Abstract
All cardiac magnetic resonance (CMR) techniques aim to create still depictions of a dynamic and ever-adapting organ. Most CMR methods rely on cardiac gating to capture information during fleeting periods of relative cardiac quiescence, at end diastole or end systole, or to acquire partial images throughout the cardiac cycle and average these signals over several heart beats. Since the inception of clinical CMR in the early 1980s, priority has been given to improving methods for image gating. The aim of this work is to provide a basic understanding of the ECG acquisition, demonstrate common ECG-related artifacts and to provide practical methods for overcoming these issues. Meticulous ECG preparation is essential for optimal CMR acquisition and these techniques must be adaptable to the individual patient.
Keywords: Magnetic resonance imaging, ECG, Trigger
Introduction
All cardiac magnetic resonance (CMR) techniques aim to create still depictions of a dynamic and ever-adapting organ. Most CMR methods rely on cardiac gating to capture information during fleeting periods of relative cardiac quiescence, at end diastole or end systole, or to acquire partial images throughout the cardiac cycle and average these signals over several heart beats. This process requires absolute precision. Since the inception of clinical CMR in the early 1980s, priority has been given to improving methods for image gating [1–5].
Cardiac gating has enabled the discrimination of internal cardiac anatomy, the evaluation of cardiac function and permitted the exploration and development of more rigorous quantitative CMR imaging techniques such as T2* and T1 measurements. Image contrast is controlled by basic parameters, like repetition time (TR), which must be individualized to the patient’s own cardiac cycle and gated to every heartbeat, necessitating a method of predicting the mechanical events of the cardiac cycle [2, 6–11].
The electrocardiogram (ECG) reflects changes in electrical potential generated by the conduction system of the heart which reliably precede mechanical contraction by milliseconds. Any phase of the cardiac cycle can be easily determined and the ECG signal can be used to trigger image acquisition. Adequate ECG synchronization is the crucial first step in acquiring high quality CMR images [1, 12, 13].
Standard ECG gating using four leads has been updated and most centers are now using vectorcardiogram (VCG)-based wireless, with three-dimensional summation vector acquisition, or fiber-optic systems with noise filtration to reduce gradient and radiofrequency pulse noise using CMR [6, 14]. To date, extensive work has been done in dealing with the numerous ECG-related artifacts [2, 6–11, 13]. The aim of this work is to provide a basic understanding of the ECG acquisition, demonstrate common ECG-related artifacts and to provide practical methods for overcoming these issues. Meticulous ECG preparation is essential for optimal CMR acquisition and these techniques must be adaptable to the individual patient.
Practical guidance to correct CMRI ECG gating
Preparing the exam
Skin preparation
Prior to the placement of ECG leads, skin preparation is essential. An accurate detection of the small potentials generated by the myocardium requires minimal skin impedance [2]. This aim is achieved through optimal electrode contact. Factors causing interference, adding noise to the ECG baseline and leading to erroneous triggering during the CMR exam, include naturally occurring oils and chest hair and products applied by the patient such as creams and ointments.
Patients should be instructed to avoid applying these products on the day of their exam. Immediately prior to electrode placement, chest hair must be shaved and the skin surface scrubbed with a mildly abrasive soap; several commercially prepared gels are available for this purpose. Ideally the skin should be bare and clean prior to electrode application. Workflow can be optimized by performing this step outside of the scanning room.
ECG placement
Standard vendor recommended electrode configurations are typically based on study reports of locations with the greatest overall success rates [7]. While these groupings are often adequate, during daily practice poor initial ECG tracings often prompt a search for alternative electrode placement. Since most systems allow the user to choose alternative lead combinations, the first step is to test each channel of incoming signal for the best ECG trace option. If none of the existing combinations are adequate, a physical alteration in electrode placement becomes necessary.
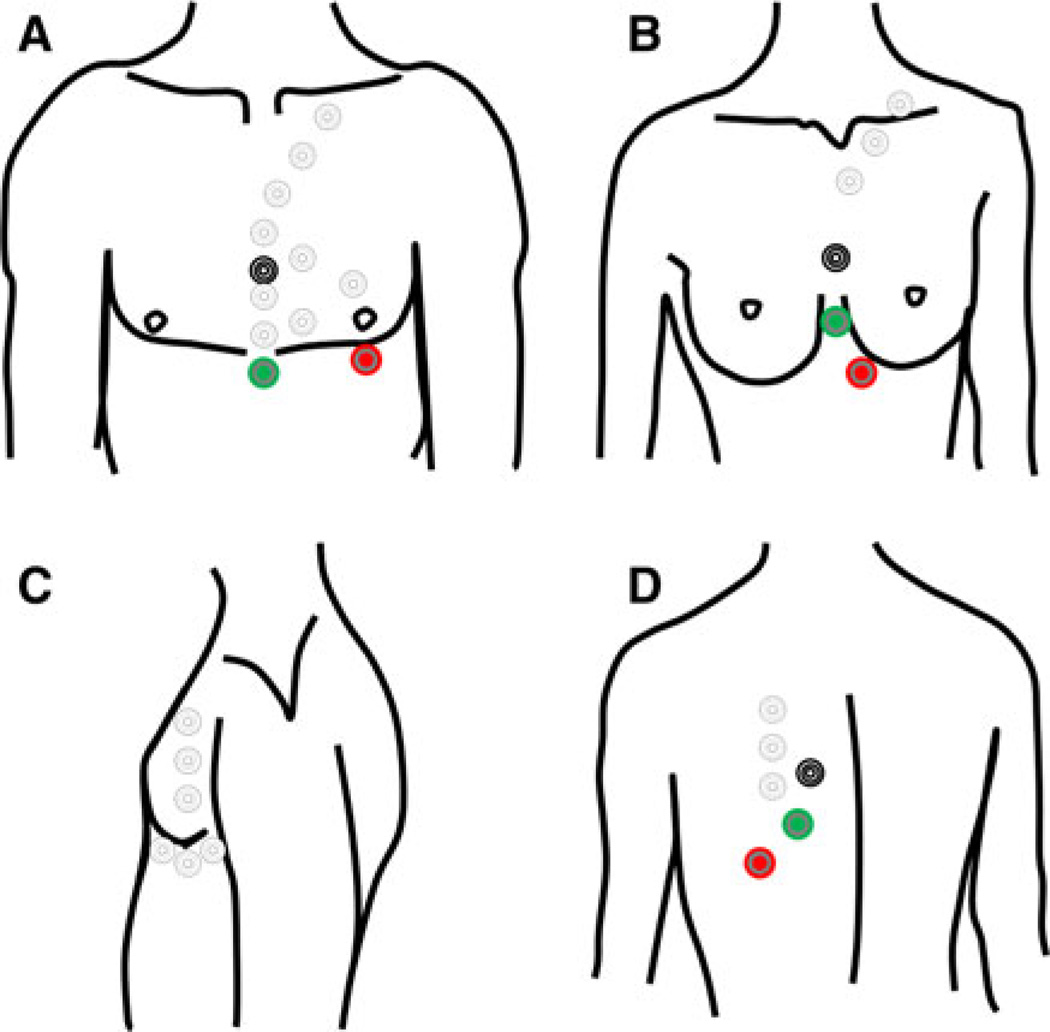
These positional changes may be subtle or may require a creative and skillful replacement of the entire electrode leads. Fatty tissue within the breast or body wall of obese patients can be especially challenging. Depending upon the patient’s body habitus it may be necessary to place the electrodes on the lateral chest wall, the back or even the neck (Fig. 1). Optimal electrode positioning is often found through trial and error.
Fig. 1.
Black, green and red leads denote the standard position of the conventional electrodes. Uncolored circles represent alternative placements which can range from the mid-sternal region to the neck, lateral chest wall and back. a Frontal view. Alignment of the leads along the parasternal midline often increases the R-wave amplitude. b Frontal view. In women, it is often best to avoid the fatty breast tissue, so lead placement can be adapted to breast size and position. c Lateral positions. d Posterior view
If repositioning of the electrodes is necessary it takes some time for the scanner to adapt to the new ECG signal. Therefore a short period of time has to elapse to evaluate if signal detection improved after repositioning.
Once the electrodes are connected to the ECG device, and a satisfactory tracing is confirmed, the connectors and cable should be aligned without any loops and minimal overlap. These cables, if poorly positioned, have the potential to add radiofrequency noise or, of even greater concern, generate heat that may cause burn injuries to the patient.
During the exam
Technical considerations
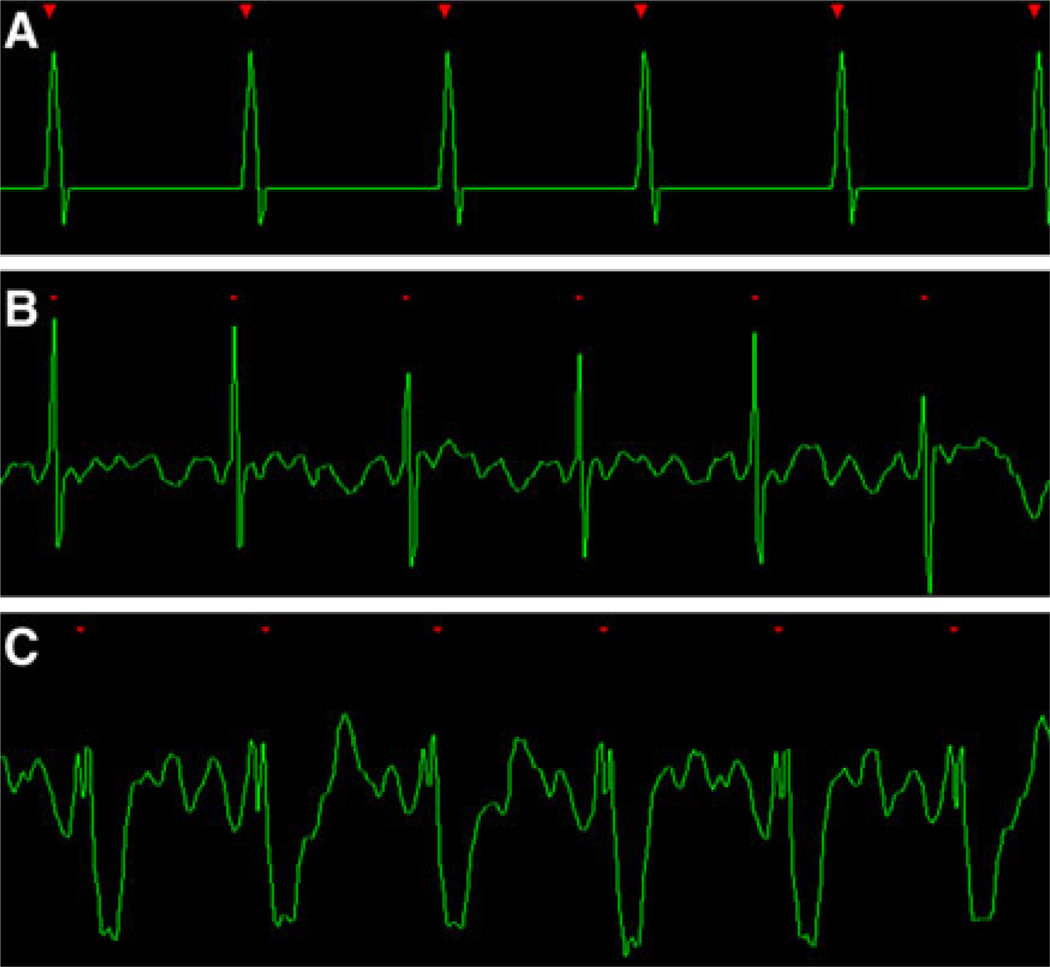
An ECG tracing demonstrates the electrical potential that is generated by the heart and conducted to the skin surface. Typically the largest ECG deflection is the R wave, which depicts an alternation in the electrical potential heralding the initiation of mechanical systole. CMR triggering relies on this electrical event to initiate scanning and is dependent on adequate coupling between the ECG device and the scanner console. The ideal ECG tracing would depict tall, evenly spaced R waves separated by a flat baseline (Fig. 2). In reality, these types of tracing are easily achieved with a simulator but usually not observed in vivo.
Fig. 2.
a An ideal ECG created by a simulation program. The R waves are tall and sensed every time, the R–R intervals are exactly evenly spaced and the intervening baseline is flat without any noise. b A excellent in vivo example. High peak amplitude R-waves are separated by minimal baseline noise. c The QRS complex is inverted; however, the high amplitude of the R-wave will serve as a reliable trigger for the acquisition
The goal is to achieve an in-bore ECG with clear R wave and flat or negative T waves. The construction of an in-bore ECG with flat or negative T waves can be easily achieved through a negative-positive direction in left caudal direction (e.g. changing lead II closer or far apart) and the third electrode on the upper left side of the chest, but this is not necessary the best positioning in all cases.
Prospective cardiac gating
In prospective ECG gating, the pulse sequence can be adapted to both morphological and functional acquisitions.
In prospectively gated morphological imaging, an R-wave initiates the capture of signal after a suitable delay period; signal acquisition coincides with diastasis, the most mechanically still portion of the cardiac cycle (Fig. 3a) which corresponds to the mid-diastolic phase of the ECG.
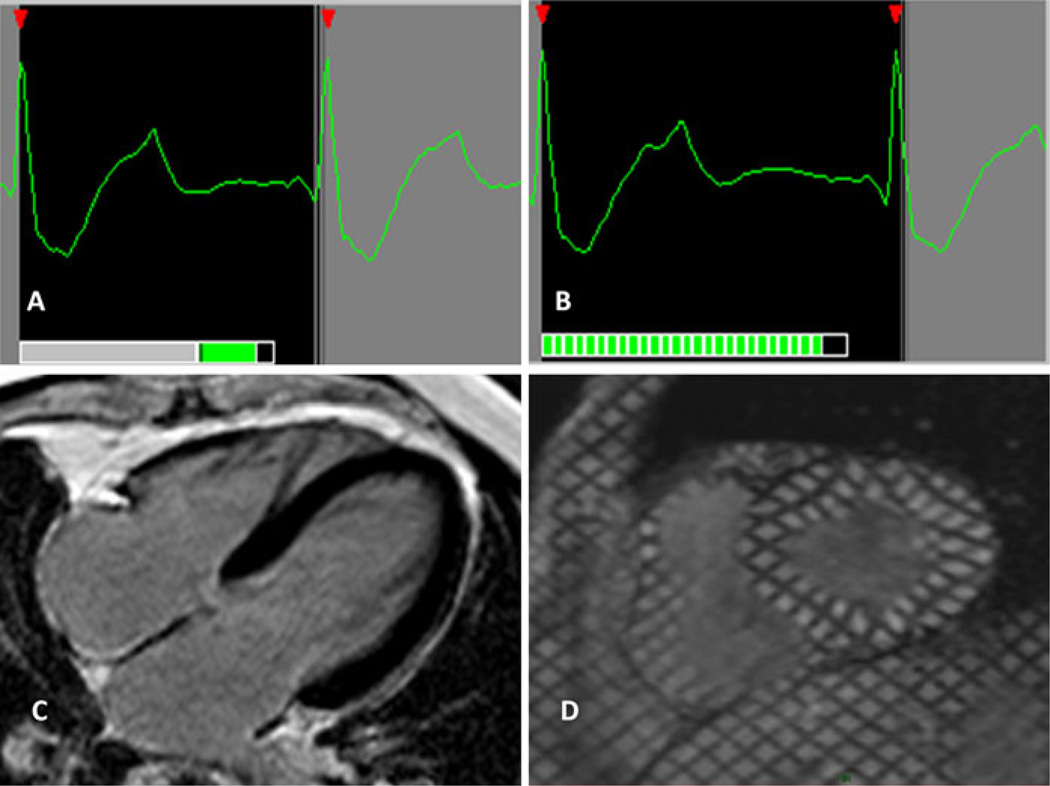
Fig. 3.
a Prospective gating for morphological imaging. The signal is acquired during the mid diastolic phase, between the T-wave (cardiac repolarization) and the a-wave (atrial contraction). b Prospective gating for functional imaging. A cine series is acquired beginning with the R-wave. The end of diastole is missed, since the acquisition has to be terminated in order not miss the next R-wave trigger. c Delayed Gadolinium Enhancement is an example of prospective gating. d Tagging cine-MR is an example of prospective cine gating
Prospective functional imaging relies on partial k-space acquisitions for a series of images spanning most of the cardiac cycle; k-space is ultimately filled by the averaging of signals from the same cardiac phase over several heart beats. This functional set begins with the R-wave and by necessity cannot acquire the end diastolic events. Since the duration of the R–R-interval slightly varies from beat to beat the final 10% of the R–R-interval is usually not sampled in order not to miss the next R-wave [15] (Fig. 3b).
Prospective gating has some advantages in filtering noise. An interval is often programmed when the scanner deliberately ignores any high peak amplitude pulses, preventing false triggering by ECG signals other than the R-wave. This robust ability to ignore the ECG trace is problematic in patients with arrhythmia. In patients with a premature beat, the early R-wave is missed because the gradient pulses triggered by the previous R-wave are still running; this process further prolongs the acquisition which lengthens the necessary breath hold period. Signal is inappropriately assigned to the wrong cardiac phase resulting in image blurring [16].
Heart rate variability
Prospective gating is vulnerable to R–R interval variations occurring not only over the whole exam but also within one breath hold. For example, the scanner software might measure the R–R interval for a short period after the ECG is attached, and use this measurement in the software planning a cine study. If the true R–R interval has lengthened since the measurement was taken, which occurs often when a nervous patient relaxes, the software may unnecessarily restrict the number of cine images and prolong the skipped diastolic period.
When planning the positioning of the acquisition window for prospectively gated morphological image heart rate variability during breath hold has to be taken into consideration. In order not to miss the next R-wave the position of the acquisition window should not be too close to the next R-wave. An acquisition window which is placed too close to the next R-wave may reach into the R-wave when the next R–R-interval is only slightly shorter (Fig. 4).
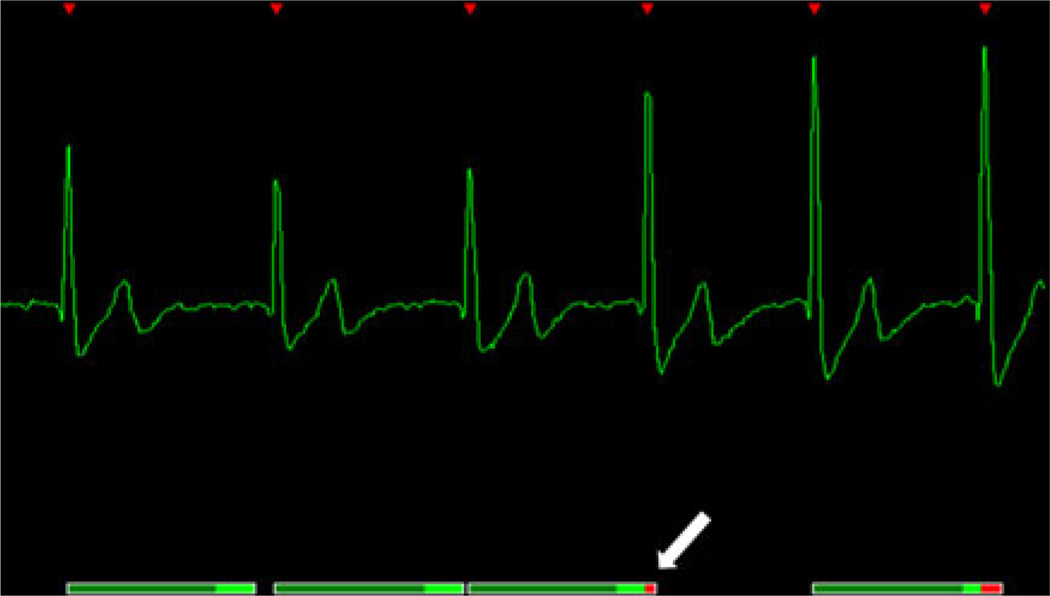
Fig. 4.
An acquisition window which is placed too close to the next R-wave may reach into the R-wave when the next R–R-interval is only slightly shorter (arrow). This problem particularly occurs during breath hold. Before acquiring the sequence it is necessary to move the acquisition window towards the mid-diastole, usually by shortening the TR time (ms)
Therefore knowledge of the patient’s heart rate variability with breath holding is crucial; depending upon the patient’s physical condition, dramatic changes can occur. Often a test breath hold is needed to capture an appropriate cycle length prior to image acquisition. Throughout the exam, the operator must be vigilant to variations in the cardiac cycle and adapt prospectively gated images appropriately.
Retrospective cardiac gating
Retrospective cardiac gating involves the continuously acquisition of data. Both image and ECG signal is collected over several consecutive heart beats and used during image reconstruction for the appropriate grouping of cardiac phase information. This demands dynamic pulse sequence and acquisition software decisions, because the method of data acquisition changes as it runs. Retrospective gating uses complex methods to filter signal interference [1, 16].
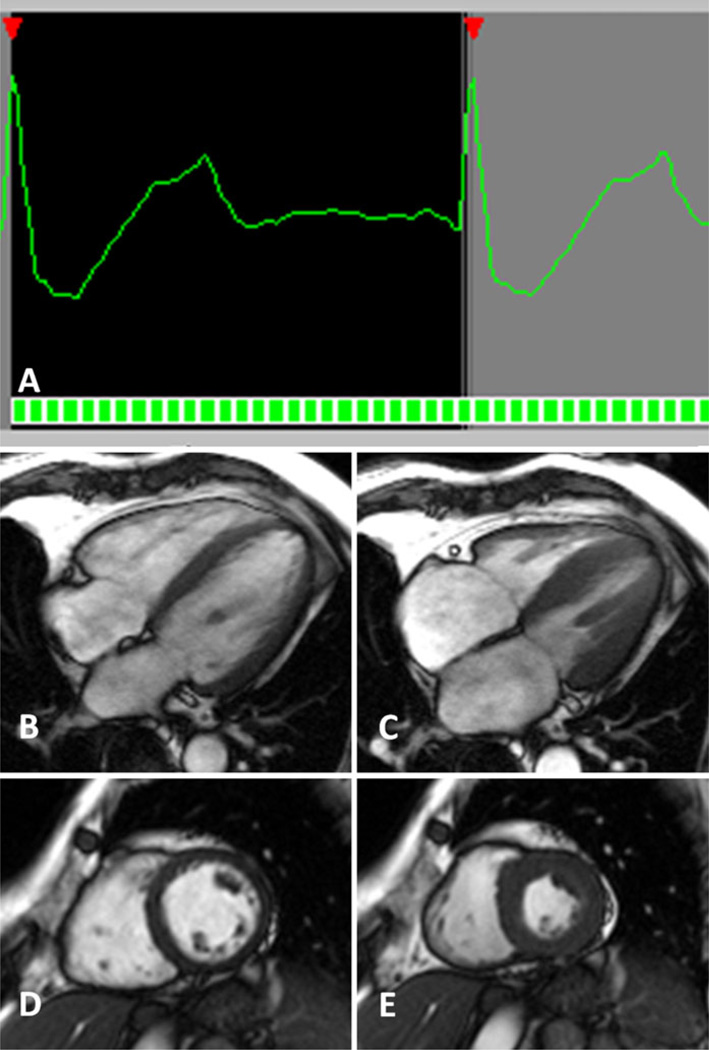
A major advantage of a retrospective acquisition is the ability to collect information from all cardiac phases (Fig. 5). Similar to prospective gating, retrospective methods may have difficulty with arrhythmias and low amplitude R-waves. With a variable heart rate, or arrhythmic event, the systolic and diastolic periods are unequally changed and the retrospective software is unable to compensate with appropriate segmentation of the cardiac phase data. Inappropriate trigger events will introduce additional errors in data segmentation.
Fig. 5.
a Retrospective gating for functional imaging. Complex post-processing methods are used to meld the imaging and ECG signals. The cine series contains all phases of the cardiac cycle. Four chamber cine-MR in end diastole (b) and end systole (c). Short axis cine-MR in end diastole (d) and end systole (e)
Cine-MR and its important parameters
A major advance on cine-MR is the ability to measure multiple K-space lines per cardiac phase, termed segmented cine acquisition. Three parameters need to be adjusted: temporal resolution (TR), acquisition time (TA) and the numbers of lines per cardiac phase (segments). The goal is to balance two competing requirements: TR and TA, because the desirable sequence should have the lowest TR as possible for a breath-hold time reasonable for the patient. Thus, if we increase the number of segments, the resulting TR increases and TA decrease. For patients with bradycardia, with longer R–R intervals, the TA may be too long for breath-holding. In that case, the number of segments may be increased to reduce overall acquisition time.
Artifacts
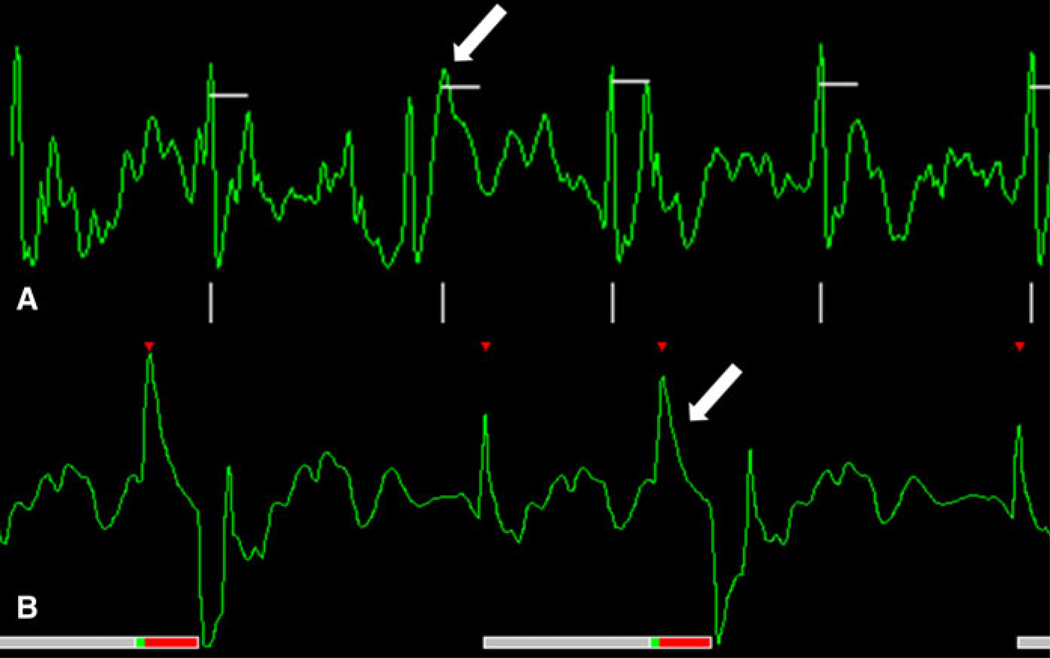
Artifacts can be caused by both patient- and system-related factors (Fig. 6). Intra-cardiac blood flow can induce a voltage and create hydrodynamic artifacts (Fig. 7) [7]. The magneto-hydrodynamic effect occurs because blood is an electric conductor fluid and its motion produces an electric current which adds on to the cardiac conduction signal. This generates an electrical signal that usually affects the T wave. As the T wave occurs at a time of rapid blood movement out of the heart, this interaction is responsible for the T wave distortion. VCG ECG usually assesses the 3 dimensional spatial orientation of electrical activity. VCG is superior to standard ECG for MR gating because cardiac electrical activity and the voltage generated by magnetohydrodynamic effects occur in different planes, and can therefore be distinguished from one another.
Fig. 6.
a Poor electrode placement. Poor electrode placement can result in low amplitude R-wave which is difficult to discern from the baseline noise. These tracings are often mistaken for arrhythmias. Before deciding that a patient’s scan must be cancelled, many electrode positions must be tried (see Fig. 4). b The same patient as in Fig. 5a with a better choice of electrode placement results in a normal ECG trace. c The result of the poor electrode placement on a short axis cine-MR. d The image of the same patient after correction of the ECG placement
Fig. 7.
a A normal tracing is seen in this patient outside the magnet bore. b Once the patient is positioned in the magnet‘s center without changing electrode position a hydrodynamic artifact occurs. c For the patient in Fig. 6a, b, electrode positions on the front and side were unsuccessful in eliminating the ECG artifacts. In this subject the ECG tracing was normalized within the magnet’s bore when the electrodes were place on the back
Unfortunately, cardiac gating is dependent on specific MR techniques to acquire MR signal. Even with VCG ECG, important limitations on the acquired data may be present. Single phase techniques (e.g. spin echo/gradient echo/inversion recovery sequences) or multiphase techniques (gradient echo (GRE) or steady state free precession (SSFP)) are the most common. Sequences may be changed if an ECG trace provides poor gating: for example, an SSFP sequence may be changed to GRE to improve gating quality.
Other patient issues, such as abnormal cardiac conduction or unrelated physiological activities, like muscle tremor, are additive to this baseline noise. Extrinsic events, like high amplitude system noise or small electric currents spuriously induced by the changing magnetic field gradients, are also of concern (Fig. 8). Optimal image quality necessitates electrode placement that maximizes the R-wave peak while minimizing these baseline artifacts. This can be accomplished by placing the leads of the right arm and left leg away from each other on the mid line of the chest; however it does not necessary overcome the problem because of the magneto-hidrodynamic effect.
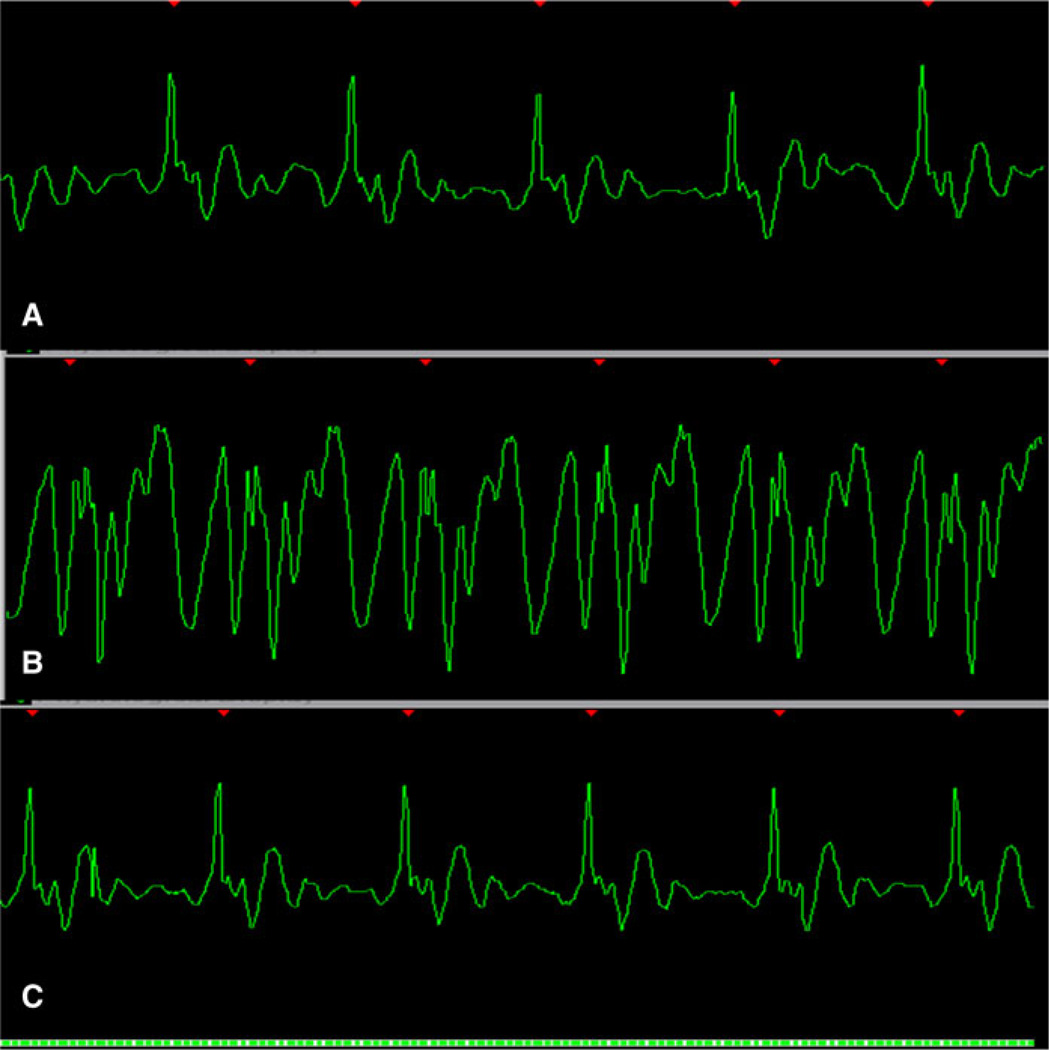
Fig. 8.
Depending on the intensity and how the sequence changes the magnet field with the radiofrequency pulse we can see that in the same patient the artifacts can occur. a Cine-MR retrospective gating without artifact. b Spectroscopy CMR prospective gating using two R–R intervals with a base line noise correlated to the pulse sequence (white arrow). If the amplitude of the R wave is not sufficient it can lead to a false triggering (not in this case)
Some scanners have the arrhythmia rejection capability and the acceptance window must be specified allowing some R–R variation. This usually increase TA and, as with everything else in MR, the decision on shorter scan time or better image quality has to be made and the number of segments need to be increased to reduce TA. However, extreme variations of the R–R interval should be overcome using prospective triggering instead of retrospective. Usually, the shorter R–R interval should be used for planning the trigger acquisition window, which needs to be slightly shorter than the shortest R–R interval. The resulting cine-MR will represent an incomplete cardiac cycle, however, calculation of ejection fraction will be possible.
These extraneous effects are often reduced by a closely spaced electrode configuration on the patient’s trunk [17]. To reduce the magnetic field distortion, electrodes and associated connectors and cables should be made from non-ferromagnetic material.
ECG displays are available at both at the patient couch and the operator’s console. Continuous monitoring of the waveform and triggering events is essential for the early detection of problems. Interference can falsely trigger the scanner and lose synchronization with the heart [11, 16] (Fig. 9). ECG triggering may be highly variable in patients with arrhythmia (Figs. 10, 11); both prospective and retrospective arrhythmia rejection has been incorporated into the acquisition and reconstruction software for many applications. However, using non-gated sequences enables to overcome all kind of trigger irregularities but for the trade off of a lower spatial resolution.
Fig. 9.
Two different cases. a A low amplitude R wave is skipped (white arrow) by the scanner when the sequence pulse is done adding noise to a T wave that is wrongly triggered. b A low amplitude R wave is skipped by the scanner as in figure (a), however now triggering on the A wave
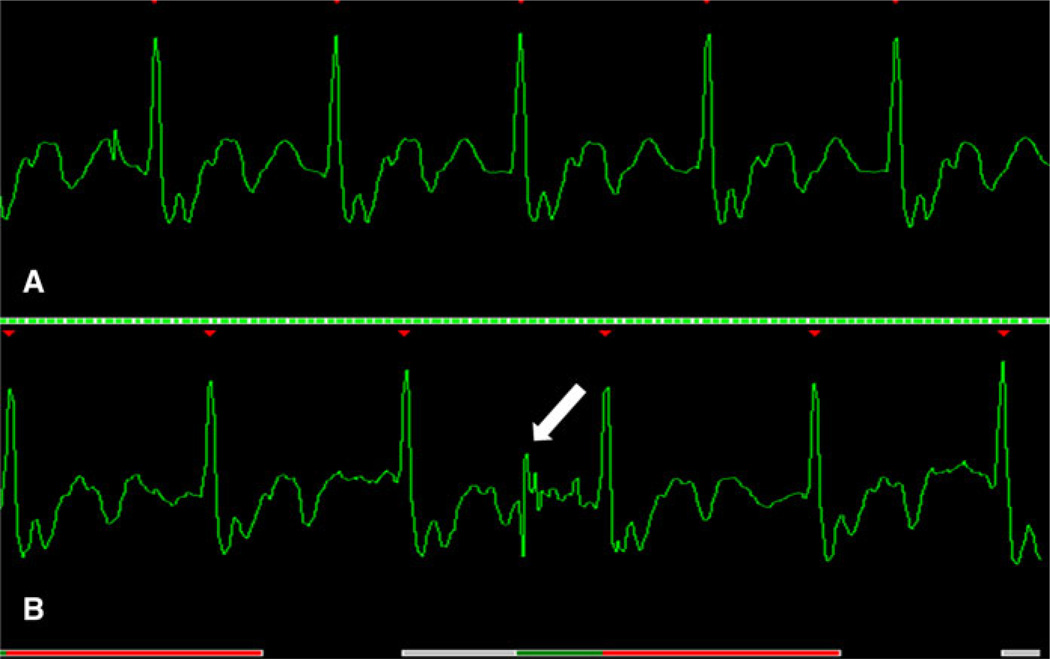
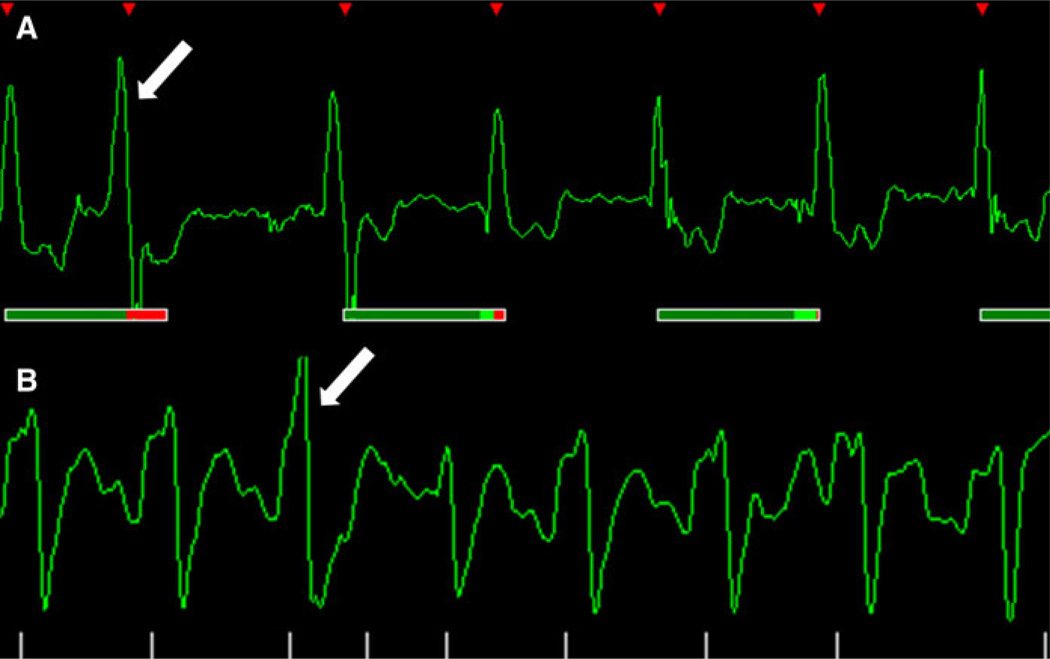
Fig. 10.
Extra heartbeat. a Observe the difference within the second (white arrow) and third QRS complex. b Premature ventricular contraction (white arrow) observed in the third QRS complex. When extra-beats occur the scanner does not trigger correctly and the image appears blurred depending on the frequency of this occurrence
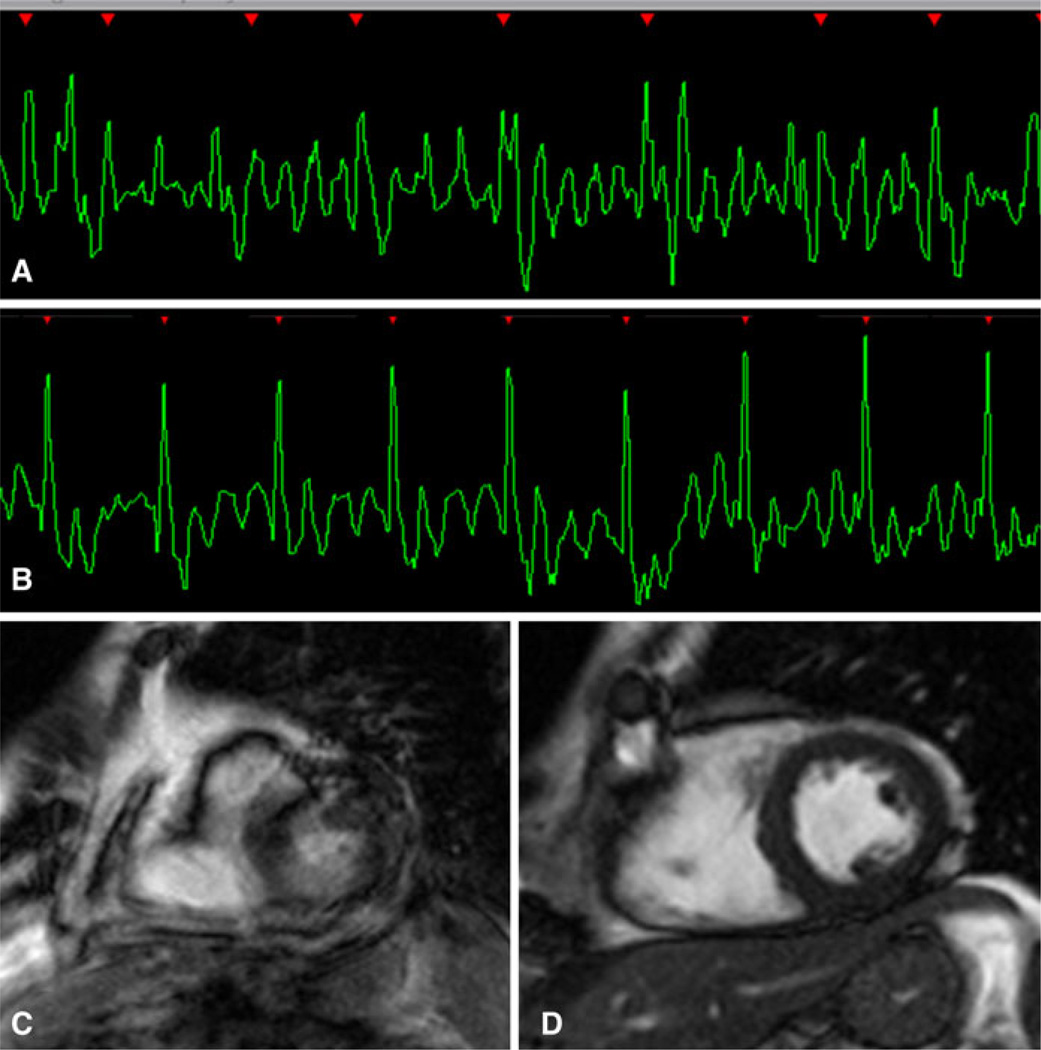
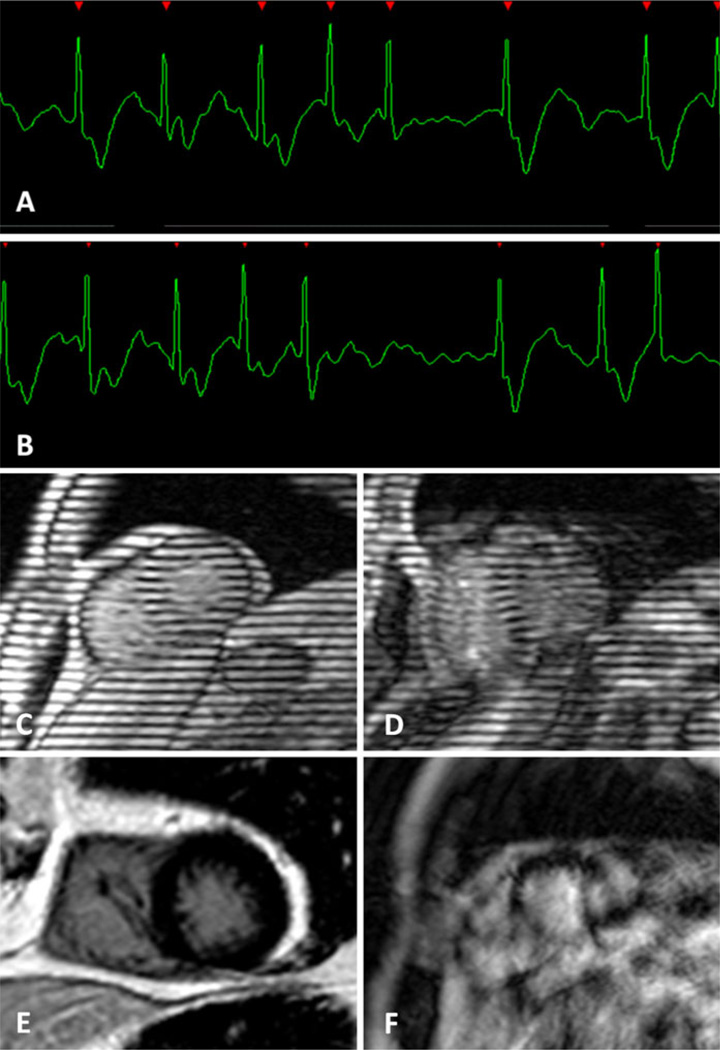
Fig. 11.
Atrial fibrillation. a, b Length of the R–R intervals vary to an extent that proper k-space sampling is hampered. Cine-MR tagging with a c normal ECG trace and on an d atrial fibrillation patient. Delayed enhancement with a e normal ECG trace and on an f atrial fibrillation patient
Future possibilities
Several approaches to “real-time” imaging have been developed [10, 13, 18–21]. These techniques aim to produce a high temporal and spatial resolution images independent of external cardiac gating. With further improvements to both hardware and software, fast “real-time” analysis may rival the capabilities of echocardiography.
Final considerations
CMR is a powerful tool in both qualitative and quantitative analysis. Its accuracy depends on meticulous ECG preparation and vigilance during imaging. Through an understanding of the basic technical considerations and possible artifacts, study acquisition can be tailored to the individual patient maximizing diagnostic utility and optimizing care.
Acknowledgments
Funded by the National Institutes of Health (NIH) Intramural program.
Footnotes
Conflict of Interest The authors declare that they have no competing interests.
Contributor Information
Marcelo Souto Nacif, Radiology and Imaging Sciences, National Institutes of Health Clinical Center, 10 Center Drive, Building 10, Rm 1C355, Bethesda, MD 20892-1182, USA; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Radiology Department, Universidade Federal Fluminense, Niterói, Brazil.
Anna Zavodni, Radiology and Imaging Sciences, National Institutes of Health Clinical Center, 10 Center Drive, Building 10, Rm 1C355, Bethesda, MD 20892-1182, USA.
Nadine Kawel, Radiology and Imaging Sciences, National Institutes of Health Clinical Center, 10 Center Drive, Building 10, Rm 1C355, Bethesda, MD 20892-1182, USA.
Eui-Young Choi, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
João A. C. Lima, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
David A. Bluemke, Email: bluemked@nih.gov, Radiology and Imaging Sciences, National Institutes of Health Clinical Center, 10 Center Drive, Building 10, Rm 1C355, Bethesda, MD 20892-1182, USA; National Institute of Biomedical Imaging and Bioengineering, Bethesda, MD, USA.
References
- 1.Lanzer P, Botvinick EH, Schiller NB, et al. Cardiac imaging using gated magnetic resonance. Radiology. 1984;150:121–127. doi: 10.1148/radiology.150.1.6227934. [DOI] [PubMed] [Google Scholar]
- 2.Amoore JN, Ridgway JP. A system for cardiac and respiratory gating of a magnetic resonance imager. Clin Phys Physiol Meas. 1989;10:283–286. doi: 10.1088/0143-0815/10/3/009. [DOI] [PubMed] [Google Scholar]
- 3.McNamara MT, Higgins CB. Cardiovascular applications of magnetic resonance imaging. Magn Reson Imaging. 1984;2:167–183. doi: 10.1016/0730-725x(84)90002-x. [DOI] [PubMed] [Google Scholar]
- 4.Osbakken M, Yuschok T. Evaluation of ventricular function with gated cardiac magnetic resonance imaging. Cathet Cardiovasc Diagn. 1986;12:156–160. [PubMed] [Google Scholar]
- 5.Stark DD, Higgins CB, Lanzer P, et al. Magnetic resonance imaging of the pericardium: normal and pathologic findings. Radiology. 1984;150:469–474. doi: 10.1148/radiology.150.2.6691103. [DOI] [PubMed] [Google Scholar]
- 6.Brau AC, Wheeler CT, Hedlund LW, Johnson GA. Fiber-optic stethoscope: a cardiac monitoring and gating system for magnetic resonance microscopy. Magn Reson Med. 2002;47:314–321. doi: 10.1002/mrm.10049. [DOI] [PubMed] [Google Scholar]
- 7.Dimick RN, Hedlund LW, Herfkens RJ, Fram EK, Utz J. Optimizing electrocardiograph electrode placement for cardiac-gated magnetic resonance imaging. Invest Radiol. 1987;22:17–22. doi: 10.1097/00004424-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dinsmore RE. Quantitation of cardiac dimensions from ECG-synchronized MRI studies. Cardiovasc Intervent Radiol. 1987;10:356–364. doi: 10.1007/BF02577346. [DOI] [PubMed] [Google Scholar]
- 9.Laudon MK, Webster JG, Frayne R, Grist TM. Minimizing interference from magnetic resonance imagers during electrocardiography. IEEE Trans Biomed Eng. 1998;45:160–164. doi: 10.1109/10.661264. [DOI] [PubMed] [Google Scholar]
- 10.Park H, Park Y, Cho S, Jang B, Lee K. New cardiac MRI gating method using event-synchronous adaptive digital filter. Ann Biomed Eng. 2009;37:2170–2187. doi: 10.1007/s10439-009-9764-4. [DOI] [PubMed] [Google Scholar]
- 11.Shetty AN. Suppression of radiofrequency interference in cardiac gated MRI: a simple design. Magn Reson Med. 1988;8:84–88. doi: 10.1002/mrm.1910080110. [DOI] [PubMed] [Google Scholar]
- 12.Amparo EG, Higgins CB, Farmer D, Gamsu G, McNamara M. Gated MRI of cardiac and paracardiac masses: initial experience. AJR Am J Roentgenol. 1984;143:1151–1156. doi: 10.2214/ajr.143.6.1151. [DOI] [PubMed] [Google Scholar]
- 13.Yamamura J, Frisch M, Ecker H, et al. Self-gatingMR imaging of the fetal heart: comparison with real cardiac triggering. Eur Radiol. 2010;21:142–149. doi: 10.1007/s00330-010-1911-7. [DOI] [PubMed] [Google Scholar]
- 14.Chia JM, Fischer SE, Wickline SA, Lorenz CH. Performance of QRS detection for cardiac magnetic resonance imaging with a novel vectorcardiographic triggering method. J Magn Reson Imaging. 2000;12:678–688. doi: 10.1002/1522-2586(200011)12:5<678::aid-jmri4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Finn JP, Nael K, Deshpande V, Ratib O, Laub G. Cardiac MR imaging: state of the technology. Radiology. 2006;241:338–354. doi: 10.1148/radiol.2412041866. [DOI] [PubMed] [Google Scholar]
- 16.Gatehouse PD, Firmin DN. The cardiovascular magnetic resonance machine: hardware and software requirements. Herz. 2000;25:317–330. doi: 10.1007/s000590050025. [DOI] [PubMed] [Google Scholar]
- 17.Wendt RE, III, Rokey R, Vick GW, III, Johnston DL. Electrocardiographic gating and monitoring in NMR imaging. Magn Reson Imaging. 1988;6:89–95. doi: 10.1016/0730-725x(88)90528-0. [DOI] [PubMed] [Google Scholar]
- 18.Becker M, Frauenrath T, Hezel F, et al. Comparison of left ventricular function assessment using phonocardiogram- and electrocardiogram-triggered 2D SSFP CINE MR imaging at 1.5 T and 3.0 T. Eur Radiol. 2010;20:1344–1355. doi: 10.1007/s00330-009-1676-z. [DOI] [PubMed] [Google Scholar]
- 19.Larson AC, White RD, Laub G, McVeigh ER, Li D, Simonetti OP. Self-gated cardiac cine MRI. Magn Reson Med. 2004;51:93–102. doi: 10.1002/mrm.10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odille F, Uribe S, Batchelor PG, Prieto C, Schaeffter T, Atkinson D. Model-based reconstruction for cardiac cine MRI without ECG or breath holding. Magn Reson Med. 2010;63:1247–1257. doi: 10.1002/mrm.22312. [DOI] [PubMed] [Google Scholar]
- 21.Ridgway JP. Cardiac magnetic resonance physics for clinicians: part I. J Cardiovasc Magn Reson. 2010;12:71. doi: 10.1186/1532-429X-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]