Abstract
The biguanide metformin is widely used for the treatment of type II diabetes. Its anti-proliferative and pro-apoptotic effects in various tumor cells suggest its potential candidacy for cancer chemoprevention. Here we report that metformin significantly inhibited human epidermoid A431 tumor xenograft growth in nu/nu mice, which was associated with a significant reduction in proliferative biomarkers PCNA and cyclins D1/B1. This tumor growth reduction was accompanied by the enhanced apoptotic cell death and an increase in Bax:Bcl2 ratio. The mechanism by which metformin manifests anti-tumor effects appears to be dependent on the inhibition of nuclear factor kappa B (NFkB) and mTOR signaling pathways. Decreased phosphorylation of NFkB inhibitory protein IKBα together with reduced enhancement of NFkB transcriptional target proteins, iNOS/COX-2 were observed. In addition, a decrease in the activation of ERK/p38-driven MAP kinase signaling was seen. Similarly, AKT signaling activation as assessed by the diminished phosphorylation at Ser473 with a concomitant decrease in mTOR signaling pathway was also noted as phosphorylation of mTOR regulatory proteins p70S6K and 4E-BP-1 was significantly reduced. Consistently, decreased phosphorylation of GSK3β which is carried out by AKT kinases was also observed. These results suggest that metformin blocks SCC growth by dampening NFkB and mTOR signaling pathways.
Keywords: Metformin, anti-diabetic agents, mTOR, skin, UVB, NFκB
Introduction
Metformin, a biguanide is among the most commonly prescribed glucose-lowering chemical agents, with proven efficacy and limited side effects (1). Retrospective epidemiological studies provide evidence that type II diabetic patients receiving metformin have substantially lower cancer incidence and mortality than those on other treatments (2, 3). Studies also suggest that metformin may inhibit neoplastic growth in experimental animal models including those of colon, breast, prostate and lung cancers (4–7). In these studies besides the known effects of metformin on AMP-activated protein kinase (AMPK), the tumor growth regulatory effects were associated with a reduction of the mTOR signaling pathway (8). The prototypic mechanism of mTOR regulation in mammalian cells occurs through the activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway, but mTOR receives input from multiple other signaling pathways, including the liver kinase β1 (LKB1)/AMPK pathway (9, 10). The activation of PI3K/Akt/mTOR pathway axis mediates novel crosstalk between insulin receptor (IR) and insulin receptor substrate (IRS-1), followed by activation of protein kinase B (PKB)/Akt, and translocation of GLUT4 glucose transporters to the plasma membrane (11, 12).
The pathogenesis of non-melanoma skin cancer (NMSC), the most common human neoplasm, involves disruption of multiple pathways including activation of the mTOR signaling pathway (13). The incidence of NMSCs is increased in organ transplant recipients (OTRs) to about 100–250 fold of the general population. In OTRs, patients receiving rapamycin, an mTOR inhibitor, manifest a significant reduction in the incidence of these cancers (13). In addition, rapamycin also reduces skin cancer growth in murine models (13). Based on this, we tested whether metformin administration retards the growth of cutaneous SCCs in a human tumor xenograft highly immunosuppressed nu/nu murine model. In this study we employed human A431 epidermoid carcinoma cells for developing xenograft tumors. A431 cells carry UVB signature mutations in p53 and possess amplified EGFR signaling conforming to the UVB-dependent etiology of human cutaneous SCCs. Our results suggest that metformin administration reduces the growth of human cutaneous xenograft SCCs which is associated with a diminution in mTOR and AKT signaling pathways activation. The enhancement in AMPK expression and reduction in the expression of NFkB transcriptional targets iNOS/COX-2 and cell cycle regulatory proteins were also associated with tumor growth reduction.
Materials and Methods
Reagents and Antibodies
Metformin (>99% pure) used in this study was purchased from LKB laboratories, Inc. The primary antibodies purchased were as follows: Cyclin B1, cdc2, p38, p-Akt1/2/3(Ser473), Akt1/2/3, p-GSK3β, GSK3β from Santa Cruz Biotechnology Inc, (Santa Cruz, CA); GLUT1 and GLUT4 from Abcam; COX-2 from Cayman chemical; Cyclin D1 from Neomarkers; and Bax, Bcl-2 (C-21), iNOS, P44/42 MAPK (ERK1/2), p-ERK, p-p38, p-PI3K 85Kda, PI3K 85Kda, PI3K 110Kda, mTOR, mTOR (Ser 2448), mTOR (Ser 2481), p-p70S6 kinase, p70S6 kinase, p-4E-BP-1, 4E-BP-1, p-AMPK and AMPK from Cell Signaling Technology, (MA).
Cell line
Human epidermoid carcinoma, A431cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 µg/ml of streptomycin in a humidified atmosphere of 5% CO2 / 95% air at 37°C.
Tumor xenograft study
Female athymic NCr-nu/nu mice (3–5 weeks old; 25–30g) were purchased from NCI-Frederick Animal Production Program (Frederick, MD, USA). Animals were housed under standard conditions (fluorescent lighting, 12 hours/day; room temperature, 23–25°C; and relative humidity, 45%–55%). All experimental protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Animals were divided into two groups of 6 mice each. Each mouse from both groups received 5X106 cells in 200 µl of PBS subcutaneously in both flanks. Starting 24 hours post-tumor cell inoculation, group 1 mice received an injection of vehicle (PBS) whereas group 2 received metformin (5 mg/mouse in PBS, I.P.) daily 5 days/week for three weeks. Tumors >1mm in diameter were measured by digital calipers thrice weekly and tumor volumes calculated using the formula volume = length X width X height, plotted as a function of days on test. At the termination of the experiment, mice were sacrificed and tumors were harvested for analysis.
Tumor xenograft histology and immunohistochemistry
All tumor xenograft tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 5-µm sections. Tissue slides were stained with hematoxylin and eosin (H&E) for histology. PCNA staining of formalin-fixed tumor tissue was performed by Vectastain ABC kit (Vector Laboratories, Inc. CA) as per manufacturer’s instructions. Sections were counterstained with Harris hematoxylin (Sigma-Aldrich), dehydrated and mounted using Permount (Fisher Scientific).
Terminal deoxynucleotidyl transferase–mediated nick end labeling (TUNEL)
TUNEL assay in tumor xenograft tissue was performed using in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instruction. Sections were counterstained with DAPI and mounted.
Western blot analysis
The tumor tissue was homogenized in ice cold lysis buffer (50mM Tris pH 7.5, 1% Triton X-100, 0.25% NaF, 10mM β-glycerol phosphate, 1mM EDTA, 5mM sodium pyrophosphate, 0.5mM Na3VO4, 10mM DTT, 1% PMSF, and protease inhibitors). The homogenate was centrifuged at 13,000g for 20 minutes at 4 °C and then the supernatant was aliquotted and stored at −80°C. For western blot, 40–80µg proteins were resolved on 8–12% polyacrylamide gel (BioRad, CA, USA). The proteins were transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% non-fat milk in Tris-buffered saline with 0.1% Tween-20 (TBST) and then the membranes were incubated with primary antibody overnight at 4°C. After washing with TBST the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibody (Pierce, Rockford, IL, USA) for 1 hour. The immune-complex was detected with chemiluminescent substrate (Pierce, Rockford, IL, USA) and was exposed to HyBlot CL autoradiography film (Denville Scientific Inc, NJ, USA). Membranes were then stripped and re-probed with β-actin antibody to verify equal protein loading. In instances where a blot is stripped multiple times and probed with different antibodies but the data are presented as a part of more than one figure, the same β-actin image was placed at the bottom of these different figures. Relative density of western blot bands was analyzed by using IMAGE J software downloaded from http://rsbweb.nih.gov/ij/.
Statistical analysis
Statistical analysis was performed using Microsoft Excel software. The significance between two test groups was determined using Student’s t test. A p- value of <0.05 was considered to be significant.
Results
Metformin inhibits growth and cell cycle regulatory proteins in human epidermoid A431 xenograft tumors
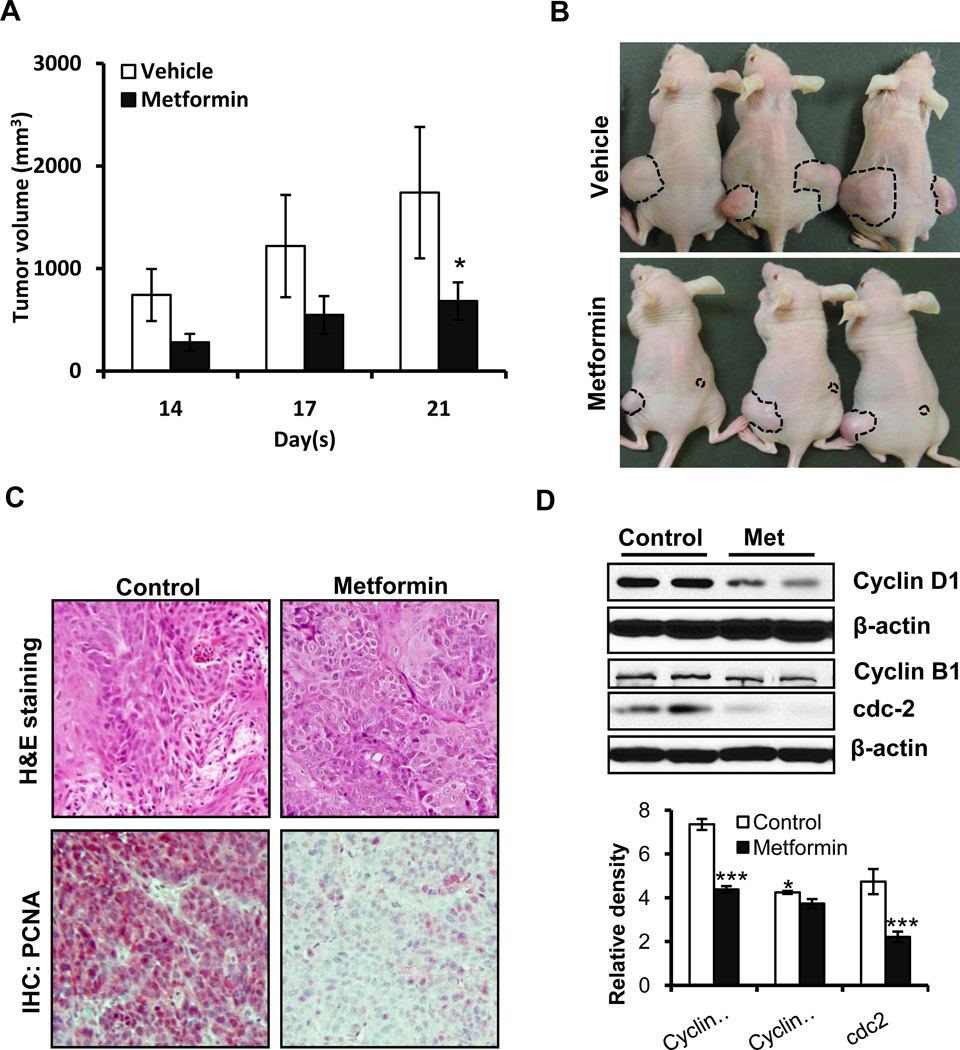
We assessed whether metformin inhibits the growth of A431 human epidermoid tumor xenografts in nu/nu mice. These animals were implanted with A431 cells and divided into two cohorts receiving vehicle or metformin. Treatment with metformin significantly reduced the development of xenograft tumors in these highly immunosuppressed mice. As shown in Figure 1A&B, tumor volumes were significantly smaller on days 3 to 21. At termination of the experiment, tumor volume in metformin-treated mice was reduced by 60.8%. The mean tumor volume in metformin-treated mice was 682.6±183.0mm3 as compared to 1741.2±641.2mm3 in vehicle-treated controls (p<0.05). No significant difference in the body weights of mice treated with metformin or vehicle was observed (data not shown). Tumors developed in metformin-treated animals and in vehicle-treated controls had similar histology as seen in their H&E staining (Figure 1C). However, metformin treatment reduced the expression of proliferation-related biomarkers. Proliferation cell nuclear antigen (PCNA) expression as assessed by immunohistochemistry as shown in Figure 1C. Similarly, the G1-associated cyclin D1 and G2/M progression-associated cyclin B1 and its partner kinase cdc2 were decreased significantly in the metformin-treatment group as compared to controls (Figure 1D).
Figure 1. Metformin reduces SCC growth by dampening cell cycle progression and blocking proliferation.
Each mouse was subcutaneously injected with 5×106 cells in PBS on both flanks. Two days later, either vehicle (150 µl) or metformin (5mg/mouse in 150 µl PBS; I.P.) was administered every day for 5 days/week for three weeks. (A) Average tumor volume (mm3) ±SEM/mouse; (B) representative pictures of mice showing xenograft tumors. The A431 tumor xenograft tissues were harvested at the termination of the experiment. Tumor lysates were subjected to western blot analysis; (C) H&E staining and immunohistochemical analysis of proliferation marker PCNA in paraffin-fixed tumor tissue sections. Metformin treatment resulted in a reduction in the number of PCNA-positive cells (magnification 20×); (D) the expression levels of cyclin D1, cyclin B1, and cdc2 from different xenograft tumor groups. Relative density of bands was analyzed using Image J software, normalized to the respective β-actin band intensities to account for sample loading variation, and shown as a bar graph. Statistical significance of difference between control and metformin groups was analyzed by student’s t-test. β-actin was used to confirm equal loading of the samples. *p<0.05 and ***p<0.001.
Metformin induces apoptosis and enhances Bax:Bcl2 ratio in xenograft human SCCs
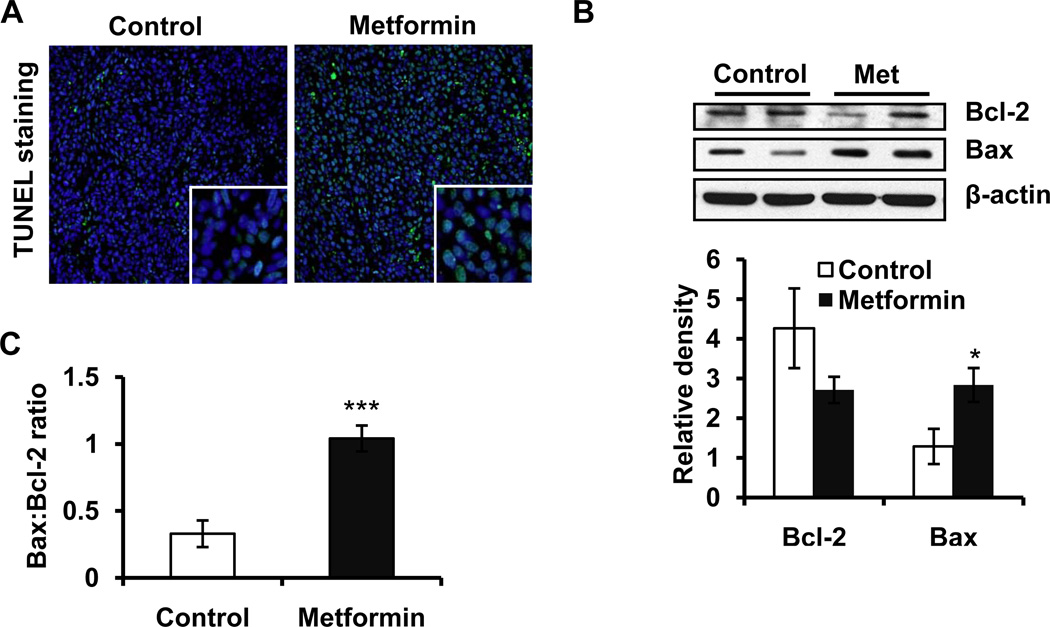
We assessed the induction of apoptosis using TUNEL assay. The number of TUNEL-positive cells was greater in metformin-treated tumors as compared to vehicle-treated control tumors (Figure 2A). The expression of anti-apoptotic Bcl2 and pro-apoptotic Bax as assessed by western blot analysis favored the apoptotic response (Figure 2B). The ratio of Bax:Bcl2 is detrimental to the life/death signal following apoptotic stimuli which was significantly increased (p<0.001) in metformin-treated tumors(Figure 2C).
Figure 2. Metformin enhances Bax:Bcl2 ratio and apoptosis as evidenced by the accumulation of TUNEL-positive cells in metformin-treated SCCs.
(A) Effect of metformin on the number of TUNEL-positive cells in tumor xenograft tissue harvested at the termination of experiment; (B) Western blotting showing expression of pro-apoptotic Bax and anti-apoptotic Bcl2; (C) Bax:Bcl2 ratio was calculated by using densitometric analysis data and expressed as mean±SE of three individual values. β-actin was used to confirm equal loading of the samples. Relative density of bands was analysed using Image J software, normalized to the respective β-actin band intensities to account for sample loading variation, and shown as a bar graph. *p<0.05 and ***p<0.001.
Metformin targets NFkB and MAPK signaling pathways
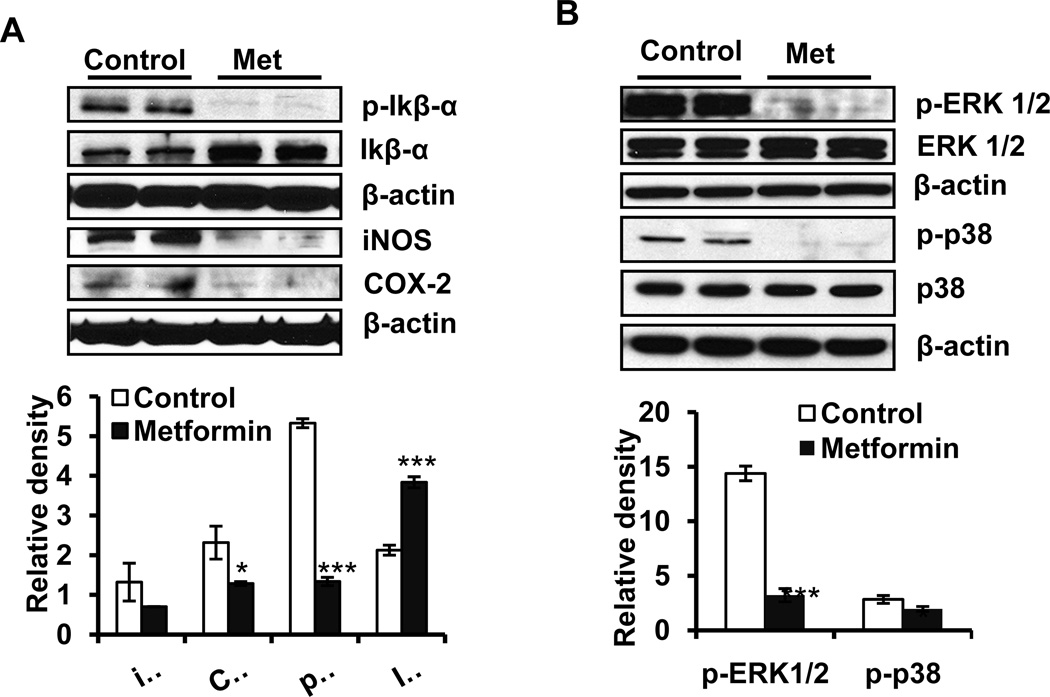
NFkB is a transcription factor which is known to regulate both proliferation and apoptosis (14). This transcription factor, when inactive, resides in the cytoplasm as a heterotrimeric complex comprised of p50/p52, p65 and inhibitory kappa B (IkB). Upon phosphorylation of IkB through the activation of upstream kinases, this complex is disrupted. Dissociated p-IkB is ubiquitinated and degrades while the remaining heterodimeric complexes comprised of p50-p65 and p52-p65 are translocated to the nucleus to perform transcription functions (15). In this study although we have not evaluated NFkB signaling following metformin treatment in depth, the phosphorylation status of IkBα and expression of NFkB transcription target proteins iNOS and COX-2 were assessed. A significant decrease in the expression of p-IkBα with a concomitant increase in IkBα was observed suggesting a reduction in NFkB activation. This was further confirmed by the significant decrease in the expression of iNOS and COX-2 (Figure 3A). Mitogen-activated protein kinase (MAPK) signaling cascade is also a target of NFkB signaling. MAPKs are serine/threonine kinases that participate in regulating various cellular responses, such as cell proliferation and apoptosis during the pathogenesis of skin cancer (16 and therein). The effects of metformin on the phosphorylation-dependent activation of ERK1/2 and p38 in A431 tumor xenografts are shown in Figure 3B. ERK1/2 and p38 phosphorylation were reduced by 77.7% and 32.1%, respectively (p<0.05) in the metformin treatment group (Figure 3B).
Figure 3. Metformin targets NFκB and MAPK signaling pathways.
The A431 tumor xenograft tissues were harvested at the termination of the experiment, and tumor lysates were subjected to analyses of phosphorylation of ERK1/2 and p38 protein using western blot analysis. (A): the expression levels of p-Ikbα, Ikbα, iNOS and COX-2 proteins; (B): the phosphorylated forms of ERK1/2 and p38. β-actin was used to confirm equal loading of the samples. *p<0.05 and ***p<0.001.
Metformin modulates PI3k/Akt/mTOR signaling proteins
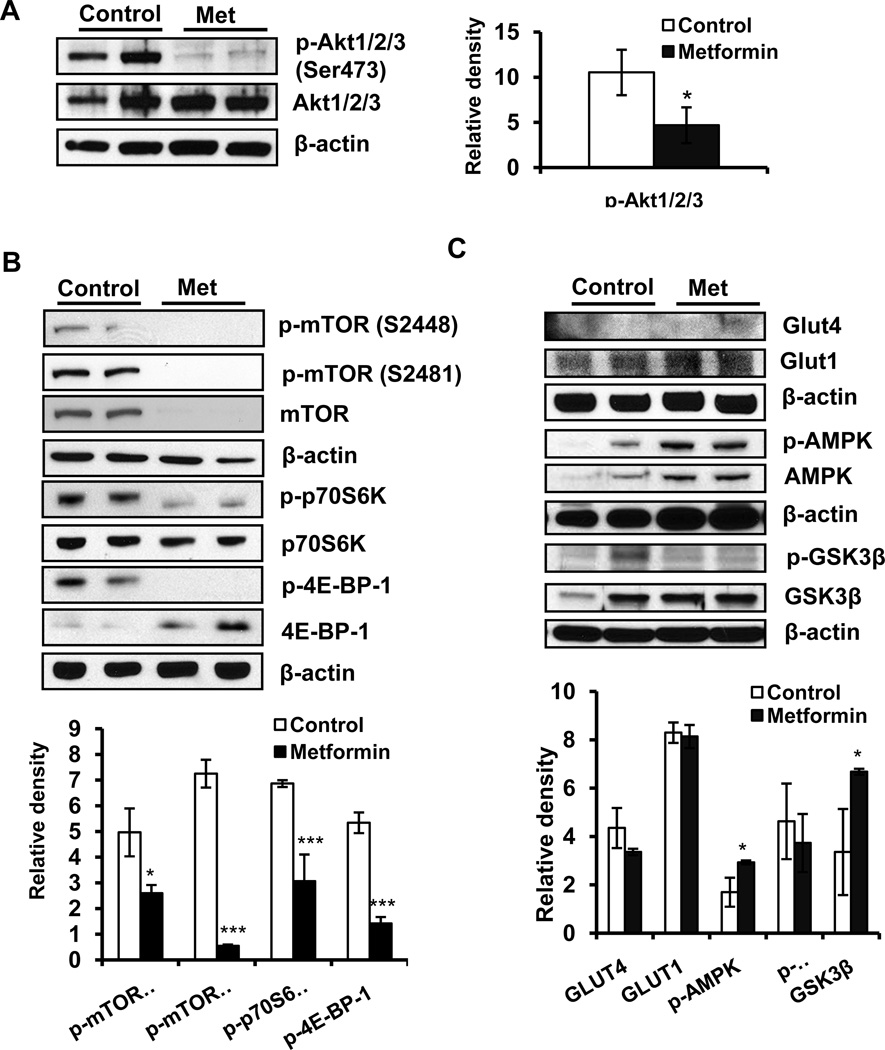
PI3K/Akt/mTOR signaling pathway is known to be activated by physiologic sensors of nutrients, regulating metabolism and tumor growth (17). Studies show that enhanced phosphorylation of mTOR (Ser2448), p70S6K (Thr389), 4E-BP-1 (Ser65 and Thr37/46) and Akt (Thr308, Ser473) induce tumor cell proliferation and growth (13). In this study, metformin treatment significantly reduced the phosphorylation of mTOR at S2448 and S2481, p70S6K, 4EBP1 and Akt at Ser473 (Figure 4 A and B). However, it did not alter the expression of p-PI3k (p85), PI3k (p85) and PI3k (p110) significantly (data not shown). Consistently, the phosphorylation of GSK3β was also reduced. Metformin treatment also activated AMPK, although no significant effects could be discerned on glucose regulatory GLUT1/4 proteins (Figure 4 C).
Figure 4. Metformin inhibits mTOR and AKT signaling activation while enhancing AMPK expression.
Western blot analysis of the metformin–treated or vehicle-treated A431 tumor xenograft tissue (A): p-AKT1/2/3 (Ser473) and AKT1/2/3 expression; (B): p-mTOR (S2448), p-mTOR (S2481), mTOR, p-p70S6 kinase, p70S6 kinase, p70S6 kinase, p-4EBP1 and 4EBP1; (C): expression levels of GLUT1/4, GSK3β, p-AMPK, AMPK. Relative density of bands was analyzed using Image J software, normalized to the respective β-actin band intensities to account for sample loading variation, and shown as a bar graph. *p<0.05 and ***p<0.001.
Discussion
Insulin and insulin receptors-dependent signaling is known to be involved in the development of various cancers of epithelial and non-epithelial origins (18–20). Based on these observations, various approaches targeting insulin receptors-dependent signaling were developed for the therapeutic intervention of these neoplasm (21, 22). The observations that patients receiving metformin manifest lower cancer incidence led us to investigate whether metformin can reduce the growth of cutaneous neoplasm in an experimental animal model system. Consistent with this notion, we found that the growth of human epidermoid carcinoma A431 xenograft tumors was significantly reduced by metformin treatment. Metformin acts by inhibiting tumor cell proliferation and reducing cell cycle progression. These responses are similar to those reported for other xenograft tumors developed by inoculating multiple human carcinoma cells in immuno-deficient mice (2, 23, 24,). The inhibition in tumor growth was accompanied by an increase in Bax/Bcl2-regulated apoptosis signaling and inhibition of activated MAP kinase, ERK1/2 and p38 proteins. Metformin-mediated inhibition in MAP kinase activity has also been shown in pancreatic and lung cancer cells (25, 26). These data suggest that MAPK signaling is a target for metformin-mediated diminution of cancer cell growth.
NFkB proteins participate in cellular growth control and neoplasia by regulating transcription of multiple genes involved in these processes (14,15). Metformin-mediated decrease in IκBα phosphorylation which was accompanied by diminished levels of iNOS and COX-2, suggest a role of metfomin in inhibiting the transcriptional activation of NFkB in SCCs as well as in the inhibition of inflammation regulatory protein expression. Donnini et al. reported a similar inhibition of iNOS and COX-2 associated with a reduction in SCC tumor growth (27). In a previous study we also showed that COX-2 inhibitors reduce UVB-induced skin carcinogenesis (28, 29).
In many of tumor cell-types, the PI3k/Akt signaling pathway is known to promote cell proliferation, cell cycle progression and to reduce apoptosis (30). In the skin, a mechanism by which Akt augments UVB-induced carcinogenesis involves mTOR activation (13). mTOR functions as an intracellular physiologic sensor of nutrients, regulating metabolism, cell division and growth (31, 32). The subunit composition of mTOR complex1 (mTORC1) regulates mRNA translation initiation and progression through p70S6 kinase 1 (p70S6K) and eIF4E-binding protein-1 (4E-BP1), thus controlling the rate of protein synthesis (32, 33). The observations in this study that metformin diminishes phosphorylation of Akt with a concomitant decrease in phosphorylated mTOR and their downstream substrates, suggest an inhibition of Akt/mTOR pathways in metformin-mediated tumor suppression.
It is known that metformin stimulates AMPK (34), which is a heterotrimeric complex composed of the catalytic kinase α subunit and two associated regulatory subunits, β and γ (35). Activated AMPK phosphorylates a number of proteins resulting in a decrease in ATP-consuming processes and an increase in ATP production through the protein synthesis, fatty acid and glucose metabolism inhibition and enhancement of glucose transport (12, 36). In addition, AMPK inhibits the activity of mTOR via tuberous sclerosis (TSC2/TSC1) tumor suppressor complex and by the direct phosphorylation of raptor (regulatory associated protein of mTOR) resulting in the disruption of its association with mTOR (37). Our observations that metformin activated AMPK in A431 tumor xenografts are consistent with other studies suggesting that it acts by turning off mTOR activity. The importance of these signaling pathways in the pathogenesis of cutaneous neoplasm is clear from a number of studies. Gurumurthy et al. provided early evidence for the importance of LKB1 in skin carcinogenesis (38). LKB1 is a central regulator of cell polarity and energy metabolism and acts via AMPK. These authors showed that LKB+/− mice are highly sensitive to two-stage DMBA/TPA-induced cutaneous chemical carcinogenesis (38). In an independent study Amornphimoltham et al. showed that rapamycin exerts remarkable anti-tumor activity in a chemically-induced skin cancer model (39). These studies demonstrate that rapamycin by causing a rapid decrease in the phosphorylation status of mTOR targets induces apoptotic death of cancer cells (39). Similarly, rapamycin was also shown to inhibit TPA-mediated tumor promotion in murine skin (40). Recent studies also show that deletion of a developmental transcription factor and tumor suppression Grhl3 in adult epidermis evokes loss of its direct target protein PTEN and leads to the pathogenesis of aggressive SCCs, which is accompanied by the activation of PI3k/Akt/mTOR (41). In a human epidermal tumor analysis (actinic keratosis, Bowen's disease and SCCs), constitutive activation of the Akt/mTOR pathway was frequent (42).
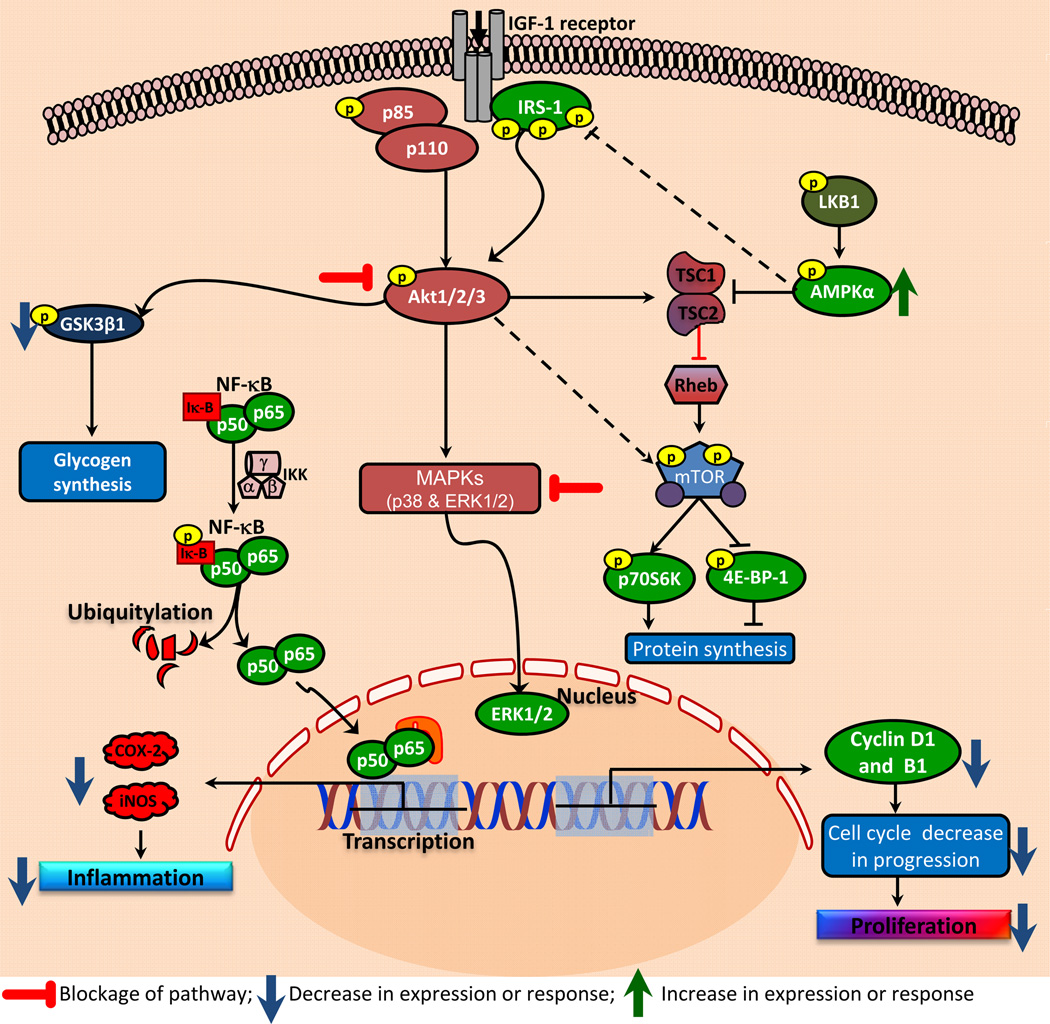
In summary, we showed that metformin administration reduces growth of human cutaneous xenograft SCCs in nu/nu mice. This reduction in the tumor growth was associated with the diminution in mTOR/Akt signaling pathway activation and enhancement in AMPK expression. This was also associated with the reduction in the expression of NFκB-mediated transcriptional target proteins, iNOS and COX-2 and cell cycle regulatory proteins as summarized in Fig. 5. These studies suggest that metformin may be an important chemopreventive agent for blocking NMSC development in humans.
Figure 5. Flow diagram showing the molecular mechanism of metformin-mediated reduction in SCC growth.
Metformin targets multiple signaling pathways to reduce the growth of cutaneous SCCs in a xenograft murine model system. The major pathways blocked by metformin treatment include mTOR, NFkB, Akt and MAP kinases. Metformin activates AMPK which blocks mTOR signaling while inhibition in NFkB pathway reduces proliferation via blocking activation of genes involved in cell cycle progression and enhancing genes that augment apoptosis.
ACKNOWLEDGMENTS
This work has been supported in part by N01-CN-43300 and R01CA138998 to Dr. Athar.
References
- 1.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 2.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 3.Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res (Phila) 2010;3:1060–1065. doi: 10.1158/1940-6207.CAPR-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1655. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomic T, Botton T, Cerezo M, Robert G, Luciano F, Puissant A, Gounon P, Allegra M, Bertolotto C, Bereder JM, Tartare-Deckert S, Bahadoran P, Auberger P, Ballotti R, Rocchi S. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2:e199. doi: 10.1038/cddis.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taubes G. Cancer research. Cancer prevention with a diabetes pill? Science. 2012;335:29. doi: 10.1126/science.335.6064.29. [DOI] [PubMed] [Google Scholar]
- 8.Li D. Metformin as an antitumor agent in cancer prevention and treatment. Journal of diabetes. 2011;3:320–327. doi: 10.1111/j.1753-0407.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiang GG, Abraham RT. Targeting the mTOR signaling network in cancer. Trends Mol Med. 2007;13:433–442. doi: 10.1016/j.molmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casaubon L, Sajan MP, Rivas J, Powe JL, Standaert ML, Farese RV. Contrasting insulin dose-dependent defects in activation of atypical protein kinase C and protein kinase B/Akt in muscles of obese diabetic humans. Diabetologia. 2006;49:3000–3008. doi: 10.1007/s00125-006-0471-5. [DOI] [PubMed] [Google Scholar]
- 12.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athar M, Kopelovich L. Rapamycin and mTORC1 inhibition in the mouse: skin cancer prevention. Cancer Prev Res (Phila) 2011;4:957–961. doi: 10.1158/1940-6207.CAPR-11-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 16.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 17.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, Naing A, Falchook GS, Moroney JW, Piha-Paul SA, Wheler JJ, Moulder SL, Fu S, Kurzrock R. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 19.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovannucci EL, Fuchs CS. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis DA, Travers JB, Somani AK, Spandau DF. The IGF- 1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene. 2010;29:1475–1485. doi: 10.1038/onc.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atzori F, Tabernero J, Cervantes A, Prudkin L, Andreu J, Rodriguez-Braun E, Domingo A, Guijarro J, Gamez C, Rodon J, Di Cosimo S, Brown H, Clark J, Hardwick JS, Beckman RA, Hanley WD, Hsu K, Calvo E, Rosello S, Langdon RB, Baselga J. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 23.Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, Hall H, Zhang D, Lu Y, Ravoori M, Kundra V, Ajani J, Lee JS, Ki Hong W, Mills GB. Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther. 2011;10:2350–2362. doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, Kobara H, Mori H, Himoto T, Okano K, Suzuki Y, Murao K, Masaki T. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012 doi: 10.1158/1535-7163.MCT-11-0594. [DOI] [PubMed] [Google Scholar]
- 25.Wang LW, Li ZS, Zou DW, Jin ZD, Gao J, Xu GM. Metformin induces apoptosis of pancreatic cancer cells. World J Gastroenterol. 2008;14:7192–7198. doi: 10.3748/wjg.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu N, Gu C, Gu H, Hu H, Han Y, Li Q. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma. 2011;58:482–490. doi: 10.4149/neo_2011_06_482. [DOI] [PubMed] [Google Scholar]
- 27.Donnini S, Finetti F, Solito R, Terzuoli E, Sacchetti A, Morbidelli L, Patrignani P, Ziche M. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J. 2007;21:2418–2430. doi: 10.1096/fj.06-7581com. [DOI] [PubMed] [Google Scholar]
- 28.Singh T, Chaudhary SC, Kapur P, Weng Z, Elmets CA, Kopelovich L, Athar M. Nitric Oxide Donor Exisulind Is an Effective Inhibitor of Murine Photocarcinogenesis. Photochem Photobiol. 2012 doi: 10.1111/j.1751-1097.2012.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang X, Kim AL, Kopelovich L, Bickers DR, Athar M. Cyclooxygenase-2 inhibitor nimesulide blocks ultraviolet B-induced photocarcinogenesis in SKH-1 hairless mice. Photochem Photobiol. 2008;84:522–527. doi: 10.1111/j.1751-1097.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 30.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 31.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 32.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2010;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 34.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 36.Hardie DG. AMPK and SNF1: Snuffing Out Stress. Cell Metab. 2007;6:339–340. doi: 10.1016/j.cmet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurumurthy S, Hezel AF, Sahin E, Berger JH, Bosenberg MW, Bardeesy N. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res. 2008;68:55–63. doi: 10.1158/0008-5472.CAN-07-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amornphimoltham P, Leelahavanichkul K, Molinolo A, Patel V, Gutkind JS. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin Cancer Res. 2008;14:8094–8101. doi: 10.1158/1078-0432.CCR-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol- 13-acetate. Cancer Prev Res (Phila) 2011;4:1011–2020. doi: 10.1158/1940-6207.CAPR-10-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT, Pandolfi PP, Pearson RB, Jane SM. Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell. 2011;20:635–648. doi: 10.1016/j.ccr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Chen SJ, Nakahara T, Takahara M, Kido M, Dugu L, Uchi H, Takeuchi S, Tu YT, Moroi Y, Furue M. Activation of the mammalian target of rapamycin signalling pathway in epidermal tumours and its correlation with cyclin-dependent kinase 2. The British journal of dermatology. 2009;160:442–445. doi: 10.1111/j.1365-2133.2008.08903.x. [DOI] [PubMed] [Google Scholar]