Abstract
The aim of this study was to evaluate the impact of preoperative devascularization of spinal metastases in relation to the preembolization tumor vascularization degree and in relation to the intraoperative blood loss. Twenty-four patients underwent preoperative transarterial embolization of hypervascular spinal metastases. Each tumor was assigned a vascularization grade (I–III) according to tumor blush after contrast agent injection in the main feeding artery. Embolization was performed with polyvinyl alcohol particles in all patients. Surgical reports were reviewed in terms of estimated blood loss. A mild hypervascularization was found in three patients (group I), medium in six patients (group II) and extensive in 15 patients (group III). In 22 out of 24 patients embolization could be performed with a complete devascularization. In two patients, only partial embolization could be performed, due to the main feeding artery arising from the artery of Adamkiewicz. In patients with complete devascularization the mean intraoperative blood loss was 1,900 ml, whereas in the two patients who were not embolized it was 5,500 ml. Intraoperative blood loss was not correlated to the vascularization grade. Angiography and embolization could be performed in all patients without causing permanent neurologic deficit, skin or muscle necrosis. The surgeons concluded that radical tumor resection after embolization was facilitated. Intraoperative blood loss is not correlated with the pre-interventional vascularization degree, if complete devascularization can be achieved with embolization. Preoperative embolization of vertebral hypervascular tumors is safe, effective and facilitates tumor resection.
Keywords: Spine, Metastases, Embolization, Interventional procedures, Blood loss
Introduction
Spinal neoplastic lesions of metastatic origin severely affect patient quality of life. Metastasizing tumors show spinal manifestation in 30–70% of cases [2]. Most common primary tumors of vertebral metastases are localized in the breast, lung, prostate and kidney. Surgical intervention with corporectomy, body replacement and stabilization, although controversial, is still the best treatment option for spinal instability, neurological complications and severe pain that cannot be managed conservatively or by local radiation [8, 9]. However these extensive surgical procedures are often complicated by massive intraoperative blood loss. Compared with normal vertebrae, tumorous vertebrae show extensive vascularization. The most highly vascularized vertebrae are those with metastases originating from renal cell and thyroid carcinoma [7]. Life-threatening blood losses have been described in cases without preoperative embolization [3, 6]. Several reports described technical aspects of vertebral tumor embolization [1, 7] and showed the efficacy of preoperative embolization on perioperative blood loss [3, 4, 10].
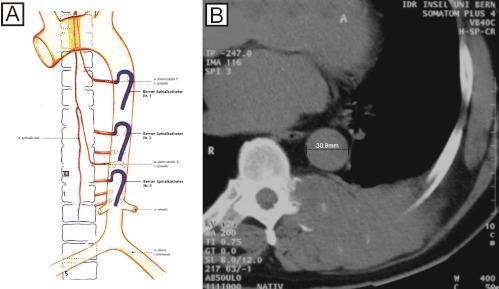
In this paper we report our personal experience with the embolization of hypervascular vertebral tumors in a series of 24 patients. The safety of preoperative embolization and the tumor resectability in the surgeons’ appreciation is evaluated. We analyze the impact of the devascularization of the lesion in relation to the pre-interventional degree of tumor hypervascularization and in relation to the intraoperative blood loss. Furthermore, we describe the use of the “Berner Spinalkatheter I-III,” a guiding catheter that was designed to obtain optimal position in the aorta abdominalis.
Materials and methods
Patient selection
We retrospectively reviewed the records of 24 patients (eight females and 16 males, mean age 64 years, range: 21–80 years, Table 1) with spinal hypervascular metastases who had undergone preoperative angiography for transarterial embolization. In 14 patients, the primary neoplasm was a renal cell carcinoma, and in four patients it was thyroid cancer. In six patients, the primary tumors were of various origins (paraganglioma, phäochromocytoma, urothelial carcinoma, melanoma, esophageal carcinoma and bronchial carcinoma). The presenting symptom was back pain in 16 patients (66.7%), neurological deficit due to spinal cord compression and nerve root compression in eight patients (33.3%).
Table 1.
Patient data (TS thoracic spine, LS lumbar spine, SS sacral spine, CS cervical spine
| Patient | Age | Sex | Primary tumor | Localization | Embolization degree | Time between embolization and surgery (days) | Vascularization degree |
|---|---|---|---|---|---|---|---|
| 1 | 76 | m | Renal cell carcinoma | TS | Partial embolization | 1 | III |
| 2 | 76 | f | Renal cell carcinoma | LS | Embolization | 1 | III |
| 3 | 77 | m | Renal cell carcinoma | LS | Embolization | 1 | III |
| 4 | 27 | m | Phäochromocytoma | TS | Embolization | 0 | III |
| 5 | 44 | f | Glomus tumor | SS | Embolization | 2 | III |
| 6 | 71 | m | Renal cell carcinoma | TS | Embolization | 1 | II |
| 7 | 79 | f | Renal cell carcinoma | CS | Embolization | 1 | III |
| 8 | 73 | m | Renal cell carcinoma | SS | Embolization | 1 | III |
| 9 | 76 | f | Thyroid cancer | TS | Embolization | 1 | III |
| 10 | 84 | m | Uorthelial carcinoma | SS | Embolization | 1 | I |
| 11 | 73 | m | Renal cell carcinoma | TS | Embolization | 3 | III |
| 12 | 74 | m | Melanoma | CS | Embolization | 1 | II |
| 13 | 71 | m | Renal cell carcinoma | LS | Embolization | 1 | III |
| 14 | 85 | f | Renal cell carcinoma | TS | Embolization | 1 | III |
| 15 | 73 | f | Thyroid cancer | LS | Embolization | 1 | I |
| 16 | 74 | f | Renal cell carcinoma | TS | Embolization | 2 | III |
| 17 | 71 | m | Esophageal carcinoma | LS | Embolization | 2 | I |
| 18 | 67 | m | Renal cell carcinoma | TS | Embolization | 2 | III |
| 19 | 85 | m | Thyroid cancer | CS | Embolization | 1 | II |
| 20 | 62 | m | Renal cell carcinoma | TS | Partial embolization | 14 | III |
| 21 | 59 | m | Renal cell carcinoma | TS | Embolization | 2 | II |
| 22 | 37 | m | Bronchial carcinoma | LS | Embolization | 3 | II |
| 23 | 75 | m | Renal cell carcinoma | CS | Embolization | 5 | III |
| 24 | 89 | f | Thyroid cancer | LS | Embolization | 1 | II |
Imaging and embolization
All patients received a radiological work-up with plain films, CT and additional MRI (n=13) and/or myelography (n=4). Diagnostic and interventional angiographies were performed on a CAS 500 biplane digital subtraction angiography unit (Toshiba Medical Systems, Tokyo, Japan). Patients underwent diagnostic angiography and embolization under local anesthesia using the femoral approach. Embolization was usually performed subsequent to diagnostic angiography or, occasionally, a few days later. Depending on the origin of the feeding arteries, various pre-shaped catheters were used for spinal angiography. To avoid unnecessary use of catheters for selective catheterization of the thoracic and lumbar segmental arteries, we decided to develop new catheters on the basis of a 5.2F catheter (Spinal Super Torque Johnson & Johnson, Division Cordis, Switzerland), with the same shape but different bend sizes. These different catheter sizes were developed with the support of Johnson & Johnson (Division Cordis, Switzerland). The inner diameter of the catheters (0.97 mm) allows their use as diagnostic catheters and as guiding catheters. Therefore, when stable position is achieved, direct coaxial introduction of the microcatheter (usually a Tracker 10 or 18, Target Therapeutics, Fremont, CA, USA) is possible. Prior to the embolization procedure, the diameter of the aorta at the level of the tumor was measured, based on the CT image. Depending on the aortic diameter, one of three different catheter sizes (10 mm, 13 mm or 16 mm: Berner Spinalkatheter I-III) was chosen in order to get optimal stability during the intervention (Fig. 1). The angiographic protocol included visualization of the vertebral arteries, thyrocervical trunk, costocervical trunk, and external carotid arteries for cervical lesions. This was also done in the segmental arteries within at least two levels above and below the tumor site, for thoracic and upper lumbar lesions; and in the lower lumbar arteries, medial sacral arteries and internal iliac arteries for lower lumbar lesions. In the thoracic and upper lumbar spine, additional localization of the artery of Adamkiewicz was performed to avoid damage of the spinal cord during embolization or corporectomy. Selective angiography of the suspected feeding arteries was then performed. Depending on the tumor vascularization on the arterial angiogram, all lesions were assigned to three groups by two independent radiologists (S.D.S., G.S.): group I for weak tumor blush after contrast injection, group II for medium tumor blush and group III for significant tumor blush. Polyvinyl alcohol (PVA) particles ranging in size from 45 μm to 350 μm were injected via a microcatheter (Tracker 10 or 18) coaxially inserted through the guiding catheter. In three cases coils were used to obliterate the segmental artery feeding the tumor. Intraoperative blood loss was determined by volume registration from the suction unit.
Fig. 1.
The three sizes of the Berner Spinalkatheter. A Depending on the aortic diameter, one of three catheter sizes is chosen in order to get an optimal stability during the intervention. B The inner diameter of this catheter allows its use as a diagnostic catheter and as a guiding catheter
Surgical procedures
All the surgical procedures were performed at a mean of 2 days (range 0–14 days) after embolization. Anterior, posterior or combined anterior–posterior approaches were used depending on maximal tumor mass. Blood loss was estimated by the anesthesiologist.
Results
Angiographic results
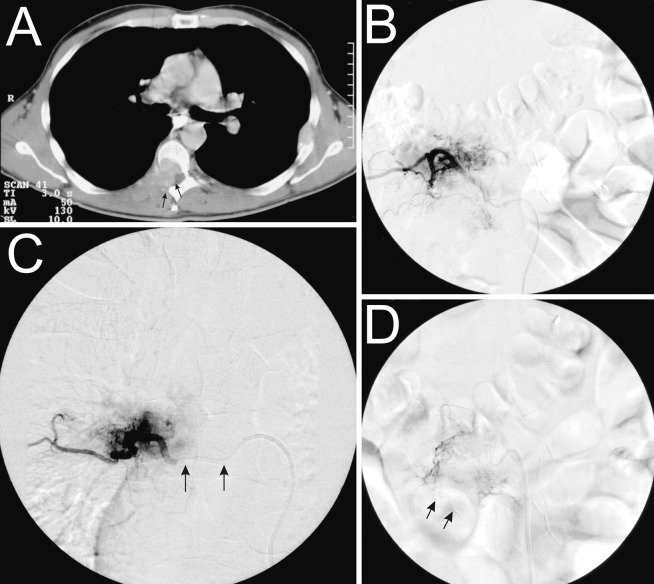
Angiography and embolization could be performed in all patients without causing permanent neurologic deficit, skin or muscle necrosis. All tumors showed hypervascularization compared with the normal vertebral blush on selective spinal angiograms. All tumors were extradural, with three localized in the sacrum, nine in the lumbar, eight in the thoracic and four in the cervical spine. The blood supply to the tumors originated from branches of the iliac artery in two patients, the lumbar segmental artery in seven patients, the intercostal artery in ten patients and from supraaortic vessels in five patients. Tumor vascularization before embolization was weak in three patients (group I), medium in six patients (group II) and extensive in 15 patients (group III). No difference for the vascularization grade was found when comparing the lumbar, the thoracic and the cervical spine with a mean vascularization grade of 2.4, 2.8 and 2.5, respectively. In 22 of 24 patients, near complete embolization of the tumor vessels could be achieved. In two patients (cases 1 and 20), the tumor feeding artery also supplied the artery of Adamkiewicz and, consequently, only partial embolization could be performed. The use of different sizes of the Berner Spinalkatheter depending on the aortic diameter at the level of the tumor proved to be very useful—it was possible to achieve an optimal catheter stability during embolization. Fig. 2 shows an illustrative case.
Fig. 2.
Illustrative case showing a metastasis of a renal cell carcinoma on the level of T7. A The CT section through vertebra T7 shows metastatic osseous destruction, mainly in the posterior part of the vertebral body, as well as intraspinal epidural extension (arrows). B Selective injection of the segmental artery D7 through the Berner Spinalkatheter shows the tumor blush. C Through the coaxially introduced microcatheter (arrows), selective embolization of the tumor-feeding vessels and near complete devascularization can be achieved. D In this case, two coils were introduced in order to seal the segmental artery D7 (arrows)
Surgical results
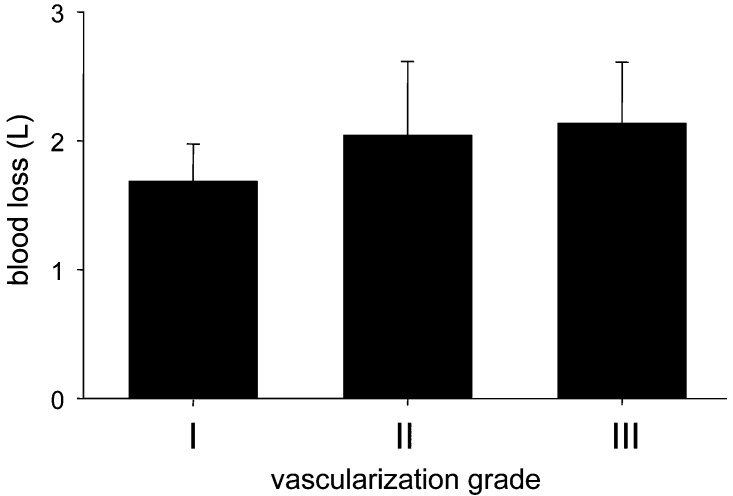
The tumors were approached from an anterior transcavitary route in six patients (25%), a posterior approach in nine patients (37.5%) and a combined approach in nine patients (37.5%). Instrumentation was placed in all patients. Sixty percent of the patients were operated on within 1 day after embolization, 95% within 5 days. Mean duration of surgery was 4.7 h. The mean blood loss for all embolized patients was 1,900 ml. For the two partially embolized patients it was 5,500 ml. The intraoperative blood loss varied significantly, with a maximum of 6,000 ml (partially embolized) and a minimum of 1,000 ml. The mean blood loss only considering the cases of renal cell carcinoma metastases was 2,400 ml. The mean blood loss estimation for all metastases except the cases of renal cell carcinoma metastases was 1,300 ml. The mean blood loss was 2,300 ml for lumbar, 2,200 ml for thoracic and 1,350 ml for cervical spine tumors. There was no statistically significant correlation between the vascularization grade and the intraoperative blood loss after transarterial embolization (Fig. 3). In two of the 24 patients (cases 1 and 20) the goal of the surgery could not be attained, due to excessive bleeding. In these patients, both of whom had hypervascularized metastases of a renal cell carcinoma, only a partial tumor reduction was achieved. The initial goal of the surgery for both patients was a vertebrectomy and instrumentation.
Fig. 3.
Bar plot showing the relation between the estimated blood loss in liters (y-axis) and the preembolization vascularization degree (x-axis). There is no statistically significant difference between the three groups
Discussion
Extensive surgical procedures in patients with hypervascular vertebral tumors are often complicated by excessive blood loss. Therefore, preoperative embolization of spinal tumors is recommended to reduce intraoperative bleeding. Several investigators have reported results with preoperative endovascular embolization of hypervascular spinal tumors and clearly showed its benefit in reduction of intraoperative blood loss [3, 4, 5, 6, 10]. In a paper by Berkefeld et al., the estimated intraoperative blood loss was 4,350 ml in non-embolized patients and 1,800 ml in patients with particle embolization [1]. More recently, in a series on spinal metastases from renal cell carcinoma, the median intraoperative blood loss was 1,500 ml (range 300–8,000 ml) [5]. In our series, the estimated mean blood loss for the 22 embolized cases was 1,900 ml. In cervical spine tumors, embolization is more difficult to perform than in lumbar or thoracic spine tumors, due to frequent anastomoses between the carotid, vertebral and subclavian arteries. The risk of cerebral or spinal cord embolization is increased [12]. In our series no patient suffered neurological deterioration after the embolization procedure. Our results show that after the embolization the mean blood loss for cervical spine tumors was 1,350 ml. This stands in contrast with surgical reports of 9.5–15 l of blood loss when preoperative embolization was not performed [3]. In two patients (cases 1 and 20), only partial embolization could be performed, and the goal of surgery could not be achieved because of excessive bleeding, with blood losses of 5,000 ml and 6,000 ml respectively. These two cases illustrate the importance of preoperative transarterial embolization of hypervascular vertebral metastases and demonstrate its potential influence on surgical success. Grading of tumor vascularization based on the tumor blush was thought to be prognostic for the intraoperative blood loss volume. However, we found that there was no significant difference in blood loss among the three groups after radiological complete devascularization (Fig. 3). This finding gives further evidence that preoperative embolization is effective.
From a qualitative, surgeons’ point of view, preoperative tumor embolization facilitated surgical resection, primarily by reducing intraoperative bleeding and, hence, ensuring a good view of the surgical field. Furthermore, it increased the cleavage between tumor and dura or bone, which resulted from tumor shrinkage.
Choosing the size of our Berner Spinalkatheter based on the diameter of the aorta at the level of the involved segmental artery resulted in reduction of the time needed for stable positioning in thoracic and upper lumbar procedures. It led to a reduction of the number of catheters used for selective catheterization of the thoracic and lumbar segmental arteries.
Conclusion
Preoperative transarterial embolization of hypervascular spinal tumors is a safe and effective procedure. Preoperative embolization in hypervascular vertebral tumors reduces the perioperative blood loss and facilitates tumor resection. Appropriate techniques allow a considerable reduction of intervention time. Adapted guiding-catheter sizes increase the catheter stability and, therefore, reduce the risk of microcatheter displacement during embolization.
Acknowledgements
Special thanks go to Mrs. Fügliskater from Johnson & Johnson, Division Cordis, Switzerland for supplying the illustration of the Berner Spinalkatheter I-III
References
- 1.Berkfeld AJNR AM J Neuroradiol. 1999;20:757. [PMC free article] [PubMed] [Google Scholar]
- 2.BolandClin Orthop 19821169957105592 [Google Scholar]
- 3.Gellad Radiology. 1990;176:683. doi: 10.1148/radiology.176.3.2389026. [DOI] [PubMed] [Google Scholar]
- 4.GRadiologe 199535557534427 [Google Scholar]
- 5.Manke AJNR AM J Neuroradiol. 2001;22:997. [PMC free article] [PubMed] [Google Scholar]
- 6.Olerud Acta Orthop Scand. 1993;64:9. doi: 10.3109/17453679308994517. [DOI] [PubMed] [Google Scholar]
- 7.ShiAJNR AM J Neuroradiol 19992029974048 [Google Scholar]
- 8.Siegal J Bone Joint Surg. 1985;67:375. [PubMed] [Google Scholar]
- 9.Sundaresan J Neurosurg. 1985;63:676. doi: 10.3171/jns.1985.63.5.0676. [DOI] [PubMed] [Google Scholar]
- 10.Sundaresan J Neurosurg. 1990;73:548. doi: 10.3171/jns.1990.73.4.0548. [DOI] [PubMed] [Google Scholar]
- 11.Tadavarthy AJR AM J Roentgenol. 1974;125:609. [Google Scholar]
- 12.Vetter Cardiovasc Intervent Radiol. 1997;20:343. doi: 10.1007/s002709900165. [DOI] [PubMed] [Google Scholar]