels of a transcription factor (Schaller et al., 1995). Two important differences distinguish the effects of bestatin in tomato from those reported here. First, the effects of most of the Ser protease inhibitors in the present work are agonist dependent and the compounds are therefore true potentiators, i.e. they are not strong inducers when added alone. Second, Ser protease inhibitors do not induce significantly the expression of the defense response genes PAL or IFR in soybean cells (Fig. 7). Elevation of GST transcripts in response to elicitor or Psg plus PMSF can be explained as a response to the more rapid accumulation of H2O2 under these conditions (Levine et al., 1994).
The observation that Ser protease inhibitors are strong potentiators of the NADPH oxidase-dependent oxidative burst in soybean suspension-cultured cells is in striking contrast to the reported inhibitory effects of protease inhibitors on NADPH oxidase activation in mammalian cells. For example, chymotrypsin inhibitors block H2O2 formation by activated human polymorphonuclear leukocytes (Frenkel et al., 1987), and the irreversible Ser protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride inhibits superoxide production from guinea pig peritoneal macrophages (Diatchuk et al., 1997). In the latter study, a range of other Ser protease inhibitors, including PMSF, 3,4-dichloroiso-coomarin, n-tosyl-l-phenylalaninechloromethyl ketone, and leupeptin, were inactive, and it was concluded that 4-(2-aminoethyl)benzenesulfonyl fluoride acted directly to interfere with the binding of the p47phox and/or the p67phox subunits to the catalytic Cyt b559 component of the oxidase (Diatchuk et al., 1997). These results indicate that Ser protease inhibitors such as 4-(2-aminoethyl)benzenesulfonyl fluoride and PMSF may have previously unforeseen sites of action, and this qualification must be borne in mind when considering the results of the present study.
We have previously shown that SA is a strong, agonist-dependent potentiator of the oxidative burst at physiological concentrations (Shirasu et al., 1997). The present results implicate proteolysis of a regulatory component in the signal pathway leading to the oxidative burst as a further site of control. SA probably acts at a different site from PMSF, in view of the observations that the agonist-dependent effects of SA and Ser protease inhibitors are additive (Fig. 5) and that SA potentiates the appearance of PAL transcripts in response to Psg:avrA (Shirasu et al., 1997), whereas PMSF does not (Fig. 7). Therefore, a picture emerges of the involvement of multiple endogenous signals, which need to be perceived together for the commitment of the plant to a program of metabolic changes that involve generation of potentially toxic active oxygen species and phytochemicals for defense.
Relationship between the Oxidative Burst and Phytoalexin Accumulation
Our data show that generation of H2O2 via the oxidative burst is necessary but not sufficient for phytoalexin accumulation in soybean cells. For example, DPI inhibits the phytoalexin response but the potentiated burst obtained with Psg:avrC in the presence of PMSF does not lead to phytoalexin accumulation (Figs. 1 and 8). Clearly, active oxygen species must act in concert with another signal specified by the interaction of the avrA gene with the Rpg3 resistance gene present in soybean before phytoalexin accumulation can occur. However, consistent with previous observations that H2O2 is not itself an inducer of genes encoding phytoalexin biosynthetic enzymes in soybean cells (Levine et al., 1994; Shirasu et al., 1997), increased phytoalexin accumulation after the early and sustained accumulation of H2O2 in protease-inhibitor-potentiated cells does not result from increased expression of PAL or IFR. If H2O2 were the signal for phytoalexin biosynthetic gene activation, one would expect a more rapid appearance of the PAL and IFR transcripts in the presence of PMSF, whereas the converse was observed (Fig. 7).
In conclusion, phytoalexin accumulation in soybean cell cultures requires, at minimum, the oxidative burst acting in concert with a second signal generated by the interaction of a race-specific or nonspecific elicitor with its cognate receptor(s). Our results also suggest that phytoalexin accumulation in the soybean cell system is controlled by mechanisms additional to the transcriptional induction of the phytoalexin biosynthetic machinery.
ACKNOWLEDGMENTS
We thank Drs. Madan Bhattacharyya and Ignacio Maldonado-Mendoza for critical reading of the manuscript.
Abbreviations:
- DFP
diisopropylfluorophosphate
- DPI
diphenylene iodonium
- GST
glutathione S-transferase
- IFR
isoflavone reductase
- PAL
l-Phe ammonia-lyase
- SA
salicylic acid
Footnotes
This work was supported by the Samuel Roberts Noble Foundation. Z.-J.G. is a Noble Foundation/Salk Institute Plant Biology Postdoctoral Fellow.
LITERATURE CITED
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Pennell R, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;9:773–786. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Apostol I, Heinstein PF, Low PS. Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Plant Physiol. 1989;90:109–116. doi: 10.1104/pp.90.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP. The origin of the oxidative burst in plants. Biochem Soc Trans. 1996;24:438–442. doi: 10.1042/bst0240438. [DOI] [PubMed] [Google Scholar]
- Chandra S, Low PS (1995) Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proc Natl Acad Sci USA 92: 4120–4123 [DOI] [PMC free article] [PubMed]
- Czarnecka E, Nagao RT, Key JL, Gurley WB. Gmhsp-26a, a stress gene encoding a divergent heat shock protein of soybean: heavy metal-induced inhibition of intron processing. Mol Cell Biol. 1988;8:1113–1122. doi: 10.1128/mcb.8.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Merida J, Legendre L, Low PS, Heinstein P. Independent elicitation of the oxidative burst and phytoalexin formation in cultured plant cells. Phytochemistry. 1993;32:607–611. [Google Scholar]
- Devlin WS, Gustine DL. Involvement of the oxidative burst in phytoalexin accumulation and the hypersensitive reaction. Plant Physiol. 1992;100:1189–1195. doi: 10.1104/pp.100.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- Doke N, Miura Y, Sanchez LM, Park H-J, Noritake T, Yoshioka H, Kawakita K. The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defense—a review. Gene. 1996;179:45–51. doi: 10.1016/s0378-1119(96)00423-4. [DOI] [PubMed] [Google Scholar]
- Frank RL, Vodkin LO. Sequence and structure of a phenylalanine ammonia-lyase from Glycine max. DNA Seq. 1991;1:335–346. doi: 10.3109/10425179109020788. [DOI] [PubMed] [Google Scholar]
- Frenkel K, Chrzan K, Ryan CA, Wiesner R, Troll W. Chymotrypsin-specific protease inhibitors decrease H2O2 formation by activated human polymorphonuclear leukocytes. Carcinogenesis. 1987;8:1207–1212. doi: 10.1093/carcin/8.9.1207. [DOI] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG. rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant Cell. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Guo Z-J, Lamb C, Dixon RA. Release and biological activity of diffusible signal compounds from elicited plant cells. J Plant Physiol. 1997;151:699–710. [Google Scholar]
- Guo Z-J, Lamb C, Dixon RA. A serine protease from suspension-cultured soybean cells. Phytochemistry. 1998;47:547–553. doi: 10.1016/s0031-9422(97)00441-x. [DOI] [PubMed] [Google Scholar]
- Keen NT, Buzzell RI. New disease resistance genes in soybean against Pseudomonas syringae pv glycinea: evidence that one of them interacts with a bacterial elicitor. Theor Appl Genet. 1991;81:133–138. doi: 10.1007/BF00226123. [DOI] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes an intrinsic plasma membrane protein with Ca2+-binding and RanGAP1 domains. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994;26:1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon RA, Lamb CJ. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response as a local trigger of programmed cell death and a diffusible inducer of cellular protectant genes. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW. The role of activated oxygen species in plant disease resistance. Physiol Plant. 1996;98:365–374. [Google Scholar]
- Mithofer A, Daxberger A, Fromhold-Treu D, Ebel J. Involvement of an NADP(H) oxidase in the elicitor-inducible oxidative burst of soybean. Phytochemistry. 1997;45:1101–1107. [Google Scholar]
- O'Donnell VB, Tew DG, Jones OTG, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva NL, Edwards R, Sun Y, Hrazdina G, Dixon RA. Stress responses in alfalfa (Medicago sativa L.). XI. Molecular cloning and expression of alfalfa isoflavone reductase, a key enzyme of isoflavonoid phytoalexin biosynthesis. Plant Mol Biol. 1991;17:653–667. doi: 10.1007/BF00037051. [DOI] [PubMed] [Google Scholar]
- Park HH, Hakamatsuka T, Sankawa U, Ebizuka Y. Involvement of oxidative burst in isoflavonoid metabolism in elicited cell suspension cultures of Pueraria lobata. Z Naturforsch. 1995;50c:824–832. [Google Scholar]
- Pepper A, Delaney T, Washburn T, Poole D, Chory J. Det1, a negative regulator of light-mediated development and gene expression in Arabidopsis, encodes a novel nuclear-localized protein. Cell. 1994;78:109–116. doi: 10.1016/0092-8674(94)90577-0. [DOI] [PubMed] [Google Scholar]
- Ryals J, Lawton KA, Delaney TP, Friedrich L, Kessmann H, Neuenschwander U, Uknes S, Vernooij B, Weymann K. Signal transduction in systemic acquired resistance. Proc Natl Acad Sci USA. 1995;92:4202–4205. doi: 10.1073/pnas.92.10.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schaller A, Bergey DR, Ryan CA. Induction of wound response genes in tomato leaves by bestatin, an inhibitor of aminopeptidases. Plant Cell. 1995;7:1893–1898. doi: 10.1105/tpc.7.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H-M, Gundlach H, Fiedler F, Zenk MH. Elicitation of benzophenanthridine alkaloid synthesis in Eschscholtzia cell cultures. Plant Cell Rep. 1987;6:410–413. doi: 10.1007/BF00272770. [DOI] [PubMed] [Google Scholar]
- Schwacke R, Hager A. Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta. 1992;187:136–141. doi: 10.1007/BF00201635. [DOI] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb CJ. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–93. [Google Scholar]
- Van Gestelen P, Asard H, Caubergs RJ. Solubilization and separation of a plant plasma membrane NADPH-O2− synthase from other NAD(P)H oxidoreductases. Plant Physiol. 1997;115:543–550. doi: 10.1104/pp.115.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E. Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell. 1997;9:249–259. doi: 10.1105/tpc.9.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]