Abstract
Background/Purpose
To retrospectively analyze the clinical presentation, histology, treatment and outcomes of children with vaginal tumors who were treated at a single institution.
Methods
A retrospective review was conducted of medical records and pathologic materials of all children with vaginal tumors treated at St Jude Children’s Research Hospital between 1970 and 2009.
Results
Eighteen patients (median age 3.7 years, range 0.1–15) were identified. Three different histologies were found: rhabdomyosarcoma (RMS) (N=13), germ cell tumor (GCT) (N=3) and clear cell adenocarcinoma (CCA) (N=2). Bleeding or blood-tinged discharge was the most common clinical presentation (66%) followed by a protruding mass (39%). Vaginal and uterine salvage was 44.4% (8 of 18 patients). Thirteen patients (72.2%) remain disease-free with a median follow-up of 23.2 years (range, 2–39). Four patients (22.2%) died of disease progression (1 RMS, 2 GCT and 1 CCA) and 1 RMS patient died of colon cancer 12 years after the primary diagnosis had been made.
Conclusions
Vaginal tumors are extremely rare in the pediatric population. Early recognition of symptoms like bleeding and a protuding vaginal mass may prevent morbidity and mortality. Our findings confirm the good prognosis of vaginal RMS.
Keywords: vaginal tumors, rhabdomyosarcoma, germ cell tumor, adenocarcinoma
Introduction
Gynecologic tumors in children are rare and represent less than 5% of all pediatric neoplasms (1). Vaginal tumors may have a variable clinical presentation, including abdominal pain, abdominal mass, chronic genital ulcer, bloody discharge or tissue protruding from the vagina (Figure 1) (1,2). Rhabdomyosarcoma (RMS) is the most common malignant neoplasm of the vagina followed by germ cell tumor (GCT) and clear cell adenocarcinoma (CCA) (2). The management of pediatric vaginal tumors has evolved from radical surgery in the 1970s and early 1980s to neoadjuvant chemotherapy followed by local control with surgery or radiotherapy (3) in the last decades. We reviewed our institutional experience over a 39-year period to more completely characterize the clinical presentation, treatment, and outcome of vaginal tumors in pediatric patients.
Figure 1.
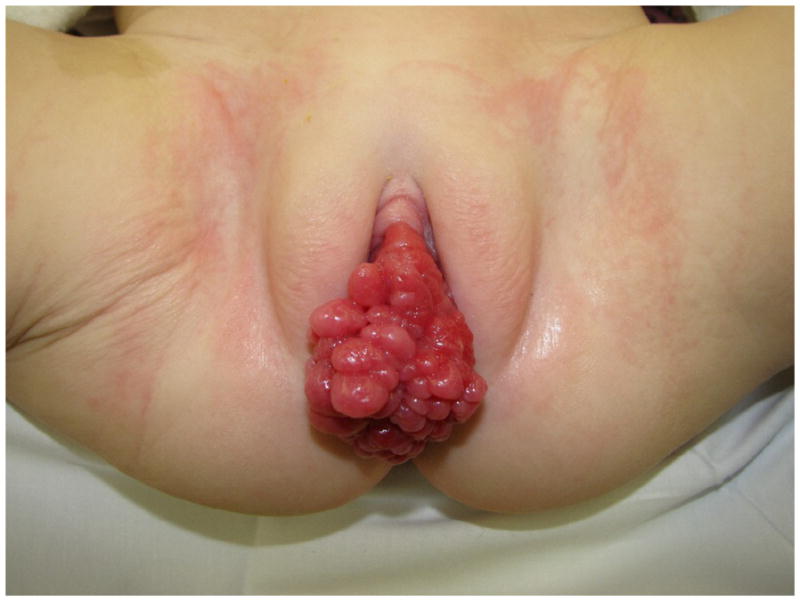
Clinical presentation with a mass protruding from the vagina
Material and methods
A review of the St. Jude Solid Tumor database identified 18 patients diagnosed with vaginal tumors among the 4485 patients under 21 years of age evaluated for malignant solid tumors (excluding brain tumors) between 1970 and 2009. The following information was collected retrospectively: patient age at diagnosis, symptoms, stage of disease, histological findings, therapy, clinical course, and outcome. This study was approved by the St. Jude Institutional Review Board.
Rhabdomyosarcoma was classified according to the international classification as botryoid, embryonal or alveolar (4) and was staged according to the tumor, lymph node, metastasis (TNM) system of the International Union Against Cancer (UICC) and the Intergroup Rhabdomyosarcoma Study (IRS) clinical grouping system (4,5), which assigns group I for localized tumor that is completely removed with pathologically clear margins and no regional lymph node involvement, group II for localized tumor that is grossly removed with microscopic disease at the margin, involved and grossly removed regional lymph nodes or both, group III for localized tumor with gross residual disease after incomplete removal or biopsy only and group IV for metastatic disease.
Results
Table 1 lists the clinical characteristics, treatment and outcomes of the 18 patients.
Table 1.
Clinical Characteristics, Treatment, and Outcome of 18 Pediatric Patients With Vaginal Tumors
| Patient | Age at diagnosis (years) | Clinical symptoms | Histology | IRS group | Initial therapy | Surgery | Postsurgery therapy | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 | Vaginal bleeding | B-RMS | I | V,D,C | APE | V,D,C | Died of colon cancer 12 yrs |
| 2 | 0.5 | Vaginal bleeding | B-RMS | II | V,A,C,E | APE | V,C,D,I,E EBRT 46 Gy | NED 8 yrs |
| 3 | 0.7 | Vaginal mass | B-RMS | III | V,A,C | _ | V,A,C | NED 4 yrs |
| 4 | 2.8 | Bloody discharge | B-RMS | II | V,A | _ | V,A | NED 3 yrs |
| 5 | 0.1 | Vaginal bleeding | B-RMS | IV | V,A,C,I,E EBRT 12.8 Gy | H/V | V,A,C,I,E EBRT 27.1 Gy | NED 39 yrs |
| 6 | 11.5 | Vaginal mass | B-RMS | I | V,D,C | H/V | V,D,C | NED 37 yrs |
| 7 | 0.5 | Vaginal bleeding | B-RMS | I | V,A,C EBRT 25 Gy | H/V | V,A,C | NED 34 yrs |
| 8 | 12.5 | Vaginal mass | E-RMS | II | V,D,C | H/V | V,D,C EBRT 39.6 Gy | NED 28 yrs |
| 9 | 4.2 | Vaginal mass | B-RMS | II | H/V | _ | V,A,C EBRT 47.1 Gy | DOD 0.7 yrs |
| 10 | 0.6 | Vaginal bleeding | E-RMS | II | V,D,C | H/V | V,D,C | NED 27 yrs |
| 11 | 1.5 | Vaginal bleeding | B-RMS | II | V,A,C,D | _ | V,A,C,D BT 54Gy | NED 24 yrs |
| 12 | 1.7 | Bloody discharge | E-RMS | II | V,A,C,D | _ | V,A,C,D BT 52.4 Gy | NED 24 yrs |
| 13 | 2.5 | Vaginal mass | B-RMS | II | Mass resection | _ | V,A | NED 8 yrs |
| 14 | 0.6 | Vaginal bleeding | GCT-EST | _ | CB,E,B | _ | CB,E,B | NED 2 yrs |
| 15 | 1 | Bloody discharge | GCT-EC | _ | V,A,C | Mass resection | V,A,C EBRT 44 Gy | DOD 3 yrs |
| 16 | 1.2 | Vaginal mass | GCT-EST | _ | _ | H/V | E,I EBRT 49Gy | DOD 2.8 yrs |
| 17 | 13 | Vaginal bleeding | CCA | _ | Partial vaginectomy | _ | CP, PT,CB EBRT 54 Gy | NED 10 yrs |
| 18 | 7.4 | Bloody discharge | CCA | _ | V,D,C | H/V | V,D,C EBRT 50Gy | DOD 3.5 yrs |
B-RMS: botryoid rhabdomyosarcoma, E-RMS: embryonal rhabdomyosarcoma, GCT-EST: germ cell tumor-endodermal sinus tumor, GCT-EC: germ cell tumor-embryonal carcinoma, CCA: clear cell adenocarcinoma, IRS: International Rhabdomyosarcoma Study, V: vincristine, A: actinomycin, C: cyclophosphamide, D: doxorubicin, I: ifosfamide, E: etoposide, CB: carboplatin, CP: cisplatin, PT: paclitaxel, B: bleomycin, APE: anterior pelvic exenteration, H/V: hysterectomy and vaginectomy, EBRT: extermal beam radiotherapy, BT: brachytherapy, NED: no evidence of disease, DOD: died of disease.
Rhabdomyosarcoma
Thirteen of the 18 children with malignant vaginal tumors had RMS, 10 botryoid subtype and 3 embryonal subtype. They presented at a median age of 3.7 years (range 0.16–15). Bleeding or blood-tinged discharge from the vagina was the most common clinical presentation (61%), followed by a protruding mass (39%). According to the IRS postsurgical grouping system there were three patients in group I, eight patients in group II, one patient in group III and one patient in group IV. As initial therapy, eleven of thirteen patients received chemotherapy (Table 1). The most common chemotherapy agents used were vincristine, dactinomycin, cyclophosphamide, doxorubicin and more recently ifosfamide and etoposide. The surgical approach was variable. Six patients underwent hysterectomy and vaginectomy (1 at initial diagnosis, 5 later during therapy), 2 underwent anterior pelvic exenteration, and 5 had only a biopsy. Five patients received external beam radiation therapy (EBRT) with a median dose of 39.5 Gy (range 12.8–47.1) and two patients received 54 and 52.4 Gy of brachytherapy. Only one patient experienced local tumor recurrence. This patient had undergone conservative surgery for tumor excision and received no adjuvant therapy. She was successfully salvaged with chemotherapy and hysterectomy/vaginectomy. Eleven patients of thirteen (84%) remain disease-free with a median follow-up of 21.4 years (range, 3–37). Two patients died, one of them who underwent anterior pelvic exenteration and ileal conduit reconstruction without radiation therapy succumbed to colon cancer 12 years after vaginal RMS diagnosis. The other patient did not receive planned postsurgical chemotherapy due to parental refusal and died of progressive metastatic disease. The remaining 11 patients have no evidence of disease. Two patients were cured without local treatment except for initial biopsy Uterine salvage was 30% (4 of 13 patients), but most of the patients undergoing hysterectomy were treated in the 1970s and early 1980s. Only 2 of the 6 patients treated in the last 25 years have required extensive surgical resection, either during treatment or for long-term complications.
Surgical complications included recto-vaginal fistula, vesico-vaginal fistula and urinary incontinence in one patient who required hysterectomy, vaginectomy and cystectomy 15 years after diagnosis.
Germ Cell Tumor
Germ cell tumor was found in 3 patients, endodermal sinus tumor subtype in two cases and embryonal carcinoma in one case. They presented at a median age of 0.9 years (range 0.6–1.2). Bleeding or blood-tinged discharge from the vagina was seen in two patients and a protruding mass in one patient. Serum alpha-fetoprotein (AFP) was markedly elevated in all these patients at diagnosis. Two patients received neoadjuvant chemotherapy (carboplatin/etoposide/bleomycin and VAC regimen, respectively) and the other patient had primary hysterectomy and vaginectomy. Two patients had postoperative external radiotherapy with doses ranging from 44 to 49 Gy. Two of the 3 patients developed local recurrences in rectum and vagina, 8 and 26 months after diagnosis, respectively, and both died of disease progression. The only survivor received carboplatin/etoposide/bleomycin and did not require surgical resection. A postchemotherapy biopsy was negative for tumor. She is alive 2 years after diagnosis without complications.
Clear Cell Adenocarcinoma
Clear cell adenocarcinoma was found in 2 of 18 patients. They presented at a median age of 10.2 years (range 7.4–13). Bleeding from the vagina was observed in both patients. One patient underwent a marginal resection via partial vaginectomy and received adjuvant cisplatin/paclitaxel chemotherapy and 54 Gy brachytherapy. She is alive 11 years after diagnosis. The other patient had neoadjuvant chemotherapy (vincristine, actinomycin-D, cyclophosphamide) to which a partial response was achieved, followed by hysterectomy and vaginectomy. Microscopic residual disease was present after surgery and she received additional postoperative chemotherapy with the same preoperative regimen and 50 Gy brachytherapy. She presented with vaginal and rectal recurrence 24 months after diagnosis and died of rectal bleeding from the tumor. Neither of these patients had documented prenatal exposure to diethylstilbestrol (DES).
Discussion
Vaginal tumors are uncommon in adults, comprising 1% to 2% of gynecologic malignancies and mainly represented by squamous cell carcinoma and adenocarcinoma, two distinct diseases with a different pathogenesis and natural history (1,2). The 18 pediatric patients with vaginal tumors treated at St. Jude Children’s Research Hospital over a 39-year period illustrate the rarity of this malignancy in childhood. In our patient cohort, the most common neoplasm of the vagina was rhabdomyosarcoma followed by germ cell tumor and clear cell adenocarcinoma. Bleeding or blood-tinged discharge was the most common clinical presentation; a protruding vaginal mass was the second most common presentation.
Rhabdomyosarcoma is the most common malignancy of the pediatric female genital tract and it generally presents in the first few years of life, with vaginal bleeding, discharge, and/or a vaginal mass (1–3). RMS is a small, round, blue-cell neoplasm with malignant skeletal muscle differentiation (4). The botryoid subtype, which is frequently seen in vaginal primary tumors, has a typical “grape-like” appearance due to a layer of spindle cells pushing up beneath the mucosa in polypoid masses (5).
Pelvic exenteration was the accepted surgical approach for vaginal RMS before 1972 (6,12). Since then, the management has progressed from radical surgery to neoadjuvant chemotherapy followed by surgery or radiotherapy after the IRS Group began to enter patients on prospective clinical trials (7,8). Hays et al. (9) reported 22 cases of nonmetastatic vaginal RMS treated on the IRS I and II protocols between 1972 and 1984. This included six patients who underwent biopsy only without extensive surgical resection. The response rate to chemotherapy was greater than 90% and when combined with a subsequent excision the results relative to survival were equally good. Andrassy et al. (10) reviewed 25 years of publications in vulvovaginal RMS and confirmed the role of organ-preserving approach. The rate of radical surgery decreased from 100% in 1972–78 to 13% in 1988–96. The prognosis was excellent; 25 of 27 girls with vulvovaginal tumors survived >5 years, with an 88% preservation rate for the uterus. All patients were treated with chemotherapy and 11 received additional radiotherapy. Recently, Arndt et al. (3) evaluated the factors affecting outcome in 151 patients with RMS of the female genital tract treated on IRS protocols I–IV. The rate of hysterectomy decreased from 48% in IRS-I/II to 22% in IRS-III/IV and the overall 5-year survival was 82%. Analysis of prognostic factors revealed that an age of 1–9 years at the time of diagnosis, noninvasive tumors, and the use of IRS-II or IRS-IV treatments (based on VAC regimen, a conservative surgical approach, and the use of radiation therapy for selected patients) were associated significantly with better outcome. Martelli et al. (11) reported the results of a conservative multimodal approach in 38 girls with nonmetastatic RMS of the genital tract (vulva, vagina, uterus), treated between 1984 and 1994 in the International Society of Pediatric Oncology (SIOP) protocol. Twenty-seven patients presented with a vulvovaginal RMS and 25 of them survived with a uterine salvage rate of 88%.
Our findings confirm the excellent prognosis of RMS arising in the vagina and emphasize the efficacy of a conservative approach to local therapy that leads to a high rate of cure. In the present study, 11 of 13 girls with vaginal RMS survived without evidence of disease at follow up of 3–39 years.
Although the overall survival of patients with vaginal RMS has improved, little is known about the late effects of treatment in long-term survivors. Spunt et al. (24) reviewed the spectrum and severity of late effects in female survivors of pelvic RMS treated at our institution and found significant late effects that reduced the quality of life and the functional capacity. These included vaginal stenosis, fistulas, gastrointestinal strictures, and bladder dysfunction.
From review of our patients, we conclude that biopsy and primary chemotherapy constitute the current initial treatment of choice for vaginal RMS, reserving surgical resection for truly persistent or recurrent disease. Radiotherapy, which is associated with significant long-term sequelae, should be avoided whenever possible.
Malignant GCT represent less than 3% of all pediatric malignancies, with endodermal sinus tumor being the most commonly observed histological subtype (13–18). The vagina is an extremely rare primary location, ranging from 3% to 8% of GCT. Clinical presentation is usually vaginal bleeding in girls under the age of 3 years. Our patient series supports this observation since all of our patients were under 2 years of age. Alpha-fetoprotein (AFP) is considered a reliable marker for diagnosis, evaluation of treatment response and remission status (14). Before 1965, surgery and/or irradiation were the usual treatment. Copeland et al. (15) reported 6 cases with a total of 4 survivors, all with tumor resection at diagnosis and VAC chemotherapy. Only one with a small tumor had preservation of the vagina. Hwang et al. (16) reported 2 children with small tumors treated with excision at diagnosis with organ preservation and VAC. The introduction of cisplatin-based chemotherapy in the 1980s has significantly improved the survival in children with malignant GCT at all anatomic sites (17). Platinum and bleomycin containing chemotherapy regimens replaced the historical VAC regimen due to the excellent response rate and tolerable toxicity (18). Both of our GCT patients who died were treated before the development of cisplatin-based chemotherapy. Current survival rate at 2 years for vaginal malignant GCT has been reported to be 70% (18). Initial biopsy followed by cisplatin-based chemotherapy and organ preservation in patients showing a complete tumor response including AFP normalization is the current approach for vaginal malignant GCT.
Clear cell adenocarcinoma is very rare before adolescence, and often has a very aggressive pattern in young children (19–23). Larger masses generally present as polypoidal and nodular or sometimes as flat and ulcerated tumors. Histology is characterized by solid sheets of clear cells due to dissolution of glycogen. The most common symptom of pediatric patients at presentation is vaginal bleeding. The development of this tumor in the vagina of young females has been historically linked to prenatal exposure to diethylstilbestrol. The age of the DES-exposed patients has varied from 7 to 34 years. Disease characteristics in young girls seem to be similar to older patients, except that young patients had a slightly higher incidence of advanced-stage disease. Surgical resection and lymph node dissection have an important role in the treatment of CCA (19). McNall et al. (20) reported one case and reviewed 37 published cases of vaginal and cervical mesonephric adenocarcinoma or CCA in patients under the age of 18 years. Overall survival was higher for patients who underwent complete tumor resection. The role of neoadjuvant chemotherapy in the treatment of vaginal CCA is unclear and combinations of platinum compounds and paclitaxel have been used (21,22). Tumor excision rather than radical hysterectomy or exenteration is preferred when feasible. Pelvic and para-aortic lymph node dissections are recommended even in patients with early stage disease (20). The 2-year event-free survival estimate is 90% for patients with Stage I disease, 71% for patients with Stage II disease, and 29% for those with Stage III/IV disease (20,22). Radiotherapy in children with vaginal CCA should be reserved for the treatment of microscopic residual disease or lymphatic metastasis and bilateral oophoropexy is recommended if postoperative EBRT is considered (20).
Conclusion
Our small group of patients with vaginal tumors illustrates the rarity of malignancies at this anatomic site in childhood. Abnormal vaginal bleeding in girls should be promptly investigated through the use of pelvic examination, appropriate imaging and tumor markers evaluation (AFP, HCG). Over time, the management of pediatric vaginal tumors has progressed from radical surgery in the 1970s and early 1980s to biopsy followed by neoadjuvant chemotherapy, particularly with the VAC regimen for RMS and cisplatin-based chemotherapy for malignant GCT, in the last decades. A higher incidence of advanced-stage disease is noticed in younger patients with CCA, in which surgical resection and lymph node dissection have an important role.
Our retrospective study has several limitations that should be considered in the interpretation of our findings, including the small number of patients in the study cohort and the referral bias inherent in our status as a tertiary referral center. Prospective studies are needed to define the best systemic therapies for these tumors and to determine when radical surgery and/or radiation therapy are warranted. Further studies are also needed to define the relationship between treatment exposures and long-term outcomes, including fertility and sexual function. There is a great need for interventions to support survivors who may have long-term anatomic and functional abnormalities.
Footnotes
Conflict of interest: NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hassan E, Creatsas G, Michalas S. Genital tumors during childhood and adolescence: a clinical and pathological study of 71 cases. Clin Exp Obstet Gynecol. 1999;26:20–21. [PubMed] [Google Scholar]
- 2.Hellman K, Silfverswa C, Nilsson B, et al. Primary carcinoma of the vagina: factors influencing the age at diagnosis. The Radiumhemmet series 1956–96. Int J Gynecol Cancer. 2004;14:491–501. doi: 10.1111/j.1048-891x.2004.014310.x. [DOI] [PubMed] [Google Scholar]
- 3.Arndt CA, Donaldson SS, Anderson JR, et al. What constitutes optimal therapy for patients with rhabdomyosarcoma of the female genital tract? Cancer. 2001;91:2454–68. [PubMed] [Google Scholar]
- 4.Qualman SJ, Coffin CM, Newton WA, et al. Intergroup Rhabdomyosarcoma Study: update for pathologists. Pediatr Dev Pathol. 1998;1:550–61. doi: 10.1007/s100249900076. [DOI] [PubMed] [Google Scholar]
- 5.Newton WA, Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification-an Intergroup Rhabdomyosarcoma Study. Cancer. 1995;76:1073–8. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Atra A, Ward HC, Aiten K, et al. Conservative surgery in multimodal therapy for pelvic rhabdomyosarcoma in children. Br J Cancer. 1994;70:1004–1008. doi: 10.1038/bjc.1994.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer HM, Moon T, Donaldson M, et al. The Intergroup Rhabdomyosarcoma Study: A Preliminary Report. Cancer. 1977;40:2015–2026. doi: 10.1002/1097-0142(197711)40:5<2015::aid-cncr2820400505>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Crist W, Gamsey L, Beltangady M, et al. Prognosis in children with rhabdomyosarcoma: A report of the INS Studies I and II. J Clin Oncol. 1990;8:443–452. doi: 10.1200/JCO.1990.8.3.443. [DOI] [PubMed] [Google Scholar]
- 9.Hays DM, Shimada H, Raney RB, et al. Sarcomas of the vagina and uterus: The Intergroup Rhabdomyosarcoma Study. J Pediatr Surg. 1985;20:718–724. doi: 10.1016/s0022-3468(85)80032-4. [DOI] [PubMed] [Google Scholar]
- 10.Andrassy RJ, Hays DM, Raney RB, et al. Conservative surgical management of vaginal and vulvar pediatric rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study III. J Pediatr Surg. 1995;30:1034–1037. doi: 10.1016/0022-3468(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 11.Martelli H, Oberlin O, Rey A, et al. Conservative treatment for girls with nonmetastatic rhabdomyosarcoma of the genital tract: A report from the Study Committee of the International Society of Pediatric Oncology. J Clin Oncol. 1999;17:2117–22. doi: 10.1200/JCO.1999.17.7.2117. [DOI] [PubMed] [Google Scholar]
- 12.Crist W, Gehan EA, Ragab A, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 13.Rescorla FJ. Germ cell tumors. Semin Pediatr Surg. 1997;6:29–37. [PubMed] [Google Scholar]
- 14.Davidoff AM, Hebra A, Bunin N, et al. Endodermal sinus tumor in children. J Pediatr Surg. 1996;31:1075–1078. doi: 10.1016/s0022-3468(96)90090-1. [DOI] [PubMed] [Google Scholar]
- 15.Copeland IJ, Sneige N, Ordonez NG. Endodermal sinus tumor of the vagina and cervix. Cancer. 1985;55:2558–2565. doi: 10.1002/1097-0142(19850601)55:11<2558::aid-cncr2820551107>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Hwang EH, Han SJ, Lee MK. Clinical experience with conservative surgery for vaginal endodermal sinus tumor. J Pediatr Surg. 1996;31:219–222. doi: 10.1016/s0022-3468(96)90000-7. [DOI] [PubMed] [Google Scholar]
- 17.Ablin AR, Krailo MD, Ramsay NK, et al. Results of treatment of malignant germ cell tumors in 93 children: A report from the Children’s Cancer Study Group. J Clin Oncol. 1991;9:1782–1792. doi: 10.1200/JCO.1991.9.10.1782. [DOI] [PubMed] [Google Scholar]
- 18.Rescorla F, Billmire Deborah, Vinocur Charles, et al. The effect of neoadjuvant chemotherapy and surgery in children with malignant germ cell tumors of the genital region: a pediatric intergroup trial. J Pediatr Surg. 2003;38:910–912. doi: 10.1016/s0022-3468(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 19.Hicks ML, Piver MS. Conservative surgery plus adjuvant therapy for vulvovaginal rhabdomyosarcoma, diethylstilbestrol clear cell adenocarcinoma of the vagina, and unilateral germ cell tumors of the ovary. Obstet Gynecol Clin North Am. 1992;19:219–33. [PubMed] [Google Scholar]
- 20.McNall RY, Nowicki PD, Miller B, et al. Adenocarcinoma of the cervix and vagina in pediatric patients. Pediatr Blood Cancer. 2004;43:289–294. doi: 10.1002/pbc.20113. [DOI] [PubMed] [Google Scholar]
- 21.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 22.Duenas-Gonzalez A, Lopez-Graniel C, Gonzalez-Enciso A, et al. A phase II study of multimodality treatment for locally advanced cervical cancer: Neoadjuvant carboplatin and paclitaxel followed by radical hysterectomy and adjuvant cisplatin chemoradiation. Ann Oncol. 2003;14:1278–1284. doi: 10.1093/annonc/mdg333. [DOI] [PubMed] [Google Scholar]
- 23.Senekjian EK, Frey KW, Anderson D, et al. Local therapy in stage I clear cell adenocarcinoma of the vagina. Cancer. 1987;60:1319–1324. doi: 10.1002/1097-0142(19870915)60:6<1319::aid-cncr2820600626>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Spunt SL, Sweeney TA, Hudson MM, et al. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J Clin Oncol. 2005;23:7143. doi: 10.1200/JCO.2005.12.096. [DOI] [PubMed] [Google Scholar]


