Abstract
Imatinib has an oral bioavailability >90% despite being monocationic under the acidic conditions in the duodenum. In vitro, we found that imatinib is transported by the intestinal uptake carrier OATP1A2, and that this process is sensitive to pH, rosuvastatin, and genetic variants. In humans, however, imatinib absorption was not associated with OATP1A2 variants, and was unaffected by rosuvastatin. These findings highlight the importance of verifying drug-transporter interactions from in vitro tests in a clinical setting.
Keywords: Imatinib, OATP1A2, pharmacogenetics, rosuvastatin
INTRODUCTION
Clinical use of the tyrosine kinase inhibitor imatinib is associated with a high interindividual pharmacokinetic variability. It was recently demonstrated that patients not responding to imatinib generally have lower systemic concentrations of imatinib compared with responding patients.1, 2 Furthermore, plasma levels of imatinib below a certain threshold value are possibly associated with a reduced response and survival.2, 3 Previous studies indicate that imatinib is almost completely absorbed and has an oral bioavailability >90%.4 This is somewhat unexpected considering the affinity of imatinib for a number of intestinal efflux transporters such as ABCB1 (Pgp) and ABCG2 (BCRP),5, 6 and the notion that under the acidic condition in the duodenum, imatinib is predominantly charged (~90% monocationic at pH 5–6).7 Such conditions suggest the involvement of facilitated uptake of imatinib across the intestinal wall by one or more carriers. Using preclinical studies, we previously identified imatinib as a substrate of the organic anion transporting polypeptide OATP1A2 (formerly, OATP-A, OATP1, OATP),8 which is expressed in duodenal enterocytes.9 Here, we further characterized imatinib transport by OATP1A2 in vitro and explored the hypothesis that imatinib absorption in humans is affected by (i) common genetic variants in the OATP1A2 gene, SLCO1A2, and (ii) concurrent administration of rosuvastatin, an inhibitor of OATP1A2.
RESULTS
Quantitative PCR analysis confirmed that the descending portion of the human duodenum showed the highest expression of OATP1A2 mRNA as compared to the other tissues involved in absorption of xenobiotics, including tongue, stomach, small intestine, colon and rectum (Figure 1A). Several additional human tissues were found to express significant levels of OATP1A2, the highest being in the spinal cord, intracranial artery, optic nerve, brain, lung, retina, uvula, and pituitary tissue (Figure 1A).
Figure 1.
(A) Expression of the OATP1A2 gene SLCO1A2 in a panel of 48 human tissues. (B) Uptake of [3H]imatinib by cRNA-injected Xenopus laevis oocytes at pH 7.4 (left) and 5 (right). * P = 0.01; ** P = 0.001; *** P = 0.0001. (C) Influence of rosuvastatin on OATP1A2-mediated transport of [3H]imatinib in Xenopus laevis oocytes.
In Xenopus laevis oocytes expressing the human OATP1A2 or rodent Oatp1a4, but not rodent Oatp1a1, the uptake of imatinib at physiological pH was significantly higher as compared to that observed in water-injected control oocytes (Figure 1B). Compared to pH 7.4 (1.5-fold vs control; P = 0.0008), a substantially increased uptake of imatinib by human OATP1A2 was seen at pH 5 (2.4-fold; P = 0.0001), and a similar pH-dependence of transport was seen with the Oatp1a4 cRNA-injected oocytes (Figure 1B). When OATP1A2 expressing oocytes were co-incubated with a mixture of imatinib and rosuvastatin (Figure 1C), significant inhibition of imatinib transport occurred at both pH 7.4 and pH 5 (P < 0.0001 at both pH values).
The uptake of imatinib in HeLa cells transiently transfected with 6 known OATP1A2 variants was reduced in 4 of the 6 variants, as compared to cells transfected with wildtype OATP1A2*1 (Figure 2A). Imatinib uptake was completely abolished in HeLa cells expressing the OATP1A2*3, OATP1A2*5, and OATP1A2*6 variants. In the case of OATP1A2*7, imatinib accumulation was reduced in a pH-dependent manner, with 77% reduction at pH 7.4 (P = 0.0001) and 46% reduction at pH 5 (P = 0.033), as compared to OATP1A2*1 at each respective pH value. As predicted based on the in vitro data, the average steady-state concentration of imatinib in 94 white patients was not significantly associated with OATP1A2*2 variant (38T>C) (Figure 2B). However, it is noteworthy that the only patient that carried two copies of this variant had a steady-state plasma concentration of imatinib that was below 1,000 ng/mL, previously identified as a threshold level associated with response to treatment.3 Although OATP1A2*3 was associated with complete lack of imatinib transport in vitro, patients that carried this variant did not have altered levels of imatinib (Figure 2C).
Figure 2.
(A) Uptake of [3H]imatinib in HeLa cells transfected with the OATP1A2 variants *2, *3, *4, *5, *6, and *7 at pH 7.4 (left) and pH 5 (right). (B, C) Steady-state plasma concentrations of imatinib in a cohort of 94 white cancer patients, carrying OATP1A2 variants (Var) at the 38 (B) and 516 (C) nucleoside positions versus patients carrying two copies of the reference (Ref) allele (left panels). Each symbol represents an individual patient, and horizontal lines denote the mean. The closed symbols represent the patients being homozygous for the variant allele.
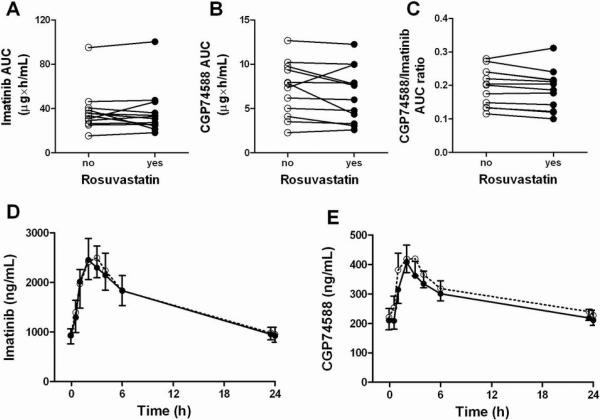
While rosuvastatin significantly inhibited OATP1A2-mediated transport of imatinib in vitro, concomitant administration of rosuvastatin had no influence on the steady-state pharmacokinetics of imatinib or its active metabolite CGP74588 in 12 patients (Figure 3). There was a trend towards lower systemic concentrations of CGP74588 (P = 0.15) when imatinib was administered with rosuvastatin (Supplementary Table 1). As a result, a lower CGP74588 to imatinib exposure ratio was seen in almost all patients, although this did not reach statistical significance (P = 0.065). Treatment-related toxicity was generally mild, with grade 2 edema and diarrhea observed in 1 patient, and grade 1 muscle cramps in 2 patients, and did not appear to be altered as a result of rosuvastatin co-administration (not shown).
Figure 3.
Individual paired areas under the plasma concentration time curves (AUC) of imatinib (A), CGP74588 (B), and CGP74588/imatinib AUC ratios (C) in 12 white cancer patients receiving concomitant administration of rosuvastatin (closed circles) or imatinib given alone (open circles). Average plasma concentration time profiles of imatinib (D) and CGP74588 (E) in the absence (open circles) and presence (closed circles) of rosuvastatin.
DISCUSSION
Over the last few years, a number of studies have established that transport of multiple drugs across the intestinal epithelium may be mediated by solute carriers, including the human organic anion transporting polypeptide OATP1A2. This protein is highly expressed in the intestine, kidney, cholangiocytes, the blood-brain barrier, and certain cancers,10 which localization suggests that OATP1A2 may be vitally important in the absorption, distribution and excretion of a broad array of clinically important drugs. In this study, we evaluated the possible relevance of this uptake transporter for the intestinal absorption of imatinib, a known substrate of this carrier.8 The current study complements previous knowledge on the interaction of imatinib with organic ion transporters, and provides further mechanistic insight into the role these proteins play in the pharmacokinetic profile of imatinib.
Using transfected Xenopus laevis oocytes we found that human OATP1A2, as well as its rodent ortholog Oatp1a4, transported imatinib in a pH-dependent manner, with increasing activity taking place at acidic pH, as found in the duodenum. This result is consistent with the finding that imatinib mainly occurs as a monocationic isomer at duodenal pH.7 A similar sensitivivity to extracellular pH has been previously reported for the in vitro transport of methotrexate by OATP1A2.11 Using transfected HeLa cells, we also found that the in vitro imatinib transport was completely absent or significantly reduced by several naturally occurring protein variants of OATP1A2.
Interestingly, the relevance of these genetic variants could not be confirmed using a pharmacogenetic-association study done in a group of 94 white cancer patients receiving treatment with imatinib. In light of the relatively few individuals studied with variant genotypes, however, the currently observed lack of significant relationships between the studied OATP1A2 variants and the steady-state pharmacokinetics of imatinib has relatively limited statistical power. It is also theoretically possible that additional genetic variants or haplotypes in OATP1A2 of importance to the pharmacokinetics of imatinib in this population are yet to be discovered, and/or that larger numbers of patients are needed to more precisely quantify genotype-phenotype associations. Nonetheless, in conjunction with the prior observation that common variants in the ABCB1, ABCG2, CYP2C9, CYP2C19, CYP2D6, CYP3A4, CYP3A5, and SLC22A1 (OCT1) genes have only a limited effect on the pharmacokinetics of imatinib,8, 12, 13 the current findings further support the possibility that intrinsic physiologic and environmental variables may have a more profound influence on the absorption and disposition of imatinib.
Based on these considerations, it was felt that understanding the possible contribution of OATP1A2 in drug interactions with imatinib absorption is of potential clinical significance, as recent data suggest that low circulating concentrations of imatinib may contribute to interindividual differences in clinical outcome.1, 2, 14, 15 In order to evaluate the impact of OATP1A2 inhibition on the pharmacokinetics of imatinib, a drug-drug interaction study was performed in patients concomitantly treated with imatinib and rosuvastatin. Unlike most statins, rosuvastatin is a hydrophilic compound16 that undergoes limited metabolism and is mainly excreted into bile as the unchanged drug.17, 18 After two weeks of concomitant rosuvastatin use, however, no significant differences in imatinib pharmacokinetics or toxicity were observed.
The collective results of this study illustrate the complications associated with translating pre-clinical pharmacological findings to the clinic. This recognition is particularly relevant in the context of the recent guidelines offered by The International Transporter Consortium regarding preclinical criteria needed to trigger the conduct of clinical studies to evaluate drug-transporter interactions.19 Theoretically, it is conceivable that the lack of a substantial effect of rosuvastatin on the steady-state concentrations of imatinib is the result of drug-drug interactions simultaneously taking place on multiple transporters (Supplementary Figure 1).8, 20, 21 For example, rosuvastatin is also known to interact with OATP1B3, expressed on the basolateral membrane of hepatocytes,22 which transporters also regulates, in part, the hepatocellular uptake of imatinib.8 The combination both of decreased intestinal and hepatic uptake transport by OATP1A2 and OATP1B3, respectively, could lead to a reduced oral bioavailability as well as to a reduced availability of imatinib for hepatic metabolism. These two mechanisms could balance each other out, thereby leaving systemic imatinib exposure unaltered. Indeed, we found a trend for reduced concentrations of the main CYP3A4-mediated metabolite of imatinib after two weeks of rosuvastatin dosing.
In conclusion, our results highlight the importance of verifying drug-transporter interactions from in vitro tests in a follow-up clinical study. The current study indicates that, although imatinib is a substrate for OATP1A2, this transporter by itself is unlikely to contribute substantially to the absorption profiles of imatinib in humans. Further study is warranted to determine the individual and collective contribution of additional, potentially redundant, intestinal carriers to the pharmacokinetics and pharmacodynamics of imatinib.
METHODS
Tissue expression of SLCO1A2
TissueScan Tissue qPCR Array plates (OriGene Technologies, Inc.) were used to assess expression of SLCO1A2 in 48 human tissues with a TaqMan Gene Expression Assay GEx probe 20× Mix for SLCO1A2 (BD Biosciences) on an Applied Biosystems 7900HT Detection System. The cDNAs are derived from a pool of at least 5 donors. Expression levels analyzed in triplicate were normalized to GAPDH.
In vitro transport studies
Uptake studies in Xenopus laevis oocytes transfected with OATP1A2 cRNA were performed with [3H]imatinib as described8 at various pH values in the presence and absence of the OATP1A2 inhibitor rosuvastatin (1 mM). The influence of SLCO1A2 variants at the 38T>C (*2), 516A>C (*3), 559G>A (*4), 382A>T (*5), 404A>T (*6), and 2003C>G (T668S, *7) loci on imatinib transport was evaluated in transiently transfected HeLa cells. These cells grown in 12-well plates (0.8×106 cells/well), infected with vaccinia in serum-free Opti-MEM I medium and allowed to adsorb for 30 min at 37°C. Cells in each well were then transfected with 1 μg of wildtype or variant SLCO1A2 cDNA packaged into a pEF6/V5-His-TOPO or pSPORT vector, along with Lipofectin, and incubated at 37°C for 16 h. The parental plasmid lacking any insert was used as vector control.
Pharmacogenetic association studies
DNA for genotyping was available from 94 white patients treated with daily oral imatinib. Pharmacokinetic parameters were determined at steady-state, as described.12 Genotypes of interest23 were determined in each sample using direct nucleotide sequencing of exon 1 (containing SLCO1A2 38T>C), exon 5 (containing SLCO1A2 502C>T and 516A>C), and exon 8 (containing SLCO1A2 968T>C and 1063A>G). The recorded genotype was termed “variant” if it differed from the reference sequence for the SNP position as acquired by GenBank. The SLCO1A2 variants at the 559G>A (*4), 382A>T (*5), 404A>T (*6), and 2003C>G (T668S, *7) loci were shown previously to have an allelic freqyency of <0.5%, and were not analyzed in the patient samples.24, 25
Clinical drug interaction studies
Twelve patients with a gastrointestinal stromal tumor were included in the interaction study and treated with daily oral imatinib dose of 400 mg for at least 4 weeks to guarantee steady-state conditions before entering the study. With this sample size, the probability is 91% that a difference in oral drug availability caused by concurrent administration of the OATP1A2 inhibitor rosuvastatin will be detected at a two-sided 5% significance level, if the true difference is 0.3 units. This is based on a within-patient standard deviation of the response variable of 0.2 units.
Additional eligibility criteria included age ≥ 18 years, WHO performance ≤ 1, and adequate hematological, renal and hepatic functions. The use of CYP3A4 and ABCB1 inhibiting or inducing medication and dietary supplements was prohibited. The study protocol was approved by the Erasmus University Medical Center review board, and all patients provided written informed consent before study entry. This clinical trial was registered at the Dutch trial registry (number NTR1504) and the European Clinical Trials Database (number 2008-002659-26). Blood samples for pharmacokinetic evaluation of imatinib and CGP74588 were taken during two 24-h periods at steady-state; one period in the absence of rosuvastatin (i.e., day 1) and one period 14 days after start of oral rosuvastatin at a dose of 20 mg/day (i.e, day 16). Plasma was isolated and analyzed for imatinib and CGP74588 by a validated method based on liquid chromatography-tandem mass spectrometry (see Supplementary Methods).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC) and US Public Health Service Cancer Center Support Grant 3P30CA021765.
We thank Peter de Bruijn, Mirjam Bakkes, and Gaia Schiavon for assistance with blood sample collection and analytical measurements.
None of the funding bodies had a role in the study design, data interpretation, or preparation of the manuscript.
This work was presented previously, in part, at the 110th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, National Harbor, MD (March 2009), and the 22nd EORTC-NCI-AACR symposium on `Molecular Targets and Cancer Therapeutics' held in Berlin, Germany (November 2010).
Footnotes
CONFLICT OF INTEREST The authors have no conflicts of interests to declare.
REFERENCES
- 1.Larson RA, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–8. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 2.Picard S, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–9. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 3.Demetri GD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27:3141–7. doi: 10.1200/JCO.2008.20.4818. [DOI] [PubMed] [Google Scholar]
- 4.Peng B, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–62. doi: 10.1177/0091270003262101. [DOI] [PubMed] [Google Scholar]
- 5.Hamada A, Miyano H, Watanabe H, Saito H. Interaction of imatinib mesilate with human P-glycoprotein. J Pharmacol Exp Ther. 2003;307:824–8. doi: 10.1124/jpet.103.055574. [DOI] [PubMed] [Google Scholar]
- 6.Burger H, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 7.Szakacs Z, Beni S, Varga Z, Orfi L, Keri G, Noszal B. Acid-base profiling of imatinib (gleevec) and its fragments. J Med Chem. 2005;48:249–55. doi: 10.1021/jm049546c. [DOI] [PubMed] [Google Scholar]
- 8.Hu S, et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–8. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 9.Glaeser H, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–70. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 10.Meyer zu Schwabedissen HE, Tirona RG, Yip CS, Ho RH, Kim RB. Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Res. 2008;68:9338–47. doi: 10.1158/0008-5472.CAN-08-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badagnani I, et al. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther. 2006;318:521–9. doi: 10.1124/jpet.106.104364. [DOI] [PubMed] [Google Scholar]
- 12.Gardner ER, et al. Association of enzyme and transporter genotypes with the pharmacokinetics of imatinib. Clin Pharmacol Ther. 2006;80:192–201. doi: 10.1016/j.clpt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Eechoute K, et al. Drug Transporters and Imatinib Treatment: Implications for Clinical Practice. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2250. [DOI] [PubMed] [Google Scholar]
- 14.Judson I, et al. Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 2005;55:379–86. doi: 10.1007/s00280-004-0876-0. [DOI] [PubMed] [Google Scholar]
- 15.Widmer N, et al. Imatinib plasma levels: correlation with clinical benefit in GIST patients. Br J Cancer. 2010;102:1198–9. doi: 10.1038/sj.bjc.6605584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McTaggart F, et al. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol. 2001;87:28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- 17.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19:117–25. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin PD, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25:2822–35. doi: 10.1016/s0149-2918(03)80336-3. [DOI] [PubMed] [Google Scholar]
- 19.International Transporter, C. et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36:2014–23. doi: 10.1124/dmd.108.021410. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34:738–42. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- 22.Ho RH, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Franke RM, Scherkenbach LA, Sparreboom A. Pharmacogenetics of the organic anion transporting polypeptide 1A2. Pharmacogenomics. 2009;10:339–44. doi: 10.2217/14622416.10.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee W, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–7. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- 25.Laitinen A, Niemi M. Frequencies of single-nucleotide polymorphisms of SLCO1A2, SLCO1B3 and SLCO2B1 genes in a Finnish population. Basic Clin Pharmacol Toxicol. 2011;108:9–13. doi: 10.1111/j.1742-7843.2010.00605.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.