Abstract
Nonmuscle myosin II-B (NM II-B) plays an important role in cardiac development and function. Genetic ablation of NM II-B in mice results in both cellular and structural defects involving cardiac myocytes. These abnormalities include a ventricular septal defect, double outlet of the right ventricle, myocyte hypertrophy and premature onset of myocyte binucleation due to abnormalities in cytokinesis. The mice die by embryonic day (E) 14.5 due to defects in heart development. Conditional ablation of NM II-B in cardiac myocytes after E11.5 allows study of NM II-B function in adult myocytes. BαMHC/BαMHC mice are born with enlarged cardiac myocytes, some of which are multinucleated. Between 6–10 months of age they develop a cardiomyopathy. Many of these mice develop a marked widening of the intercalated discs. The loss of NM II-B from the intercalated discs primarily affects the adhesion junctions rather than the gap junctions and desmosomes. Interestingly, the loss of NM II-B results in a decrease in the actin binding protein mXin which also has been shown to cause disruption of the intercalated disc in addition to cardiac arrhythmias (Gustafson-Wagner et al. Am J Physiol Heart Circ Physiol. 2007, 293:H2680-92). Finally we review the evidence showing that ablation of NM II-C (which also localizes to the intercalated disc) in mouse hearts deficient in NM II-B expression results in destabilization of N-cadherin and β-catenin in the intercalated disc.
Keywords: Nonmuscle Mysoin II, Cardiac Myocyte, Cytokinesis, Karyokinesis, Intercalated Disc, Cardiomyopathy, Knockout Mice
2. INTRODUCTION TO NONMUSCLE MYOSIN II
Nonmuscle myosin II (NM II) is the name given to the multi-subunit protein product of the MYH9, MYH10 and MYH14 genes, which encode three different myosin heavy chains (230 kDa) and the MYL6, MYL9 and MYL12 genes which encode a family of light chains (17–20 kDa each) (1). Each of the three isoforms of NM II is composed of a hexamer consisting of two heavy chains and two pairs of light chains (Fig. 1A). At present the myosin isoforms, which are named on the basis of the heavy chains, are commonly referred to as NM II-A (MYH9), NM II-B (MYH10) and NM II-C (MYH14). We assume, but have no direct evidence, that the light chains have no heavy chain specificity. In addition to diversity based on the differences in amino acid sequence of the heavy chains, both NM II-B and II-C undergo alternative splicing of their heavy chain mRNAs to yield additional isoforms, some with markedly different properties compared to the unspliced isoform (see below).
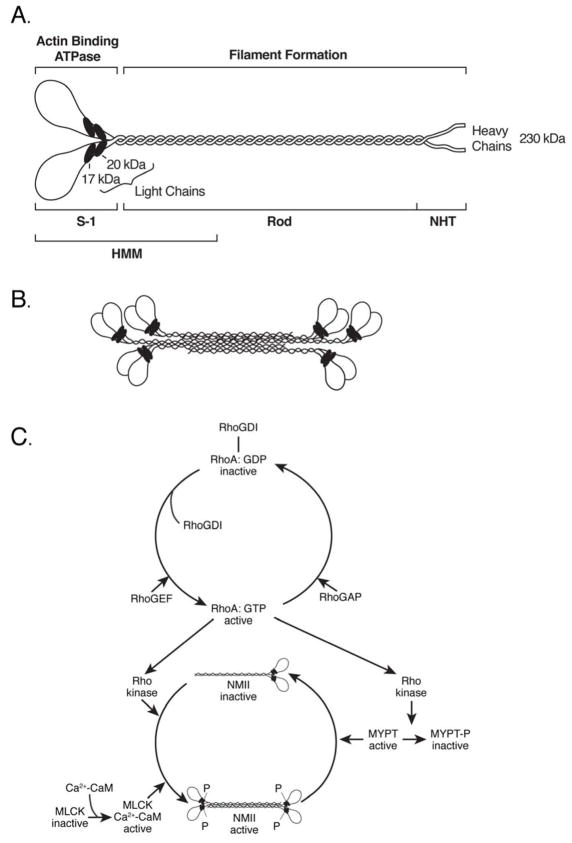
Figure 1. Diagram Depicting NM II Molecule, Bipolar Filament and Regulation.
(A) Diagram of a myosin molecule showing the globular head region, the α-helical coiled-coil rod and the short non-helical tail (NHT). The subfragment-1 (S-1), rod and heavy meromyosin (HMM) proteolytic domains are also indicated. (B) An example of a bipolar filament, which is formed by interaction among the rod domains. The actual number of molecules is greater than shown. (C) Regulation of myosin activity by phosphorylation of RMLC of NMII by myosin light chain kinase (MLCK) and Rho kinase. Whereas MLCK can only phosphorylate RMLC, Rho kinase can phosphorylate RMLC and also a subunit of myosin phosphatase (MYPT). Phosphorylation by Rho kinase activates myosin and inactivates MYPT. Both result in an increase in phosphorylated RMLC and activation of myosin. Reproduced from reference 2.
All three NM II isoforms share a number of common properties. Each can bind to actin in a MgATP-dependent manner (MgATP dissociates the complex) and thereby exert tension or translocate actin-filaments. This ability to convert the chemical energy of ATP into mechanical force is the hallmark of myosins in general. Unlike skeletal, cardiac and smooth muscle myosins, with which they share a number of properties, NM IIs are not confined to any particular tissues but are present in every cell in the human body. Indeed they are present, along with the more specialized and abundant forms of myosin II, in all three types of muscle cells, especially during early development. It is of note that NM IIs share many of the physical and biochemical properties of smooth muscle myosins, including their regulation by phosphorylation of the 20 kDa light chain (RMLC) (2).
Similarities in the properties of NM IIs (and smooth muscle myosin) derive from the 60–80% identity of the heavy chain amino acid sequences. These include the ability of single molecules to form a folded structure which, following activation by RMLC phosphorylation, can align into small bipolar filaments (Fig. 1B) (1;3). This ability to form filaments resides in the α-helical coiled coil domain located at the carboxyl-terminal end of the molecule. In contrast, the globular shaped motor domain at the amino-terminal end of the molecule, which contains both the actin and ATP binding domains is responsible for the ability of NM II to translocate actin-filaments. There are significant differences in motor activity among the three nonmuscle myosin isoforms. These are reflected in differences in their rate of ATP hydrolysis, as well as the length of time that the NM II is bound to actin during a complete contractile cycle (the duty ratio) (4;5).
Activation of the MgATPase activity of both NM II as well as smooth muscle myosin depends on the phosphorylation of Serine 19 (and sometimes, additionally Threonine 18) in the 20 kDa RMLC (Fig. 1C) (2;6). Although there are a number of different kinases that can catalyze this reaction, the two most important ones in terms of cellular function are the Ca2+-calmodulin dependent, myosin light chain kinase (MLCK), and the Rho-GTP dependent, Rho kinase (ROCK). Phosphorylation of the RMLC is required for the various functions of NM II including cell migration and cell adhesion. The state of NM II activation is also regulated by a phosphatase composed of three subunits: a catalytic subunit, a regulatory subunit (MYPT) and a third subunit of undefined function (7). Thus activation of myosin is possible by kinase activation and phosphatase inhibition. Until recently it was thought that all smooth muscle myosin and NM II actin-activated ATPase activities could be regulated by RMLC phosphorylation, but two of the alternatively spliced isoforms of NM II appear to be exceptions to this rule. Human NM II-B2, an alternatively spliced nonmuscle myosin heavy chain (NMHC) isoform that contains a 21 residue insert near its actin-binding domain cannot be activated by RMLC phosphorylation (8). In addition NM II-C2, an alternatively spliced isoform of NMHC II-C, which contains 33 residues (41 in mice) at a site analogous to the NM II-B2 insert, is active whether or not it is phosphorylated on the RMLC (9). Both of these unusual myosins are only expressed in the brain.
NM II filament formation can also be regulated by phosphorylation on the NMHC. In this case a number of different phosphorylation sites in both the helical rod region as well as the non-helical tail have been identified as well as kinases that can catalyze these reactions (10). Phosphorylation of the NMHC acts to either promote filament dissociation or to prevent filament formation.
3. FUNCTION OF NM II IN VIVO
The in vivo functions of NM II are numerous since all three isoform are widely expressed throughout various tissues (11) and both NM II-A and II-B play important roles in early mammalian development (12;13). Similar to its binding partner nonmuscle actin, NM II plays a role in cell migration, cell-cell and cell-matrix adhesion, cell polarity (10), and as recently described, receptor internalization (14) and embryonic stem cell apoptosis (15–18). Indeed there are few cellular functions that require shape change and movement that do not involve NM II. An important question that has been generated by the presence of three different genes on three different chromosomes expressing the NMHCs is whether the various NM II isoforms overlap in their functions or whether one can substitute for the other for any given function. In vivo experiments have been carried out to address this question. Of note is the finding that germline ablation of each isoform results in distinct phenotypes. Ablation of NM II-A results in lethality at embryonic day (E) 6.5 (12), ablation of NM II-B causes lethality at E14.5 (13;19) and ablation of II-C has no obvious phenotype (20) (see below). Moreover, genetic ablation of one isoform by introducing cDNA encoding a second isoform into the gene locus has allowed direct testing of the substitution of one isoform for another (21;22). These experiments, which are still in progress, have resulted in the following hypothesis: Those functions of NM II which are more static such as the cross-linking of actin-filaments, are more amenable to isoform substitution. A good example is the ability of NM II-A to rescue hydrocephalus in NM II-B ablated mice (21). In this circumstance NM II-A can replace NM II-B in maintaining cell-cell adhesion in the neuroepithelial cells lining the spinal canal. On the other hand, those functions of NM II which mainly depend on the motor domain, such as cell migration, are not amenable to substitution. Thus the facial and pontine neurons that migrate abnormally in mice deficient in NM II-B cannot be rescued by NM II-A. Recently this hypothesis has been tested using chimeric constructs of the NM II-A and II-B heavy chains in which the globular head and α-helical tail region were exchanged (22). With this brief general introduction, we now turn to the in vivo role of NM IIs in cardiac development.
4. NM II IN HEART DEVELOPMENT AND FUNCTION
4.1 NM II Isoform Expression and Function in Embryonic Heart Development
As noted above three isoforms of NM II, namely NM II-A, II-B and II-C, have been detected in mammalian species including humans and mice. NM II-A and II-B are expressed from very early mouse embryonic development including in embryonic stem cells. NM II-C is detected by immunofluorescence staining in the developing mouse pituitary around E11.5 and in the lungs at E13.5 (11;20). All three isoforms of NM II are expressed in mouse and human hearts. In cardiac non-myocyte cells, including the epicardial, endocardial and cardiac fibroblast cells, NM II-A and II-B, but not NM II-C are detected in the early embryos and adults (23). In cardiac myocytes, NM II-B is expressed during early embryonic development (19;20). NM II-C is expressed later on and NM II-A is only transiently detected in the heart tube and outflow tract regions where the newly added cardiac myocytes are localized (Fig. 2). Both NM II-B and II-C are distributed throughout the cytosol in embryonic cardiac myocytes, but decrease substantially in expression immediately after birth and become confined to the cardiac intercalated discs in adult cardiac myocytes (20;24). The transition in cellular distribution reflects distinct roles for NM II in embryonic and adult cardiac myocytes.
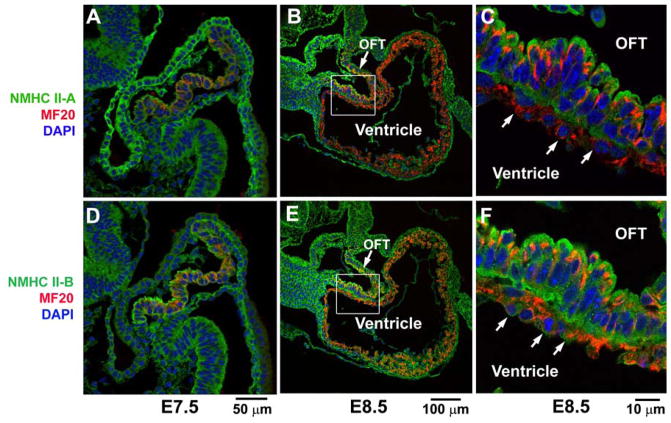
Figure 2. Expression of NM II during Early Heart Formation.
Immunofluorescence confocal images of wild-type mouse hearts at E7.5 and E8.5 stained for NMHC II-A (green, A–C) and II-B (green, D–F), and co-stained for MF20 (red, a marker for cardiac myocytes). At E7.5, the early cardiac myocytes (A,D) show co-staining of NMHC II-A and MF20 (A) or NMHC II-B and MF20 (D) indicating that both NMHC II-A and II-B are expressed in cardiac myocytes at this stage. At E8.5, the cardiac myocytes in the developing outflow tract (OFT) still express both NMHC II-A (B, magnified in C) and II-B (E, magnified in F), however in the ventricular myocytes at E8.5 (arrows, C,F) only NMHC II-B (E,F), and not NMHC II-A (B,C) is detected. Modified from reference 20.
The function of NM II during heart development becomes clearer by analyzing the phenotype following ablation of each of the isoforms and replacement of one isoform with another. Germline ablation of NM II-A results in early embryonic lethality in mice by E6.5 before organogenesis due to the failure of these embryos to form a functional visceral endoderm (12). Genetic replacement of NM II-A expression with NM II-B, using NMHC II-B cDNA to ablate NMHC II-A and thereby place the exogenous NMHC II-B under control of the NMHC II-A promoter, permits embryos to pass through gastrulation and survive to E9.5–10.5 with beating hearts (22). Interestingly, conditional ablation of NM II-A in cardiac myocytes using a Nkx2.5 promoter-driven cre-recombinase shows no defects in mouse heart development (unpublished observation). These results indicate that expression of NM II-A is not essential for mouse heart development. Mice that are germline ablated for NMHC II-B survive to E14.5 with major defects in the brain and heart (13;19;25). The B−/B− mouse brains exhibit a severe hydrocephalus with defects in neuroepithelial cell-cell adhesion in the neural tube and abnormal neuronal cell migration of three different neuronal tracts (facial, pontine and cerebellar granular cells) (26).
The B−/B− hearts manifest a ventricular septal defect (VSD) with the origin of the aorta mis-placed to the right ventricle (DORV, double outlet of the right ventricle). The cardiac myocytes of B−/B− mice show reduced proliferative activity associated with a defect in cytokinesis indicated by a premature accumulation of bi-nucleated cardiac myocytes in B−/B− embryonic hearts compared to the wild-type control hearts (19). Many of the B−/B− cardiac myocytes however remain mono-nucleated (74% vs 95% for wild type controls). Since NM II-B is ablated and NM II-A is not expressed in the cardiac myocytes, the results indicate that NM II-C can partially support cytokinesis in the cardiac myocytes, but that the expression of NM II-C alone is not sufficient for normal cytokinesis during mouse heart development. Unlike A−/A− and B−/B− embryos, mice ablated for NM II-C survive to adulthood with no obvious abnormalities. The C−/C− mouse hearts are normal with no evidence for a defect in cytokinesis in the cardiac myocytes (20). Analyses of these NM II isoform specific ablated mice show that NM II-B is essential for heart development especially for the normal alignment of the outflow tract and cytokinesis of cardiac myocytes. This is further supported by studies of Ba*/Ba* mice where NM II-B expression is replaced by NM II-A. Ba*/Ba* mouse hearts still develop a DORV and show an abnormal occurrence of premature bi-nucleated cardiac myocytes though not as severe as in B−/B− cardiac myocytes (21).
In addition to bi-nucleation, some B−/B− cardiac myocytes contains multi-lobed nuclei indicating a failure in karyokinesis. Defects in cardiac myocyte karyokinesis become more frequent when both NM II-B and NM II-C are ablated simultaneously (B−C−/B−C− mice). In NM II-B/II-C doubly ablated mice more than 90% of the cardiac myocytes in the compact myocardium contain multi-lobed nuclei (20). The cardiac myocytes of these mice have abnormal mitotic spindles with mis-alignment of the chromosomes. Similar defects in karyokinesis are also observed in cultured HL-1 cells (derived from a mouse atrial myocyte tumor) (27) when NM IIs are depleted by siRNA treatment or NM II ATPase activity is inhibited by blebbistatin. These results support a role for NM II in karyokinesis in cardiac myocytes during mouse heart development. Thus, ablation of NM II-C alone in mice does not interfere with karyokinesis in cardiac myocytes; expression of NM II-C in NM II-B ablated cardiac myocytes can support karyokinesis for the majority of the B−/B− cardiac myocytes. In summary, NM II-B is required for both cardiac myocyte cytokinesis and karyokinesis, and normal outflow tract alignment during mouse embryonic heart development. Interestingly, knocking out NM II-B and NM II-C had no obvious detrimental effect on sarcomere formation, despite the concomitant absence of NM II-A (Fig. 3). This differs from the findings using primary cultured chicken cardiac myocytes which indicate a role for NM II-B in myofibril formation (28).
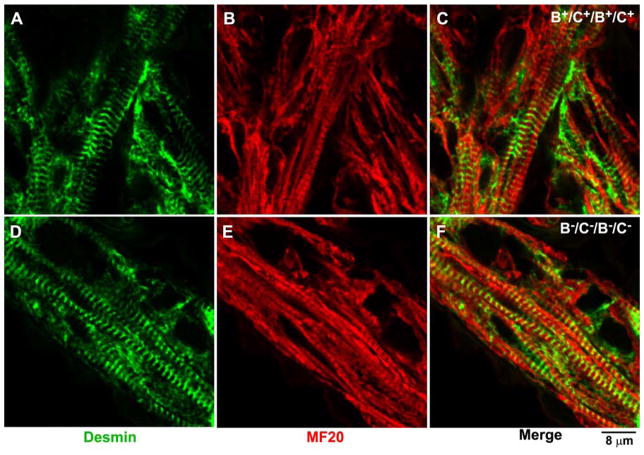
Figure 3. Normal Sarcomere Formation in B−C−/B−C− Cardiac Myocytes.
Immunofluorescence confocal images of E13.5 cardiac myocytes stained with MF20 (red, a marker for cardiac myosin II, B,E) and desmin (green, A,D) in B+C+/B+C+ (A–C) and B−C−/B−C− (D–F) mouse hearts. No difference in sarcomere formation is found between B+C+/B+C+ and B−C−/B−C− hearts. Reproduced from reference 20.
NM II is one of the major cellular motor proteins which also functions to cross-link actin-filaments. The requirement of NM II for contractile ring constriction during cytokinesis has been well demonstrated although a detailed mechanism has not been elucidated. It is generally accepted that NM II provides the major force driving contractile ring constriction, although accumulating evidence also points to a motor-independent cross-linking mechanism for cytokinesis (29). The contractile ring is highly dynamic during cytokinesis and NM II also functions to promote actin-filament turnover (30;31). The involvement of NM II in karyokinesis is much less well understood. NM II could regulate karyokinesis through an effect on microtubules. Ablation of NM II expression increases microtubule stability in both NM II ablated cardiac myocytes as well as in cultured cell lines (20;32). NM II is also reported to regulate centrosome separation and positioning after nuclear envelope breakdown in cultured cells through a contribution to cortical flow (33).
NM II appears to play a role in normal alignment of the cardiac outflow tract. NM II could contribute to myocardialization of the outflow tract, a process during which the mesenchyme of the proximal region of the outlet septum is replaced by cardiac myocytes. A failure in this process leads to the development of DORV as seen in NM II-B ablated mouse hearts. Of note is a report by Phillips et al. (34) attributing mislocalization of the aorta to the right ventricle in loop-tail mice to inhibition in NM II function due to mutations in the Vangl2 gene. Mutations in Vangl2 disrupt RhoA and ROCK1 expression thereby inhibiting NM II activity in the developing outflow tract of these mutant mice.
4.2 NM II in the Adult Heart
Fig. 4A shows immunofluorescence confocal images of an E13.5 mouse heart stained for NMHC II-A (a,d, green), II-B (b,e, green), and II-C (c,f, green) together with desmin (d–f, red), a marker for (cardiac) myocytes. Note that by this time NM II-A is only expressed in nonmyocytes (a, green) and that NM II-B is detected in both myocytes (e, red and green colocalization) and nonmyocytes (e, green) in the heart. NM II-C is detected in myocytes (f, red and green colocalization) but not in nonmyocytes (20). During postnatal heart development in mice, NM II-B expression decreases markedly in cardiac myocytes within the first week after birth but can be detected in the intercalated discs. Mass spectroscopic analysis of the relative amounts of NM II isoforms from whole heart extracts shows 37% of the total NM II is NM II-B by postnatal day (P)2 and this reduces to 5% of the total NM II in adults (20). The expression of NM II-C is so low that mass spectroscopy fails to detect NM II-C peptides in both P2 and adult mouse hearts. However, immunofluorescence confocal microscopy detects NM II-C at the intercalated discs in adult mouse hearts, while none is detected in the NM II-C ablated mice (Fig. 4B). This localization of NM II-B and II-C is consistent with their function in maintaining the integrity of the intercalated discs in adult cardiac myocytes. Additionally, mice specifically ablated for NM II-B in cardiac myocytes using an α-cardiac myosin heavy chain promoter-driven cre-recombinase (BαMHC/BαMHC mice) survive to adulthood and develop a progressive hypertrophic cardiomyopathy associated with disruption of the intercalated discs (Fig. 5A) (23). At 10 months approximately 20% of the intercalated discs of BαMHC/BαMHC mice have abnormally widened adhesion junctions, while the gap junctions and desmosomes remain unaffected (Fig. 5A). In maintaining cell-cell adhesion, NM II could act to stabilize adherens junctions by cross-linking actin-filaments (35). To maintain the adherens junctions of the intercalated discs, NM II-B may also function by affecting other components of the disc such as the actin binding protein, mXin. Loss of NM II-B results in marked reduction of mXin expression (Fig. 5B), which in turn could disrupt the intercalated disc (23). Mice ablated for mXin demonstrate a disruption in intercalated discs similar to NM II-B ablated mouse hearts (36). Ablation of NM II-B does not affect formation of the intercalated discs since BαMHC/BαMHC mice are born with normal intercalated discs. Milder defects in the intercalated discs are observed at 6 months. Therefore ablation of NM II-B together with a loss of mXin expression in the intercalated discs of BαMHC/BαMHC mice may weaken the adhesions junctions and consequently disrupt the intercalated discs, at a later time during adulthood of BαMHC/BαMHC mice. Understanding the mechanism of how the loss of NM II-B causes the decrease of mXin expression is of great interest.
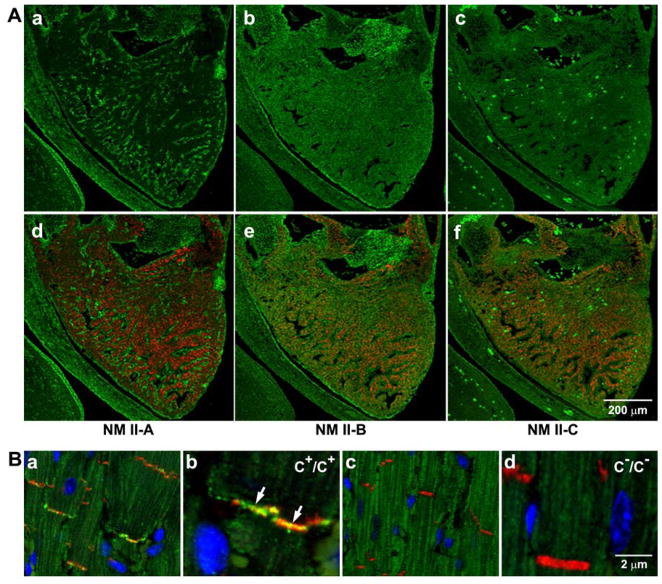
Figure 4. Expression of NM II in Embryonic Mouse Hearts and NM II-C in Adult Mouse Hearts.
(A) Immunofluorescence confocal images of an E13.5 mouse heart stained for NMHC II-A (a,d, green), II-B (b, green), and II-C (c, green) together with desmin (d–f, red), a marker for (cardiac) myocytes. NM II-A is only expressed in nonmyocytes (a,d, green). NM II-B is detected in both myocytes (e, red and green colocalization) and nonmyocytes (e, green) in the heart. NM II-C is detected in myocytes (f, red and green colocalization) but not in nonmyocytes. The bright green spots are autofluorescence from red blood cells in c and f. (B) Immunofluorescence confocal images of adult heart sections from C+/C+ (a, magnified in b) and C−/C− (c, magnified in d) mice. N-Cadherin is a marker for the intercalated disc (red). Nuclei are stained with DAPI (blue). Arrows in b indicates the presence of NMHC II-C (green) in the intercalated disc. NMHC II-C is absent from C−/C− intercalated discs (c and d). Reproduced from reference 20.
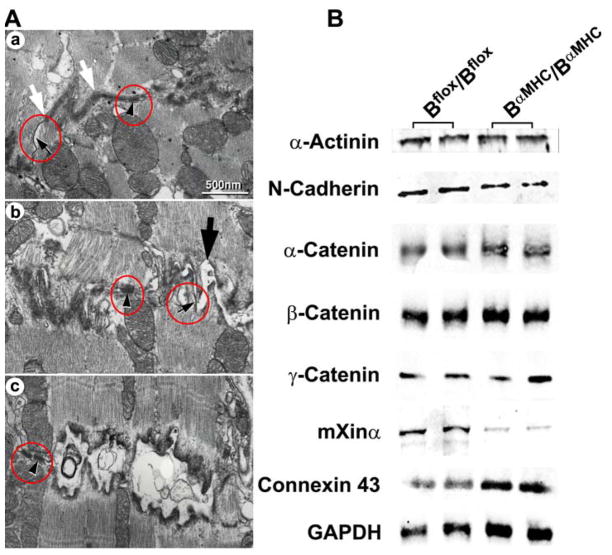
Figure 5. Abnormalities in the Intercalated Discs in BαMHC/BαMHC Mice.
(A) Electron microscope sections of Bflox/Bflox (a) and BαMHC/BαMHC (b and c) left ventricles at 10 months. b shows a less-affected intercalated disc than c. Both b (large arrow) and c show that the adhesion type junctions of the BαMHC/BαMHC cardiac myocytes are severely distorted, whereas the structures of the desmosomes (arrowheads) and gap junctions (small arrows) remain intact. The structure between the white arrows shows a normal adhesion junction (a). Similar results were found for 4 other wild-type and 3 other BαMHC/BαMHC mice. (B) Immunoblot analysis for proteins associated with the intercalated discs at 6 months. Note the decrease in mXimα in BαMHC/BαMHC mouse hearts. In contrast, expression of connexin 43 is increased, most likely due to cardiac myocyte hypertrophy. Modified from reference 23.
The role of NM II-C in cardiac myocytes depends on the level of NM II-B expression (20). In the presence of wild-type levels of NM II-B expression, ablation of NM II-C has no effect on heart development and function. However ablation of NM II-C in mice expressing 12% of wild-type NM II-B levels in the heart (BΔB1NC−/BΔB1NC− mice) markedly enhances the development of cardiac myocyte hypertrophy compared to NM II-B hypomorphic mice alone (BΔB1NC+/BΔB1NC+ mice, Fig. 6). In addition, NM II-C ablated/NM II-B hypomorphic mouse hearts show abnormal and diffuse expression and localization of adhesion molecules such as N-cadherin and β-catenin at the intercalated discs (Fig. 7). This diffuse distribution may reflect the weakening and consequent separation of the intercalated discs. A similar diffuse distribution is also observed in BαMHC/BαMHC mice which show widened intercalated discs. NM II-B hypomorphic mice show no defects in localization of N-cadherin and β-catenin. Thus the function of NM II in cardiac myocytes depends more on the total amount of NM II expressed rather than the individual isoform of NM II. This dosage dependence was nicely demonstrated in NM II-B hypomorphic mice. Mice which expressed no NM II-B in cardiac myocytes develop cardiac myocyte hypertrophy as embryos. Mice expressing 6% of wild-type levels of NM II-B in the heart show hypertrophy at 1 month and mice with 12% of wild-type NM II-B in the heart develop hypertrophy at 6 to 7 months (37).
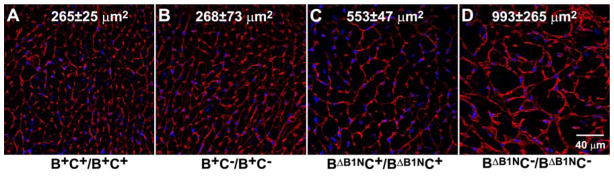
Figure 6. Ablation of NM II-C Accelerates Development of Cardiomyopathy in NM II-B Hypomorphic Mice.
Wheat germ agglutinin staining shows plasma membranes in heart sections from B+C+/B+C+, B+C−/B+C−, BΔB1NC+/BΔB1NC+, and BΔB1NC−/BΔB1NC− mice. The average cross-sectional area of the cardiac myocytes for each genotype was measured and is shown in each panel. Compared with B+C+/B+C+ cardiac myocytes (A), the average cross-sectional area for B+C−/B+C− myocytes remains unchanged (B), but it is doubled in BΔB1NC+/BΔB1NC+ myocytes (C) and increased to 4 times in BΔB1NC−/BΔB1NC− myocytes (D). Nuclei are stained with DAPI (blue). Modified from reference 20.
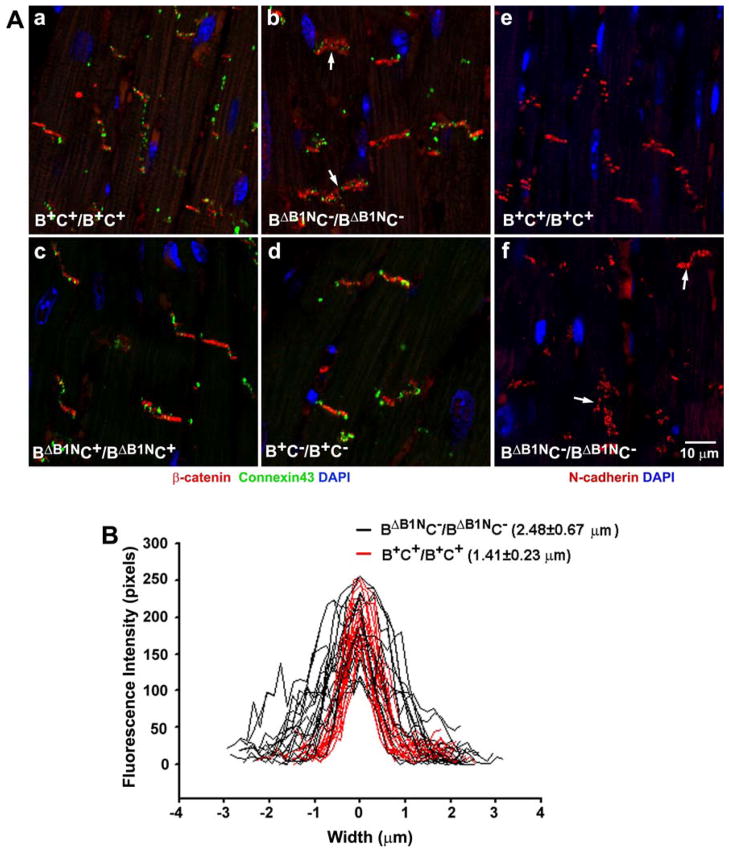
Figure 7. Impaired Localization of β-Catenin and N-cadherin in BΔB1NC−/BΔB1NC− Intercalated Discs.
(A) Immunofluorescence confocal images of adult heart sections from B+C+/B+C+, BΔB1NC−/BΔB1NC−, BΔB1NC+/BΔB1NC+, and B+C−/B+C− mice stained for β-catenin (red, a–d), connexin43 (green, a–d) and N-cadherin (red, e,f). In contrast to B+C+/B+C+ (a,e), B+C−/B+C− (d), and BΔB1NC+/BΔB1NC+ (c) cardiac myocytes where β-catenin and N-cadherin very precisely stain the intercalated discs, BΔB1NC−/BΔB1NC− myocytes (arrows, b,f) show a diffuse β-catenin and N-cadherin staining. No difference in connexin43 staining (green, a–d) is observed among these four genotypes. Nuclei were stained by DAPI (blue). (B) Profiles of β-catenin staining at the intercalated discs for B+C+/B+C+ (red lines) and BΔB1NC−/BΔB1NC− (black lines) mouse hearts. BΔB1NC−/BΔB1NC− hearts showed a diffuse β-catenin distribution manifested by widened β-catenin staining at the intercalated discs compared to the wild-type hearts. An embedded Zeiss LSM image profile tool is used to quantify the fluorescence intensity along a line drawn vertically across the intercalated discs. Reproduced from reference 20.
5. SUMMARY AND PERSPECTIVE
In this review we have attempted to cover the function of the various isoforms of NM II in cardiac development, with particular emphasis on their role using mouse models. Table 1 summarizes the phenotypes of the mouse lines mentioned in this paper. One of the most interesting outcomes of this research has been finding a role for NM II-B in the adult heart. Since NM II-B ablated mice die by E14.5 with brain and heart defects, we made use of a conditional mouse line in which NM II-B is ablated only in the heart and at a later time than the germ line II-B ablated mice. This was possible by crossing II-B floxed mice to mice expressing cre-recombinase under control of the α-MHC promoter. Under these conditions the cardiac myocyte NM II-B ablated mice survive to adulthood and most of them demonstrate abnormal widening of the intercalated discs. These experiments reveal a role for NM II-B in maintenance, but not in formation of the intercalated disc. Moreover, although there is no evidence for abnormalities of intercalated disc structure in NM II-B ablated mice, which die by E14.5, the NM II-B hypomorphic mice that are ablated for NM II-C do show destabilization of cell-cell adhesion proteins such as β-catenin and N-cadherin, demonstrating that both NM II-B and II-C (or the equivalent of their total amount of NM II) are required for normal intercalated disc formation and/or maintenance. Sorting out the exact mechanism by which NM II-B and NM II-C interact with the various proteins such as mXin that are found in the disc and contribute to stable disc structure is an immediate challenge.
Table 1.
Summary of Phenotypes of Genetically Modified Mice
| Genotype | Lethality | Phenotype |
|---|---|---|
| A−/A− | E6.5 | Defects in cell-cell adhesion and visceral endoderm formation. No gastrulation.(12) |
| B−/B− | E14.5 | Defects in heart (VSD, DORV) and brain (hydrocephalus and neuronal migration defects).(13;25) |
| C−/C− | Adult | No obvious abnormalities.(20) |
| B−C−/B−C− | E14.5 | Defects in heart and brain similar to B−/B− mice with major abnormalities in cardiac myocyte karyokinesis.(20) |
| Ab*/Ab* NMHC II-B under control of II-A promoter |
E9.5–10.5 | Visceral endoderm cell-cell adhesion defect rescued, embryos undergo gastrulation. Defects in placental development due to lack of endothelial cell migration.(22) |
| Ba*/Ba* NMHC II-A under control of II-B promoter |
E14.5 | Hydrocephalus rescued by restoring neuroepithelial cell-cell adhesion. Neuronal migration and heart defects not rescued.(21) |
| BαMHC/BαMHC | Adult | Hypertrophic cardiomyopathy; Disruption of the intercalated discs associated with a reduced mXin expression.(23) |
| BΔB1N/BΔB1N 88% reduction of NM II-B expression in heart |
Adult | Abnormal facial neuron migration; Cardiac myocyte hypertrophy at 10 months old.(37) |
| BΔB1NC−/BΔB1NC− | Adult | Earlier and more severe cardiac myocyte hypertrophy than BΔB1N/BΔB1N mice; Diffuse localization of N-cadherin and β-catenin at the intercalated discs.(20) |
Acknowledgments
The authors are grateful to Drs. Mary Anne Conti and Sachiyo Kawamoto for reading and commenting on the manuscript.
Abbreviations
- DORV
Double Outlet of the Right Ventricle
- E
Embryonic Day
- NM II
Nonmuscle Myosin II
- NMHC
Nonmuscle Myosin Heavy Chain
- RMLC
Regulatory Myosin Light Chain
- VSD
Ventricular Septal Defect
References
- 1.Conti MA, Kawamoto S, Adelstein RS. Non-Muscle Myosin II. In: Coluccio LM, editor. Myosins: A Superfamily of Molecular Motors. Springer; Watertown: 2008. pp. 223–264. [Google Scholar]
- 2.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci. 2008;121:11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]
- 3.Milton DL, Schneck AN, Ziech DA, Ba M, Facemyer KC, Halayko AJ, Baker JE, Gerthoffer WT, Cremo CR. Direct evidence for functional smooth muscle myosin II in the 10S self-inhibited monomeric conformation in airway smooth muscle cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1011784108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs M, Wang F, Hu A, Zhang Y, Sellers JR. Functional divergence of human cytoplasmic myosin II: kinetic characterization of the non-muscle IIA isoform. J Biol Chem. 2003;278:38132–38140. doi: 10.1074/jbc.M305453200. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem. 2003;278:27439–27448. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- 6.Cremo CR, Hartshorne DJ. Smooth-Muscle Myosin II. In: Coluccio LM, editor. Myosins: A Superfamily of Molecular Motors. Springer; Watertown: 2008. pp. 171–222. [Google Scholar]
- 7.Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: Many roles in cell function. Biochem Biophys Res Commun. 2008;369:149–156. doi: 10.1016/j.bbrc.2007.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim KY, Kawamoto S, Bao J, Sellers JR, Adelstein RS. The B2 alternatively spliced isoform of nonmuscle myosin II-B lacks actin-activated MgATPase activity and in vitro motility. Biochem Biophys Res Commun. 2008;369:124–134. doi: 10.1016/j.bbrc.2007.11.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jana SS, Kim KY, Mao J, Kawamoto S, Sellers JR, Adelstein RS. An alternatively spliced isoform of non-muscle myosin II-C is not regulated by myosin light chain phosphorylation. J Biol Chem. 2009;284:11563–11571. doi: 10.1074/jbc.M806574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, Goldin E, Conti MA, Sellers JR, Adelstein RS. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279:2800–2808. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- 12.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 13.Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci U S A. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, et al. Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature. 2010;467:859–862. doi: 10.1038/nature09420. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Zhou J, Wang L, Shin ME, Su P, Lei X, Kuang H, Guo W, Yang H, Cheng L, et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J Cell Biol. 2010;191:631–644. doi: 10.1083/jcb.201006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohgushi M, Matsumura M, Eiraku M, Murakami K, Aramaki T, Nishiyama A, Muguruma K, Nakano T, Suga H, Ueno M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Walker A, Su H, Conti MA, Harb N, Adelstein RS, Sato N. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat Commun. 2010;1 doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda K, Kishi H, Ma X, Yu ZX, Adelstein RS. Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ Res. 2003;93:330–337. doi: 10.1161/01.RES.0000089256.00309.CB. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Jana SS, Conti MA, Kawamoto S, Claycomb WC, Adelstein RS. Ablation of Nonmuscle Myosin II-B and II-C Reveals a Role for Nonmuscle Myosin II in Cardiac Myocyte Karyokinesis. Mol Biol Cell. 2010;21:3952–3962. doi: 10.1091/mbc.E10-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao J, Ma X, Liu C, Adelstein RS. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem. 2007;282:22102–22111. doi: 10.1074/jbc.M702731200. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Ma X, Conti MA, Liu C, Kawamoto S, Adelstein RS. Nonmuscle myosin II isoform and domain specificity during early mouse development. Proc Natl Acad Sci U S A. 2010;107:14645–14650. doi: 10.1073/pnas.1004023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X, Takeda K, Singh A, Yu ZX, Zerfas P, Blount A, Liu C, Towbin JA, Schneider MD, Adelstein RS, et al. Conditional ablation of nonmuscle myosin II-B delineates heart defects in adult mice. Circ Res. 2009;105:1102–1109. doi: 10.1161/CIRCRESAHA.109.200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Yu ZX, Qian S, Chin TK, Adelstein RS, Ferrans VJ. Nonmuscle myosin II localizes to the Z-lines and intercalated discs of cardiac muscle and to the Z-lines of skeletal muscle. Cell Motil Cytoskeleton. 2000;46:59–68. doi: 10.1002/(SICI)1097-0169(200005)46:1<59::AID-CM6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Kawamoto S, Hara Y, Adelstein RS. A point mutation in the motor domain of nonmuscle myosin II-B impairs migration of distinct groups of neurons. Mol Biol Cell. 2004;15:2568–2579. doi: 10.1091/mbc.E03-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanger JW, Kang S, Siebrands CC, Freeman N, Du A, Wang J, Stout AL, Sanger JM. How to build a myofibril. J Muscle Res Cell Motil. 2005;26:343–354. doi: 10.1007/s10974-005-9016-7. [DOI] [PubMed] [Google Scholar]
- 29.Sun SX, Walcott S, Wolgemuth CW. Cytoskeletal cross-linking and bundling in motor-independent contraction. Curr Biol. 2010;20:R649–R654. doi: 10.1016/j.cub.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guha M, Zhou M, Wang YL. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–736. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 31.Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 32.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- 34.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 35.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafson-Wagner EA, Sinn HW, Chen YL, Wang DZ, Reiter RS, Lin JL, Yang B, Williamson RA, Chen J, Lin CI, et al. Loss of mXinalpha, an intercalated disk protein, results in cardiac hypertrophy and cardiomyopathy with conduction defects. Am J Physiol Heart Circ Physiol. 2007;293:H2680–H2692. doi: 10.1152/ajpheart.00806.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uren D, Hwang HK, Hara Y, Takeda K, Kawamoto S, Tullio AN, Yu ZX, Ferrans VJ, Tresser N, Grinberg A, et al. Gene dosage affects the cardiac and brain phenotype in nonmuscle myosin II-B-depleted mice. J Clin Invest. 2000;105:663–671. doi: 10.1172/JCI8199. [DOI] [PMC free article] [PubMed] [Google Scholar]