Abstract
The introduction of the titanium mesh cage (TMC) in spinal surgery has opened up a variety of applications that are realizable as a result of the versatility of the implant. Differing applications of TMCs in the whole spine are described in a series of 150 patients. Replacement and reinforcement of the anterior column represent the classic use of cylindrical TMCs. The TMC as a multisegmental concave support in kyphotic deformities and as a posterior interlaminar spacer or lamina replacement after wide laminectomy are additional applications. Implant subsidence, pseudarthrosis and implant loosening are the complications typically encountered with use of TMCs. The versatility of the implant permits its use in unusual surgical situations.
Keywords: Titanium mesh cages, Spinal surgery, Anterior column support, Kyphotic deformity, Lamina replacement
Introduction
From both the diagnostic and surgical points of view, the complex construction of the spine, with its anatomical and mechanical interaction of relatively small structures, represents a particular challenge. The form and biomechanics of the vertebrae and the motion segments vary considerably, not only between the global anatomical sections, such as the cervical, thoracic or lumbar regions, but also within these sectors from segment to segment. Furthermore, the normal anatomy may be distorted and altered in pathologies associated with deformity of the spine. The spatial registration of the vertebra and its surrounding soft tissue requires considerable skill in the three-dimensional imagination of the surgeon.
Similar to other surgical procedures, spine surgery requires careful preoperative planning and a clear concept of the surgical intention. In this way, the experienced surgeon is able to avert unexpected events. However, unforeseen situations and individual variation can never be completely avoided, and having the skill and capacity to improvise may be the only way of circumventing difficult situations if an acceptable result is to be maintained. It is impossible to design a wide enough range of implants that can overcome all unexpected difficulties and can support all the individual differences in anatomy. The TMC not only fulfils the requirements of regular vertebral body replacement after corpectomy, but also those of more unusual situations encountered intraoperatively.
Titanium mesh cages originated in orthopaedic surgery as fenestrated metal sheets used in reconstructive maxilla facial surgery and acetabular replacement surgery more than 20 years ago [19]. These were regularly used to deal with bony defects. Appropriate cutting and moulding allowed support of the onlay bone graft in the desired position until calcification took place (Fig. 1).
Fig. 1.
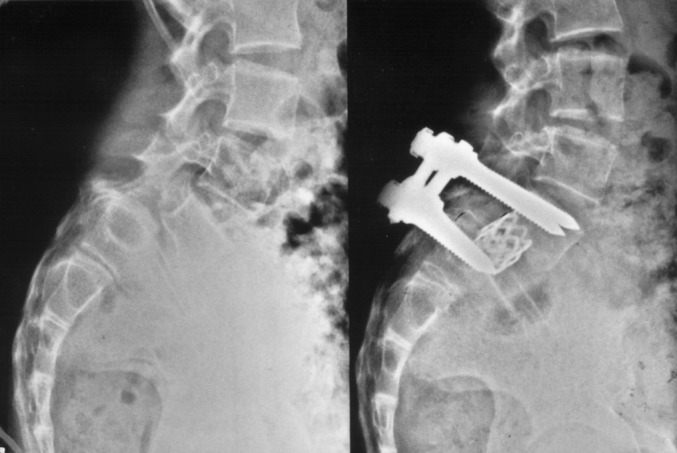
Classic indication for TMC: reconstruction of spondylolisthesis with anterior support of the vertebral space by TMC and posterior transpedicular fixation.
In 1986, Harms and Biedermann developed the first implant for the spine. The oval-shaped mesh cylinder was designed to act as a vertebral spacer. Graft fracture or collapse—the main complications with autologous anterior strut grafts from iliac crest, tibia or fibula—could be avoided with this new device and represented a significant improvement compared with previous techniques.
Autologous bone graft inside the cylinders turned this implant into a biological vertebral body replacement with metal reinforcement. The simplicity of the design, with its several diameters and its capacity to be individually tailored to suit the prevailing circumstances, conveyed upon it the advantage that it could be applied almost universally.
It is the purpose of this paper to illustrate the use of TMC in regular and special circumstances during spine surgery, by describing our experiences with the implant in a large series of patients.
Patients and methods
Between October 1992 and March 2002, 150 patients underwent implantation of TMCs at various sites along the spine: 63 (42%) in the cervical spine, 26 (17%) in the thoracic spine and 61 (41%) in the lumbar spine. The distribution of gender was almost equal: 74 (49%) women and 76 (51%) men. The average age at surgery was 49 years (SD 19, range 8–85 years).
There were various indications for TCM implantation within the series (for details see Table 1). TCM was used most commonly (n=94 cases, 63%) as a vertebral body replacement, with the most common indications being spinal stenosis (n=38), kyphosis/scoliosis (n=16), tumor/metastasis (n=12), fracture (n=8), spondylolisthesis/spondyloptosis (n=7), pseudarthrosis (n=5), herniated disc (n=4), instability (n=3), and spondylarthrosis (n=1). In 49 cases, one vertebral body was replaced, and in 50 cases, more than one vertebral body. TCM was used as an interbody spacer in 46 (31%) patients with an underlying diagnosis of spondylolisthesis/spondyloptosis (n=19), fracture (n=12), pseudarthrosis (n=6), segmental instability (n=3), kyphosis/scoliosis (n=2), spondylarthrosis (n=2), tumor (n=1), and herniated disc (n=1). TCM provided concave mechanical support in complex deformities in just four (3%) cases, for pseudarthrosis (n=2) and for spondylarthrosis (n=2). In a further five cases (3%), TCM was used as an interlaminar replacement for spinal stenosis (n=1), kyphosis/scoliosis (n=1), pseudoarthrosis (n=2) and spondylitis (n=1), and in one case, as a posterior interlaminar spacer (pseudoarthrosis (n=1).
Table 1.
Summary of indications and the corresponding use of the titanium mesh cage (TMC) for all regions of the spine
| Use of the cage | |||||||
|---|---|---|---|---|---|---|---|
| Indication | Vertebral-body replacement (1 only) | Vertebral-body replacement (>1) | Inter-body spacer | Concave mechanical support | Laminar replace-ment | Posterior inter-laminar spacer | All uses |
| Spondylolisthesis/ spondyloptosis | 5 | 2 | 19 | 0 | 0 | 0 | 26 |
| Spinal stenosis | 38 | 0 | 0 | 0 | 1 | 0 | 39 |
| Kyphosis/scoliosis | 4 | 12 | 2 | 0 | 1 | 0 | 19 |
| Tumour/ metastases | 1 | 11 | 1 | 0 | 2 | 0 | 15 |
| Pseudarthrosis | 0 | 5 | 6 | 2 | 0 | 1 | 14 |
| Instability | 0 | 3 | 3 | 0 | 0 | 0 | 6 |
| Spondylarthrosis/ osteosclerosis | 0 | 1 | 2 | 2 | 0 | 0 | 5 |
| Herniated disc | 0 | 4 | 1 | 0 | 0 | 0 | 5 |
| Fracture | 0 | 8 | 12 | 0 | 0 | 0 | 20 |
| Spondylitis | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| All indications | 48 | 46 | 46 | 4 | 5 | 1 | 150 |
The indications for surgery, in relation to the three regions of the spine, are shown in Table 2.
Table 2.
Indications for use of the titanium mesh cage (TMC), according to region of the spine
| Region of the spine | Indication | Number patients |
|---|---|---|
| Cervical spine | Spinal stenosis | 39 |
| Spondylolisthesis/spondyloptosis | 5 | |
| Tumor/metastases | 5 | |
| Kyphosis/scoliosis | 4 | |
| Instability | 3 | |
| Pseudarthrosis | 3 | |
| Fracture | 2 | |
| Herniated disc | 1 | |
| Spondylarthrosis/osteosclerosis | 1 | |
| Total n=63 | ||
| Thoracic spine | Tumor/metastases | 8 |
| Kyphosis/scoliosis | 7 | |
| Fracture | 6 | |
| Herniated disc | 3 | |
| Spondylolisthesis/spondyloptosis | 1 | |
| Spondylitis | 1 | |
| Total n=26 | ||
| Lumbar and lumbosacral spine | Spondylolisthesis/spondyloptosis | 20 |
| Pseudarthrosis | 11 | |
| Kyphosis/scoliosis | 8 | |
| Spondylarthrosis/osteosclerosis | 4 | |
| Instability | 3 | |
| Tumor, metastases | 2 | |
| Herniated disc | 1 | |
| Fracture | 12 | |
| Total n=61 |
The majority of the patients (n=99; 66%) had not previously undergone surgery of the spine; 34 (23%) had been operated on at the same segmental level; 13 (9%) at the neighbouring segmental level; and four (3%) at a distant segmental level.
At follow-up (median 9 months, range 1–70 months postoperatively), radiographs and hospital charts were reviewed. Screw-loosening, subsidence, and displacement of the TCM were evaluated and compared with the radiographs taken immediately after surgery. If any of these parameters were positive, flexion/extension views were taken to exclude intersegmental motion.
Stable fusion was assumed if there was an absence of motion, a regular implant positioning without loosening or breakage, and a stable clinical situation.
Results
Complications
As might be expected, in view of the complex pathologies in this series, the rate of complications was relatively high. The proportion of patients who fulfilled the criteria for “potentially serious” complications was: 39/120 (32%) in those with at least 3 months follow-up; 33/89 (37%) in those with >6 months; 29/75 (39%) in those with >9 months; 25/60 (42%) in those with >12 months; and 13/32 (41%) in those with >24 months.
The consequences of the complications in the subgroup of patients with more than 9 months follow-up (which was the median follow-up time for the whole group) were examined in closer detail. Subsidence of the cage into the vertebral body, with or without a change in the state of the screws and without pseudarthrosis, was noted in 12 (16%) patients. None of these had required re-intervention (Fig. 2). Ten (13%) patients showed pseudarthrosis (two cervical, one thoracic, seven lumbar/lumbosacral); two of these were symptomatic and had required revision surgery (one cervical, one lumbar/lumbosacral). seven patients (9%) showed a fracture or a change in state of the spondylodesis material, but without pseudarthrosis. (Fig. 3)
Fig. 2.

Subsidence of TMC: occurrence of discrete kyphotic deformity after corpectomy of L3
Fig. 3.

Complication after anterior reconstruction with TMC. Failure of the TMC after reconstruction of the anterior thoracolumbar spine in fracture. Additional posterior fusion would have been able to prevent this complication
Technical notes
TCM as an interbody device or as a vertebral body replacement
The anterior column can be expected to show optimal mechanical behaviour if it can be replaced at its normal anatomical location. This represents the classical indication for TMC. Its use as an interbody spacer allows placement via an anterior approach that can be minimally invasive (endoscopic) or via a posterior transforaminal approach in combination with posterior fixation. A crucial point is the TCM-vertebral endplate interface. The use of rings internally fixed at both ends of the cylinder to increase the surface area reduces the incidence of subsidence, but the risk of recurrence of kyphosis remains, especially in soft osteoporotic vertebral bone (Fig. 4a and b). The additional use of anterior or posterior fixation is necessary if there are any doubts about the bone quality. The insertion of the largest possible TMC is advocated. After cutting the cylinder on site to the appropriate length, remaining spikes of the cage must be smoothened in order to avoid injury to the surrounding soft tissue and to minimise the risk of the TMC getting stuck during insertion between the endplates. After completion, intraoperative radiographic examination is recommended, since the orientation in situ for orthograde positioning might be difficult (Figs. 5 and 6).
Fig. 4 a.

Failed back after decompression, cage insertion and wide laminectomy. b Stabilization and solid reconstruction with anterior TMC and posterior transpedicular fixation
Fig. 5 a.
Metastasis of a prostate cancer with osteolysis of the left lateral mass of the atlas. Severe neck pain. b and c Postoperative X-rays after reconstruction of the lateral mass of the atlas and occipito-cervical fixation. The patient was pain-free until he died of the cancer 19 months after the surgery
Fig. 6 a.
Metastasis of mamma carcinoma with destruction of the anterior column of C2. b and c Postoperative X-rays after transoral dissection of the body of C2 and reconstruction by TMC. Additional posterior pedicular fixation C1 to C3
To increase the chance of solid bony fusion, the TMC should be packed with bone graft. According to the literature [22, 23] there is a fair chance of bony ingrowth into the cages (Fig. 7). If at all possible, anterior bridging outside the TMC by adding bone graft should be performed in order to allow easy and reliable radiological assessment of solid fusion at follow-up.
Fig. 7 a.

Solid bony ingrowths of TMC in the cervical spine after corpectomy. The bone formation shows viable bone within the cage. b Transverse section after effective decompression in spondylotic cervical myelopathy with visible bone inside the cage
TCM as a concave support
In complex deformities of the cervicothoracic area, with multisegmental kyphosis or kyphoscoliosis, an anterior support is required for permanent stability. A direct approach to this part of the spine requires extensive dissection, including splitting of the anterior thoracic cage, with increased risk of injury of vital structures within the thorax. TCM offers an elegant solution in this situation (Fig. 8). The length of the support must extend from end-vertebra to end-vertebra and is often greater than the standard length of the commercially available TCM. Instead of ordering a custom-made, extra long cage, standardised cylinders of different diameter may be assembled and secured together with screws. Thus, any desired length may be obtained. For the insertion of the cage, two openings are necessary. The one close to the lower end-vertebra is usually located in the mid-to-lower thoracic spine. A posterolateral approach with costotransversectomy prepares the way for the cage-fitting. The cranial end-vertebra is reachable by a standard anterolateral cervical approach. The insertion of the cage is performed from caudal to cranial after blunt finger dissection of the pathway of the cage through the thorax and mediastinum. A soft rubber tube is inserted prior to the cage and this prevents soft-tissue traumatisation by slipping it over the end of the cage. Once in situ, the cage may be secured by screws or antiglide plates (Figs. 9 and 10). This type of surgery, where the TMC is placed in the vicinity of the spinal cord, heart, lung and great vessels, requires a thorough knowledge of the corresponding anatomy and must be planned carefully.
Fig. 8 a.
Patient with neurofibromatosis and severe progressive cervicothoracic kyphotic deformity. b Postoperative picture with correction of the kyphotic deformity and anterior support by TMC. c X-ray of the severely deformed thoracic spine with marked kyphotic deformity. d Postoperative X-ray after anterior kyphotic support and correction and posterior fixation. e Biological fixation: the end of the titanium cage is filled with autologous bone graft. CT scans at follow-up show solid integration of bone into the cage, thus fixing it securely in place
Fig. 9.
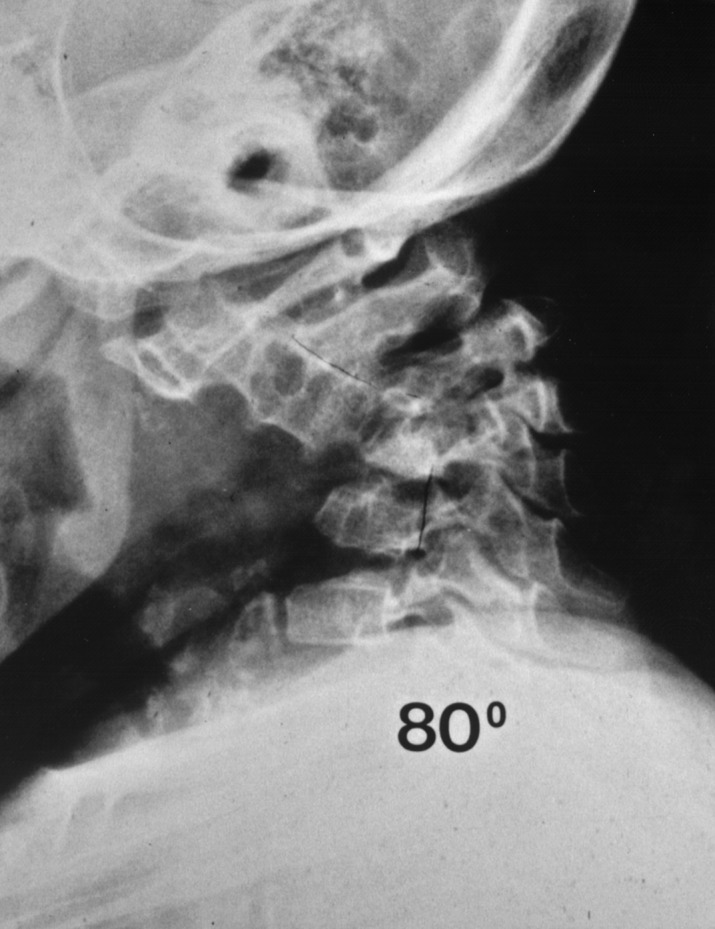
Severe kyphotic deformity due to tuberculosis in an 11-year-old child
Fig. 10.

Postoperative X-ray after reduction and anterior support with TMC
TMC as a lamina replacement
Multisegmental laminectomy with posterior fixation may be indicated in tumor surgery or severe cases of spinal stenosis. In order to prevent implant loosening, additional bone grafting is essential. The placement of bone graft might be difficult, since the implant covers the bony surface available to receive the graft. In addition, there is a risk of the morcellised bone compressing the dura and medulla by displacement after wound closure. A TMC of appropriate diameter can be split longitudinally. By opening the cylinder with two pliers, it can be formed into a lamina-like roof over the spinal canal, bridging the edges of the laminectomy on both sides of the spinal canal. Fixation of this artificial roof can be performed with screws in the lateral masses or by elasticity of the mesh pushing against the bilaterally positioned implants. Consequently the bone graft can be placed all over the TMC, which serves to protect the spinal canal and its contents (Figs. 11, 12 and 13).
Fig. 11.

Reliable protection of the medulla by TMC laminoplasty
Fig. 12.

Laminoplasty with TMC after wide laminectomy due to intraspinal tumor resection
Fig. 13.

Intraoperative situs after TMC laminoplasty. The mesh cage not only protects the dura from trauma but also allows positioning of the bone graft continuously over the fused area, decreasing the risk of pseudarthrosis
TMC as posterior interlaminar spacer
The posterior fixation of the atlantoaxial segment with transarticular screws requires a posterior midline graft. This is for biological fusion and permanent stability but serves at the same time as the third point of the omnidirectional stabilisation [7]. In circumstances where increased stability by instrumentation of the cervical or lumbar spine is required, it might be desirable to have a structured and compression-resistant implant to increase stability. A TMC cut accordingly can be used in this situation. (Fig. 14).
Fig. 14.

Three-point fixation in atlantoaxial stabilization: The two anteriorly situated transarticular screws and the wire fixation of the titanium mesh cage (filled with autologous bone) provide a stable segmental fixation in all three planes of the space
Discussion
The importance of the solid reconstruction of the anterior column was recognized early and several constructs and techniques have been reported in the literature [8, 15, 16]. Itoh et al. [10] were probably the first to publish, in 1934, “a new radical operation for Pott’s disease” describing the reinforcement and vertebral body replacement by tibial autografts. In the 1950s, Hodgson et al. [9] received international recognition for their technique of anterior column reconstruction with rib autografts, based on the same principle. Their techniques were mainly developed to surgically correct the kyphotic deformities of Pott’s disease. Further techniques for reconstruction of the anterior column were described in the literature, but the results were not convincing, with a rate of pseudarthrosis of greater than 20% [1, 6, 20]. As a result, surgeons became reluctant to use the anterior approach to the lumbar and thoracolumbar spine, except in the case of tuberculosis [18]. The cervical Pott showed better results and remained the flagship of anterior spine surgery with the classical procedures described by Smith, Robinson and Cloward [2, 21]. With the development of anterior cages, which were soon introduced using endoscopic techniques (BAK device), the anterior approach finally witnessed a revival.
One of the problems of anterior spine surgery was finding reliable material for anterior column reconstruction. The properties of autologous bone promised good results, but the reservoir of the human body for such anterior column replacements is limited and the stability was dependent on the anatomical location of the harvested site. In extensive surgery, a large amount of bone graft produced significant morbidity at the donor site. For interbody fusion, iliac crest or ribs were used [9, 18], but for corpectomies with longer distances, the insertion of fibula or tibia was necessary. The use of mechanically strong homologous femoral rings of different lengths reduced the risk of graft fracture and donor site morbidity, but at the risk of immunologic reaction, possible transmission [3] of disease and non-union [17].
The introduction of metallic implants attempted to overcome the aforementioned difficulties. Dunn [4], Kostuik [14], Kaneda [12] and others used rod and plate systems to support the bone graft and achieved improved fusion rates with this reinforcement. Typically, these implants were fixed on the anterior or lateral aspect of the spine and supported the bone graft, which was used to reconstruct the anterior column. The next generation of implants for spinal surgery was initiated by the invention of the metallic interbody spacer. This substituted for the bone graft as the weight-bearing structure. The role of the bone was transformed from one of mechanical support into one of biological adjunct to the metallic implant, with the latter taking over as the structural replacement of the anterior column. As a consequence, the volume of autologous bone graft could be significantly reduced, and donor site morbidity was lowered accordingly.
Interbody cages appeared first as cylinders that were introduced as single or double implants or as box type implants. In fracture treatment or tumor surgery, the replacement of one or several vertebral bodies was necessary and larger implants were designed.
The TCM, introduced in 1986, represented the first widely used vertebral replacement. The simple structure of the mesh graft formed to a cylinder (initially done intraoperatively by the surgeon) allowed individual tailoring in accordance with the intraoperative conditions encountered. In addition, the cylinder could be filled with autologous bone graft from the iliac crest or—in the case of corpectomy—with locally harvested bone from the excised vertebra. There is still controversy about the viability of the bone within the cage, but the few reports in the literature are encouraging and confirm the biological response of the bone in the cage [22, 23]. These advantages secured this implant a survival of more than a decade, despite the ongoing development of other, more sophisticated implants designed for the same purpose.
Apart from these classical applications with reconstruction or support of the anterior column, some non-conventional utilisation is described. Extensive laminectomy after decompressive procedure, tumor removal or columnotomy exposes the dura to potential mechanical damage or compression by scar formation and jeopardises the intended bone fusion due to significantly reduced bony surface. The readily cut and moulded TMC is able to replace the lamina by bridging the dura, using the edges of the laminectomy or the stabilising implants as support. The elasticity of the titanium keeps the construct in place. This reconstruction of the lamina protects the dura against mechanical influences forming a protective cage. Soft tissue is kept a good distance away from the dura and scar formation occurs only due to the surrounding haematoma. The reconstruction also allows bone graft to be placed over the entire site of laminectomy and to enhance stability by solid continuous bone formation. The mesh prevents the bone chips from encroaching on the spinal canal and prevents potential neurogenic compression due to ectopic bone formation.
The use of the TMC as a compression-resistant interspinous device revitalizes the well known technique of the posterior H-graft for spine fusion [5]. Translaminar screws in the lumbar spine demonstrate similar stiffness to pedicle screws, except in extension [13]. A compression-resistant graft inserted between the spinous processes is able to counteract this disadvantage. Since bone grafting is done in the majority of cases only with cancellous chips, the TMC used as spacer is able to add the required stability. In the cervical spine, the posterior implantation of the rigid TMC provides the third point of stabilisation in conjunction with atlantoaxial transarticular screws or with hook plates [11]. One of the TMC’s major drawbacks is the lack of distraction it provides when used for anterior column reconstruction. In kyphotic deformities—a frequent indication for anterior vertebral replacement—reduction has to be performed prior to insertion of the TMC. This must be performed by applying distraction with a bone spreader, directly on the endplates, or using screws in the adjacent vertebrae. This manoeuvre is associated with a risk of vertebral fracture and compromise of a solid support for the cage. Thus, newer implants offer the possibility of distraction with the implant itself, but with the consequence of a bulky design and increased cost. An individual tailoring is feasible only in relation to the length, and within a limited selection.
The series presented here illustrates the possibility of different applications of the TMC. It has been used in the classical way to replace anterior column defects, but it also helped to circumvent difficult situations where no other standardised solutions were available.
References
- 1.BohlmannClin Orthop 1981154577471590 [Google Scholar]
- 2.Cloward Spine. 1988;13:823. doi: 10.1097/00007632-198807000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Deijkers J Bone Joint Surg Br. 1997;79:161. doi: 10.1302/0301-620X.79B1.7137. [DOI] [PubMed] [Google Scholar]
- 4.Dunn Clin Orthop. 1984;189:116. [PubMed] [Google Scholar]
- 5.Fidler Eur Spine J. 1997;6:214. doi: 10.1007/BF01301441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FlynnJ Bone Joint Surg Am 1979611365861 [Google Scholar]
- 7.Grob Spine. 1992;17:480. doi: 10.1097/00007632-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Harms J, Stoltze D (1992) The indication and principles of correction of post-traumatic deformities. Eur Spine J 1 [DOI] [PubMed]
- 9.Hodgson J Bone Joint Surg Am. 1960;42:295. [Google Scholar]
- 10.Itoh J Bone Joint Surg. 1934;16:499. [Google Scholar]
- 11.Jeanneret Spine. 1991;16:S56. doi: 10.1097/00007632-199103001-00010. [DOI] [PubMed] [Google Scholar]
- 12.Kaneda Spine. 1984;9:788. doi: 10.1097/00007632-198411000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kornblatt Clin Orthop. 1986;203:141. [PubMed] [Google Scholar]
- 14.Kostuik Spine. 1983;8:512. doi: 10.1097/00007632-198307000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Matthias Clin Orthop. 1986;203:33. [Google Scholar]
- 16.Nachemson Clin Orthop. 1966;107:45. [PubMed] [Google Scholar]
- 17.Norman-Taylor J Bone Joint Surg Br. 1997;79:178. doi: 10.1302/0301-620X.79B2.7492. [DOI] [PubMed] [Google Scholar]
- 18.OClin Orthop 197712856598176 [Google Scholar]
- 19.Patel Br J Oral Maxillofac Surg. 1991;29:316. doi: 10.1016/0266-4356(91)90118-O. [DOI] [PubMed] [Google Scholar]
- 20.Sacks Clin Orthop. 1966;44:163. [PubMed] [Google Scholar]
- 21.Smith J Bone Joint Surg Am. 1958;40:607. [PubMed] [Google Scholar]
- 22.Togawa Spine. 2001;26:2. doi: 10.1097/00007632-200109150-00022. [DOI] [Google Scholar]
- 23.TogawaSpine 200328246. 10.1097/00007632-200302010-0000812567025 [DOI] [Google Scholar]





