Abstract
To evaluate the role of complement in pneumococcal and staphylococcal pneumonia, we decomplemented rats with cobra venom factor and inoculated them intratracheally with Staphylococcus aureus or type 25 pneumococci. S. aureus produced a patchy bronchopneumonia in normal Sprague-Dawley or Lewis rats, and decomplementation did not increase the severity of staphylococcal infection in either rat strain as judged by quantitative cultures of the lungs and blood at 6, 24, and 48 h after inoculation. In contrast, decomplementation markedly increased the severity of pneumonia caused by type 25 pneumococci in Sprague-Dawley and Lewis rats. In Sprague-Dawley rats, decomplementation significantly increased the number of bacteria in the lungs at 3, 6, and 24 h of infection. Bacteremia developed early in decomplemented Sprague-Dawley rats, but the higher pulmonary bacterial counts did not appear to be caused by bacteremic seeding of the lungs. Decomplemented Sprague-Dawley rats inoculated intravenously with pneumococci failed to develop the very high levels of bacteria in the lungs that were observed when the rats were inoculated intratracheally. Moreover, decomplemented Lewis rats inoculated intratracheally with pneumococci developed significantly increased numbers of pneumococci in the lungs early in infection (3 and 6 h) when they had no detectable bacteremia. These data indicate that in murine models complement plays a major protective role against type 25 pneumococci in the lung, whereas complement is not important to host defense in staphylococcal pneumonia.
Full text
PDF

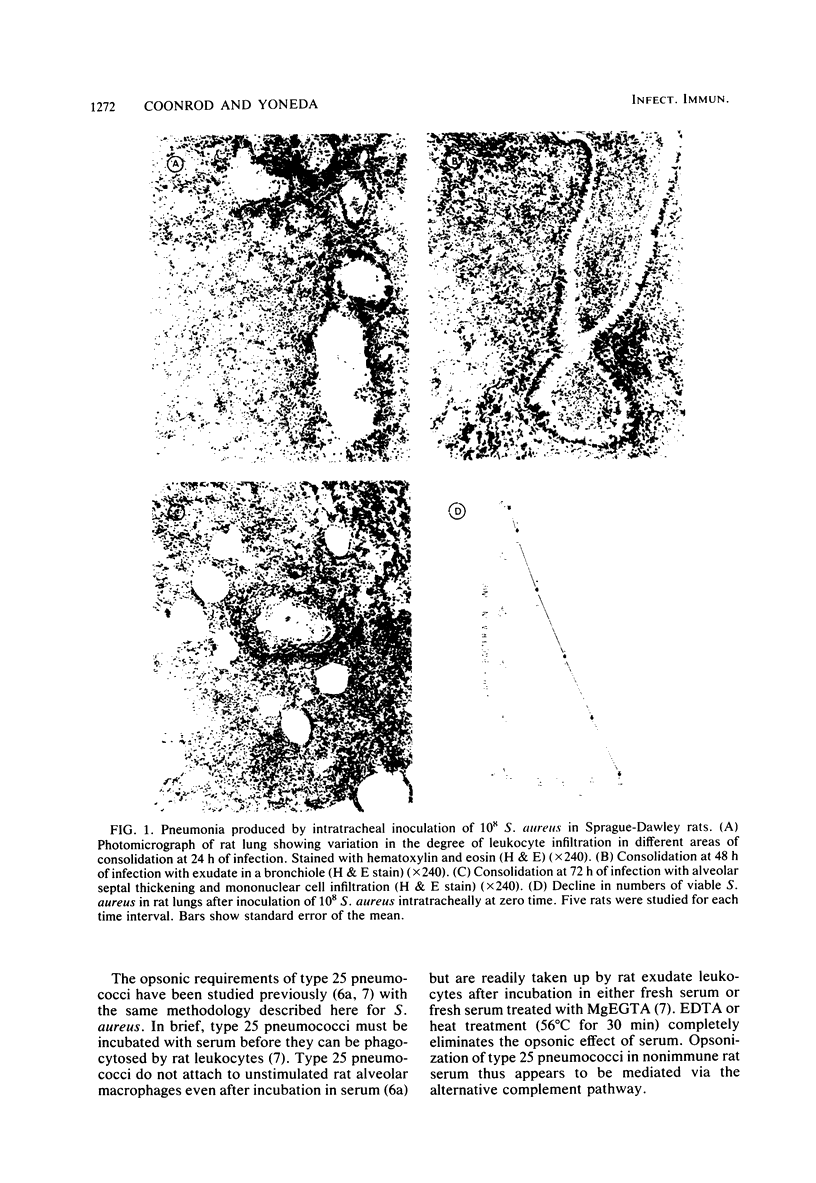

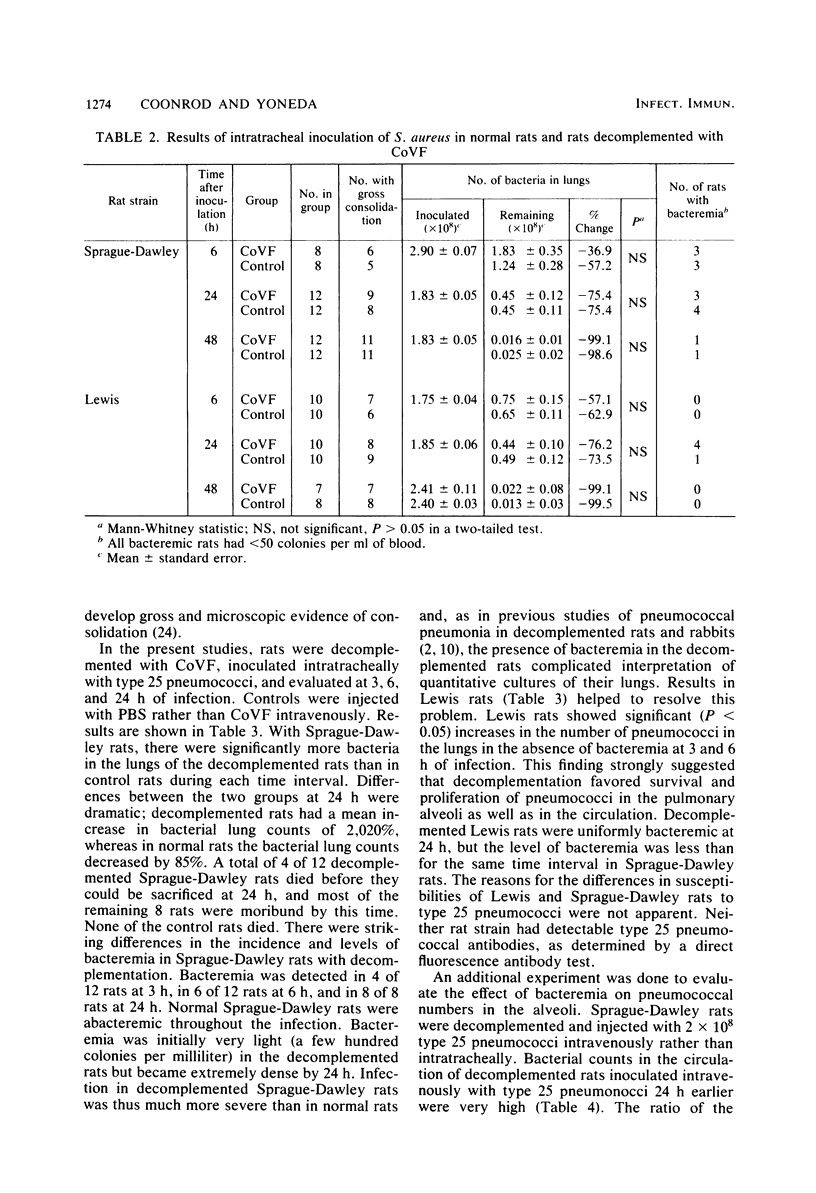
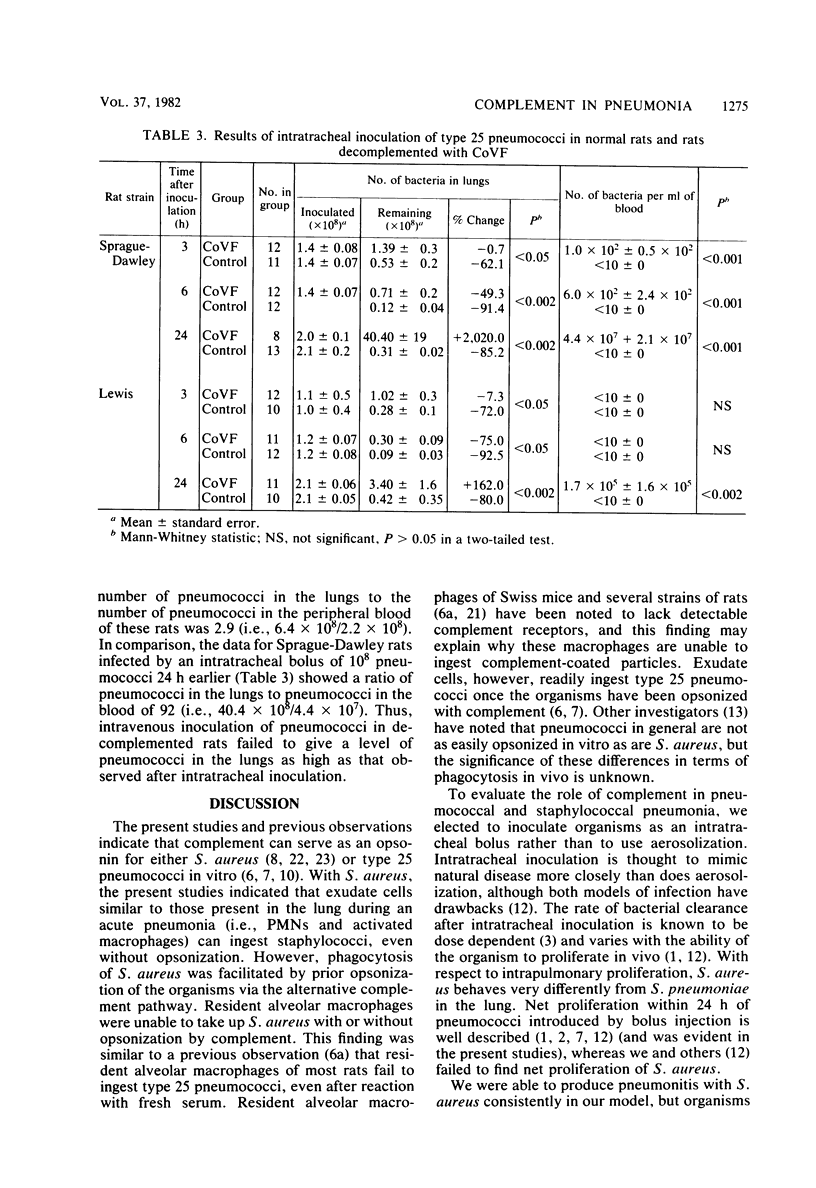


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansfield M. J., Woods D. E., Johanson W. G., Jr Lung bacterial clearance in murine pneumococcal pneumonia. Infect Immun. 1977 Jul;17(1):195–204. doi: 10.1128/iai.17.1.195-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker-Woudenberg I. A., de Jong-Hoenderop J. Y., Michel M. F. Efficacy of antimicrobial therapy in experimental rat pneumonia: effects of impaired phagocytosis. Infect Immun. 1979 Jul;25(1):366–375. doi: 10.1128/iai.25.1.366-375.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt R. F. Relationship of method of administration to respiratory virulence of Klebsiella pneumoniae for mice and squirrel monkeys. Infect Immun. 1978 May;20(2):581–583. doi: 10.1128/iai.20.2.581-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- Coonrod J. D., Jenkins S. D. Functional assay of the alternative complement pathway of rat serum. J Immunol Methods. 1979;31(3-4):291–301. doi: 10.1016/0022-1759(79)90142-x. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D. Pulmonary opsonins in Klebsiella pneumoniae pneumonia in rats. Infect Immun. 1981 Aug;33(2):533–539. doi: 10.1128/iai.33.2.533-539.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Rehm S. R. Complement receptors of rat macrophages. J Reticuloendothel Soc. 1982 Feb;31(2):107–115. [PubMed] [Google Scholar]
- Coonrod J. D., Yoneda K. Complement and opsonins in alveolar secretions and serum of rats with pneumonia due to Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):310–322. doi: 10.1093/clinids/3.2.310. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Opsonic activity in human serum chelated with ethylene glycoltetra-acetic acid. Immunology. 1974 Jun;26(6):1251–1256. [PMC free article] [PubMed] [Google Scholar]
- Gross G. N., Rehm S. R., Pierce A. K. The effect of complement depletion on lung clearance of bacteria. J Clin Invest. 1978 Aug;62(2):373–378. doi: 10.1172/JCI109138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckian J. C., Christensen G. D., Fine D. P. The role of opsonins in recovery from experimental pneumococcal pneumonia. J Infect Dis. 1980 Aug;142(2):175–190. doi: 10.1093/infdis/142.2.175. [DOI] [PubMed] [Google Scholar]
- Haneishi S. [Experimental studies on staphylococcal pneumonia. (XI). Studies on the rate of migration and the phagocytotic ability of leucocytes in experimentally induced in staphylococcal pneumonia]. Sapporo Igaku Zasshi. 1965 Jan-Feb;27(1):1–19. [PubMed] [Google Scholar]
- Harris G. D., Woods D. E., Fine R., Johanson W. G., Jr The effect of intraalveolar fluid on lung bacterial clearance. Lung. 1980;158(2):91–100. doi: 10.1007/BF02713708. [DOI] [PubMed] [Google Scholar]
- Hof D. G., Repine J. E., Peterson P. K., Hoidal J. R. Phagocytosis by human alveolar macrophages and neutrophils: qualitative differences in the opsonic requirements for uptake of Staphylococcus aureus and Streptococcus pneumoniae in vitro. Am Rev Respir Dis. 1980 Jan;121(1):65–71. doi: 10.1164/arrd.1980.121.1.65. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J. Factors influencing the immune enhancement of intrapulmonary bactericidal mechanisms. Infect Immun. 1976 Aug;14(2):389–398. doi: 10.1128/iai.14.2.389-398.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Rehm S. R., Coonrod J. D. Early clearance of pneumococci from the lungs of decomplemented rats. Infect Immun. 1982 Apr;36(1):24–29. doi: 10.1128/iai.36.1.24-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Hart D. A., Pierce A. K. Animal model of neutropenia suitable for the study of dual-phagocyte systems. Infect Immun. 1979 Jul;25(1):299–303. doi: 10.1128/iai.25.1.299-303.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Wheat L. J., Humphreys D. W., White A. Opsonization of staphylococci by normal human sera: the role of antibody and heat-labile factors. J Lab Clin Med. 1974 Jan;83(1):73–78. [PubMed] [Google Scholar]
- Yoneda K., Coonrod J. D. Experimental type 25 pneumococcal pneumonia in rats: an electron-microscopic study. Am J Pathol. 1980 Apr;99(1):231–242. [PMC free article] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]