Abstract
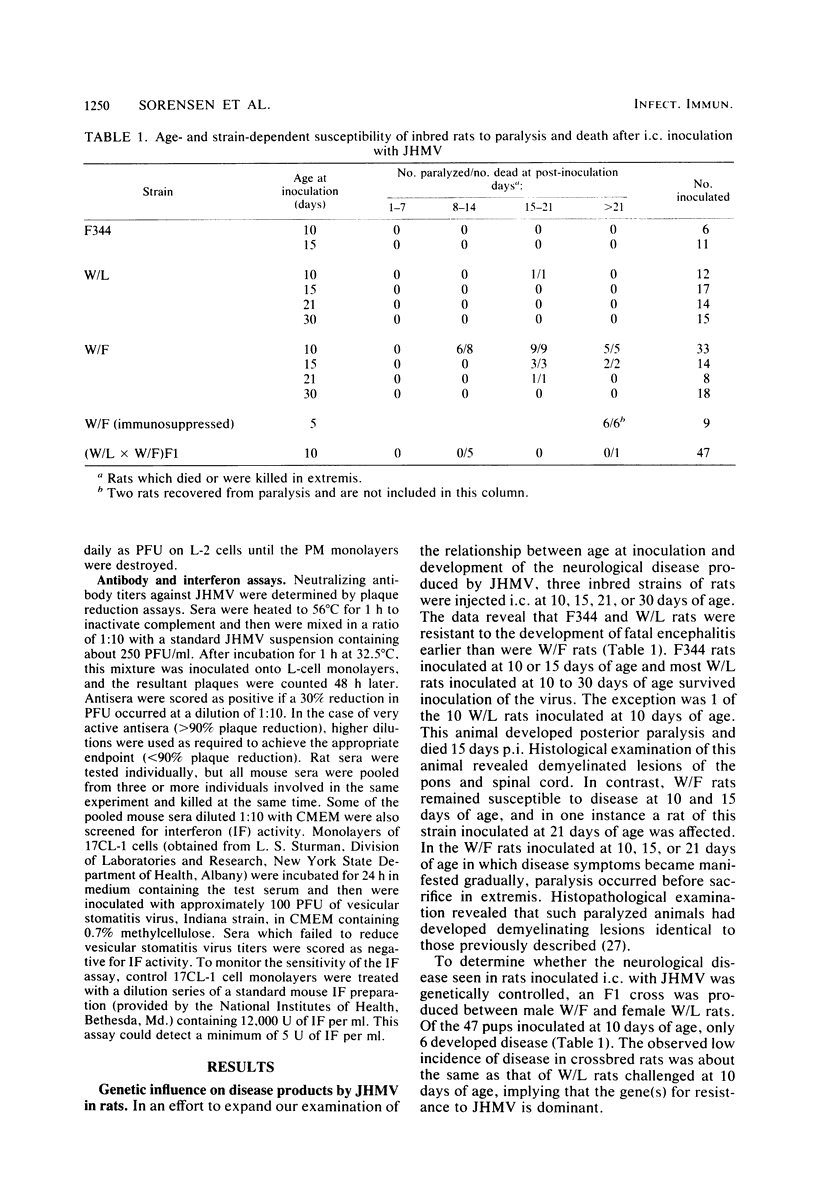
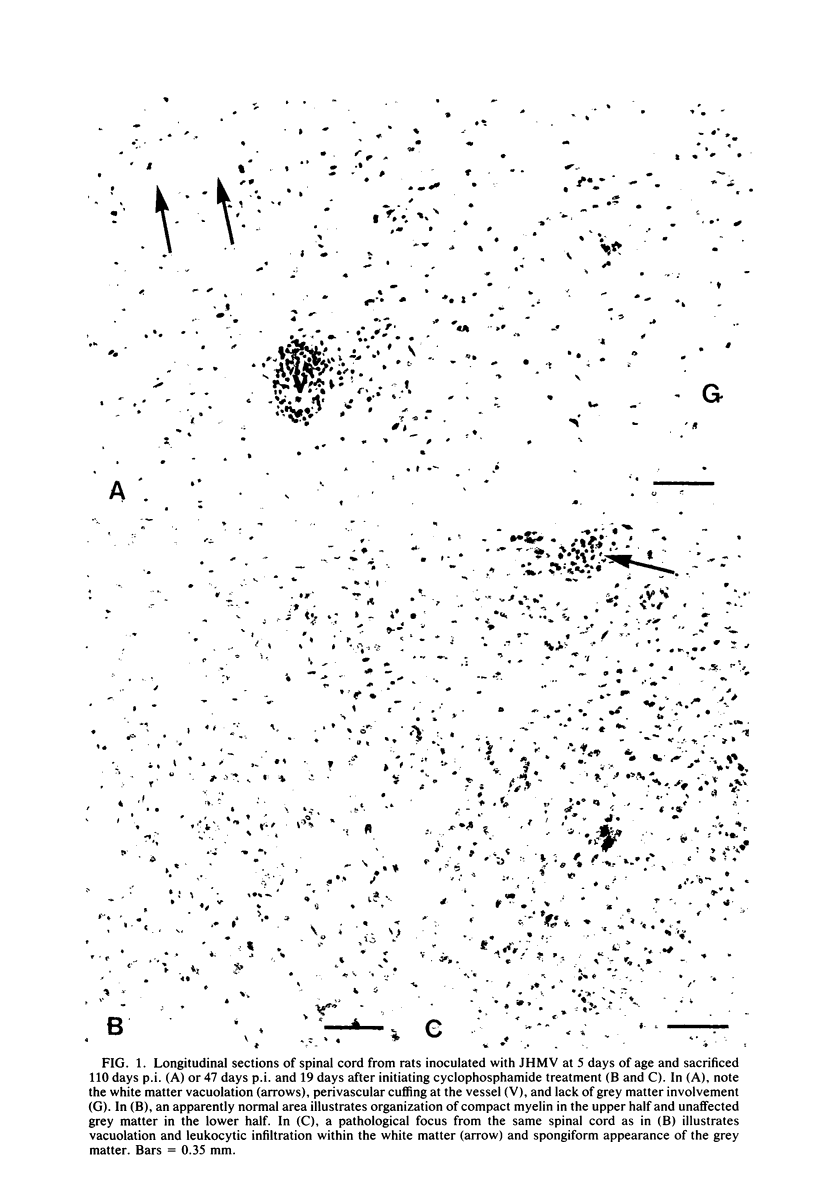
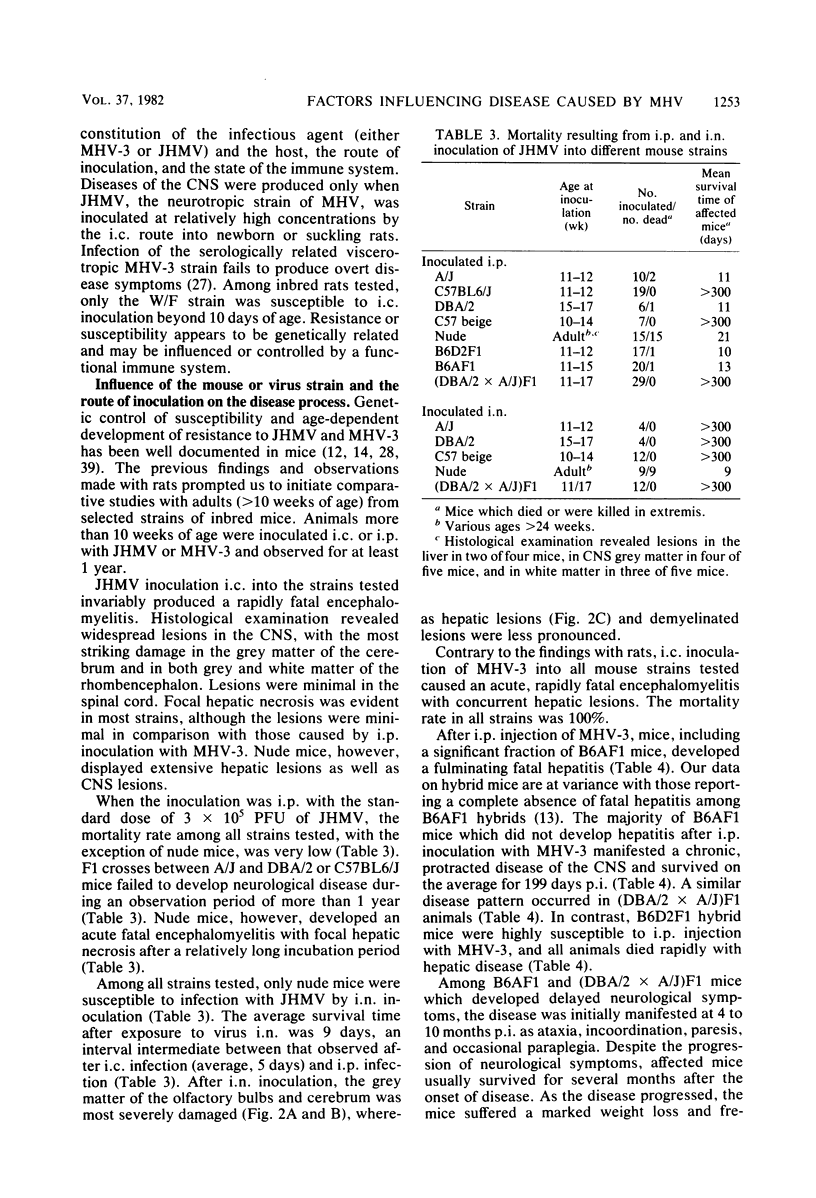
Intracerebral inoculation of JHM virus (JHMV), the neuropathic strain of mouse hepatitis virus, into Wistar Furth, Wistar Lewis, and Fischer 344 rats at various ages indicated that Wistar Furth rats are more susceptible to the virus than are the other strains. Fischer 344 and Wistar Lewis rats were more resistant to inoculation at 2 and 5 days of age and completely resistant by 10 days of age. In contrast, Wistar Furth rats which were very susceptible at both 2 and 5 days of age remained susceptible until 21 days of age. Intracerebral challenge of an F1 cross between Wistar Furth and Wistar Lewis rats at 10 days of age indicated that resistance to JHMV infection is dominant. Cyclophosphamide treatment 28 days after intracerebral inoculation exacerbated an inapparent infection, leading to paralysis in eight of nine and death in six of nine Wistar Furth test rats. In such immunosuppressed animals, grey- and white-matter lesions were noted throughout the central nervous system, in contrast to the purely demyelinating lesions noted previously. Since rats, unlike mice, were not susceptible to disease after intracerebral injection with the serorelated viscerotropic strain MHV-3, we wished to extend our understanding of the neurological disease process elicited by the two viruses in rodents. For this reason, various mouse strains, including some with recognized immunodeficiencies, were challenged by different routes of inoculation. Intraperitoneal infection of nude and beige mice with JHMV indicated that lack of natural killer cell functions does not markedly enhance the susceptibility to virus, whereas T-cell activity appears to be essential for resisting infection. JHMV and MHV-3 replication in peritoneal macrophages from highly resistant A/J mice was reduced in comparison with that noted in macrophages from susceptible C57BL6/J mice. An initial intraperitoneal inoculation of JHMV was able to protect C57BL6/J mice against fatal intracerebral challenge within 3 days, whereas A/J mice remained susceptible beyond day 3. The protective effect did not appear to result from increased levels of circulating interferon, preceded elevation in serum JHMV-neutralizing antibody titers, and persisted for at least several weeks after intraperitoneal inoculation. Based on the combined studies described here and on previous work by us and others, it appears that the factors influencing the outcome of coronavirus disease in rodents are age at inoculation, route of challenge, genetic constitution of the virus and host, and competence of the immune system, particularly cellular immunity involving T-cells.
Full text
PDF


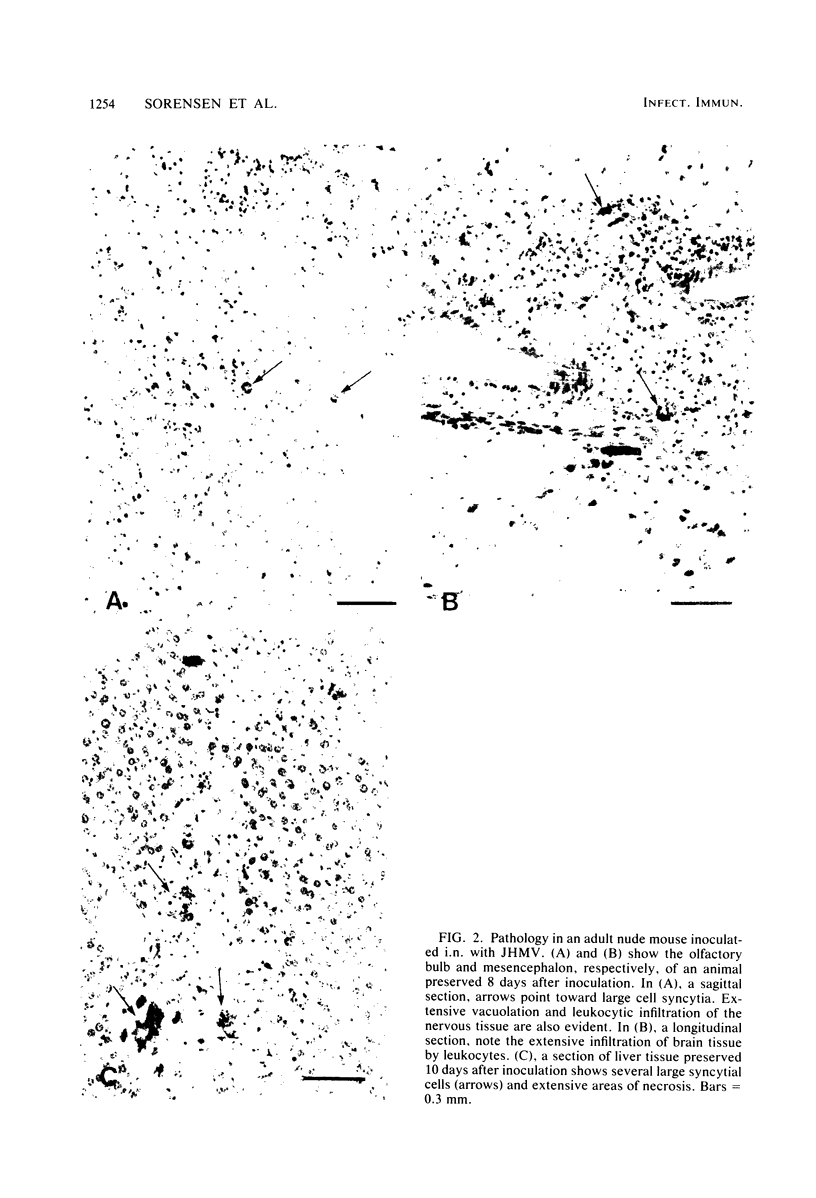
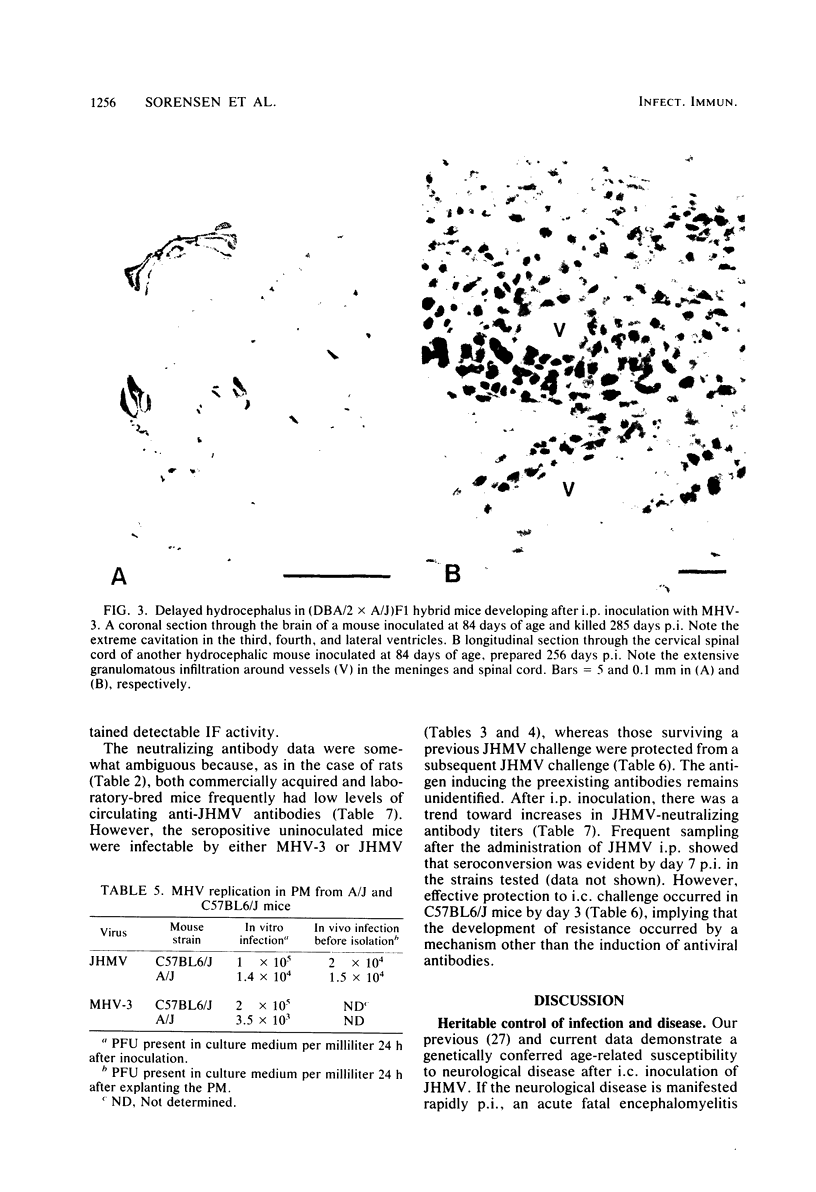
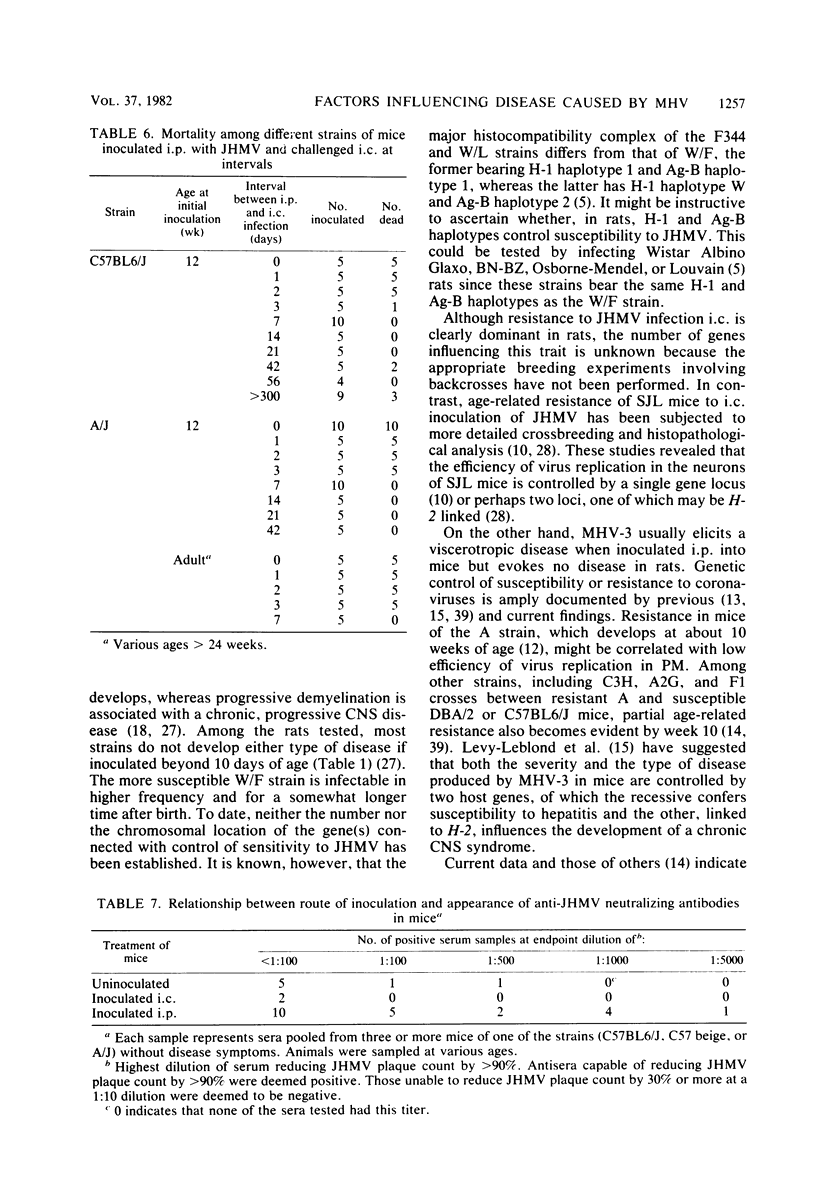



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P. N., Percy D. H., Jonas A. M. Characterization of the virus of sialodacryoadenitis of rats: a member of the coronavirus group. J Infect Dis. 1972 Aug;126(2):123–130. doi: 10.1093/infdis/126.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy J. M., Levey-Leblond E., Le Prevost C. Immunopathology of mouse hepatitis virus type 3mii. effect of immunosuppression in resistant mice. J Immunol. 1975 Jan;114(1 Pt 1):226–230. [PubMed] [Google Scholar]
- Gasser D. L. Current status of rat immunogenetics. Adv Immunol. 1977;25:93–139. [PubMed] [Google Scholar]
- Hierholzer J. C., Broderson J. R., Murphy F. A. New strain of mouse hepatitis virus as the cause of lethal enteritis in infant mice. Infect Immun. 1979 May;24(2):508–522. doi: 10.1128/iai.24.2.508-522.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Goto N., Ogawa T., Ono K., Murakami T., Fujiwara K. Hydrocephalus in suckling rats infected intracerebrally with mouse hepatitis virus, MHV-A59. Microbiol Immunol. 1980;24(9):825–834. doi: 10.1111/j.1348-0421.1980.tb02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler R. L., Haspel M. V., Oldstone M. B. Mouse hepatitis virus type 4 (JHM strains). induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981 Apr 1;153(4):832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. In vitro NK-activity and in vivo resistance to leukemia: studies of beige, beige//nude and wild-type hosts on C57BL background. Int J Cancer. 1980 Dec 15;26(6):789–797. doi: 10.1002/ijc.2910260613. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prevost C., Levy-Leblond E., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. Role of humoral and cell-mediated immunity in resistance mechanisms. J Immunol. 1975 Jan;114(1 Pt 1):221–225. [PubMed] [Google Scholar]
- Levy-Leblond E., Dupuy J. M. Neonatal susceptibility to MHV3 infection in mice. I. Transfer of resistance. J Immunol. 1977 Apr;118(4):1219–1222. [PubMed] [Google Scholar]
- Lévy-Leblond E., Oth D., Dupuy J. M. Genetic study of mouse sensitivity to MHV3 infection: influence of the H-2 complex. J Immunol. 1979 Apr;122(4):1359–1362. [PubMed] [Google Scholar]
- McKinnon K. P., Hale A. H., Ruebush M. J. Elicitation of natural killer cells in beige mice by infection with vesicular stomatitis virus. Infect Immun. 1981 Apr;32(1):204–210. doi: 10.1128/iai.32.1.204-210.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Corona virus induced subacute demyelinating encephalomyelitis in rats: a morphological analysis. Acta Neuropathol. 1978 Oct 13;44(1):63–70. doi: 10.1007/BF00691641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Wege H., Meyermann R., ter Meulen V. Demyelinating encephalomyelitis induced by a long-term corona virus infection in rats. A preliminary report. Acta Neuropathol. 1979 Mar 15;45(3):205–213. doi: 10.1007/BF00702672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C., Cross S. S., Rowe W. P. Rat coronavirus (RCV): a prevalent, naturally occurring pneumotropic virus of rats. Arch Gesamte Virusforsch. 1970;31(3):293–302. doi: 10.1007/BF01253764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell H. C., Lampert P. W. Oligodendrocytes and their myelin-plasma membrane connections in JHM mouse hepatitis virus encephalomyelitis. Lab Invest. 1975 Oct;33(4):440–445. [PubMed] [Google Scholar]
- Prévost C. L., Virelizier J. L., Dupuy J. M. Immunopathology of mouse hepatitis virus type 3 infection. III. Clinical and virologic observation of a persistent viral infection. J Immunol. 1975 Sep;115(3):640–643. [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Sebesteny A., Hill A. C. Hepatitis and brain lesions due to mouse hepatitis virus accompanied by wasting in nude mice. Lab Anim. 1974 Sep;8(3):317–326. doi: 10.1258/002367774780943733. [DOI] [PubMed] [Google Scholar]
- Sheets P., Shah K. V., Bang F. B. Mouse hepatitis virus (MHV) infection in thymectomized C3H mice. Proc Soc Exp Biol Med. 1978 Oct;159(1):34–38. doi: 10.3181/00379727-159-40278. [DOI] [PubMed] [Google Scholar]
- Shif I., Bang F. B. In vitro interaction of mouse hepatitis virus and macrophages from genetically resistant mice. I. Adsorption of virus and growth curves. J Exp Med. 1970 Apr 1;131(4):843–850. doi: 10.1084/jem.131.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Perry D., Dales S. In vivo and in vitro models of demyelinating diseases. III. JHM virus infection of rats. Arch Neurol. 1980 Aug;37(8):478–484. doi: 10.1001/archneur.1980.00500570026003. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Frelinger J. A., Weiner L. P. Resistance to fatal central nervous system disease by mouse hepatitis virus, strain JHM. II. Adherent cell-mediated protection. J Immunol. 1980 Apr;124(4):1733–1739. [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Asymptomatic infection of mouse hepatitis virus in the rat. Brief report. Arch Virol. 1979;59(3):275–279. doi: 10.1007/BF01317424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Factors involved in the age-dependent resistance of mice infected with low-virulence mouse hepatitis virus. Arch Virol. 1979;62(4):333–340. doi: 10.1007/BF01318107. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Resistance to highly virulent mouse hepatitis virus acquired by mice after low-virulence infection: enhanced antiviral activity of macrophages. Infect Immun. 1980 Jul;29(1):42–49. doi: 10.1128/iai.29.1.42-49.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Kai C., Sakaguchi A., Ishida T., Fujiwara K. The role of macrophages in the early resistance to mouse hepatitus virus infection in nude mice. Microbiol Immunol. 1979;23(10):965–974. doi: 10.1111/j.1348-0421.1979.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Héry C., Dupuy J. M. Neonatal susceptibility to MHV3 infection in mice. II. Role of natural effector marrow cells in transfer of resistance. J Immunol. 1980 Jan;124(1):418–423. [PubMed] [Google Scholar]
- Taylor C. E., Weiser W. Y., Bang F. B. In vitro macrophage manifestation of cortisone-induced decrease in resistance to mouse hepatitis virus. J Exp Med. 1981 Mar 1;153(3):732–737. doi: 10.1084/jem.153.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch Virol. 1976;50(4):279–285. doi: 10.1007/BF01317953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Dayan A. D., Allison A. C. Neuropathological effects of persistent infection of mice by mouse hepatitis virus. Infect Immun. 1975 Nov;12(5):1127–1140. doi: 10.1128/iai.12.5.1127-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Gresser I. Role of interferon in the pathogenesis of viral diseases of mice as demonstrated by the use of anti-interferon serum. V. Protective role in mouse hepatitis virus type 3 infection of susceptible and resistant strains of mice. J Immunol. 1978 May;120(5):1616–1619. [PubMed] [Google Scholar]
- Virelizier J. L. Role of macrophages and interferon in natural resistance to mouse hepatitis virus infection. Curr Top Microbiol Immunol. 1981;92:53–64. doi: 10.1007/978-3-642-68069-4_4. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Virelizier A. M., Allison A. C. The role of circulating interferon in the modifications of immune responsiveness by mouse hepatitis virus (MHV-3). J Immunol. 1976 Sep;117(3):748–753. [PubMed] [Google Scholar]
- Warner N. L., Woodruff M. F., Burton R. C. Inhibition of the growth of lymphoid tumours in syngeneic athymic (nude) mice. Int J Cancer. 1977 Jul 15;20(1):146–155. doi: 10.1002/ijc.2910200122. [DOI] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]
- Weiser W., Bang F. B. Macrophages genetically resistant to mouse hepatitis virus converted in vitro to susceptible macrophages. J Exp Med. 1976 Mar 1;143(3):690–695. doi: 10.1084/jem.143.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg D. O., Shah K. V., Bang F. B. Effect of cyclophosphamide on the genetic resistance of C 3 H mice to mouse hepatitis virus. Proc Soc Exp Biol Med. 1973 Mar;142(3):762–766. doi: 10.3181/00379727-142-37111. [DOI] [PubMed] [Google Scholar]