Abstract
Background
Previous clinical studies have shown the safety and effectiveness of balloon kyphoplasty in the treatment of pathological vertebral compression fractures (VCFs). However, they have not dealt with the impact of relatively common comorbid conditions in this age group, such as spinal stenosis, and they have not explicitly addressed the use of imaging as a prognostic indicator for the restoration of vertebral body height. Neither have these studies dealt with management and technical problems related to surgery, nor the effectiveness of bone biopsy during the same surgical procedure. This is a prospective study comparing preoperative and postoperative vertebral body heights, kyphotic deformities, pain intensity (using visual analogue scale) and quality of life (Oswestry disability questionnaire) in patients with osteoporotic vertebral compression fractures (OVCFs) and osteolytic vertebral tumors treated with balloon kyphoplasty.
Methods
Thirty-two consecutive patients, 27 OVCFs (49 vertebral bodies [VBs]) and 5 patients suffering from VB tumor (12 VBs) were treated by balloon kyphoplasty. The mean age was 68.2 years. All patients were assessed within the first week of surgery, and then followed up after one, three and six months; all patients (27 OVCFs and 5 tumor patients) were followed up for 12 months, 17 patients (14 OVCFs and 3 tumors) were followed up for 18 months and 9 patients (8 OVCFs and 1 tumor) were followed up for 24 months (mean follow up 18 months). The correction of kyphosis and vertebral heights were measured by comparing preoperative and postoperative radiographic measurements.
Results
Thirty-one patients (96.9%) exhibited significant and immediate pain improvement: 90% responded within 24 h and 6.3% responded within 5 days. Daily activities improved by 53% on the Oswestry scale. In the OVCF group, kyphosis correction was achieved in 24/27 patients (89.6%) with a mean correction of 7.6°. Anterior wall height was restored in 43/49 VBs (88%) (mean increment of 4.3 mm), and mid vertebral body height was restored in 45/49 VBs (92%) (mean increment of 4.8 mm). Edema (high intensity signal) on short tau inversion recovery (STIR) was evidenced in all OVCF patients who experienced symptoms for less than nine months and was associated with correction of deformity. Cement leakage was the only technical problem encountered; it occurred in 5/49 VBs (10.2%) of the osteoporotic group and 1/12 VBs (8.3%) of the tumor group but had no clinical consequences. The incidence of leakage to the anterior epidural space was 2%. Spinal stenosis was present in three patients (11.1%) who responded successfully to subsequent laminectomy. Retrieval of tissue samples for biopsy was successful in 10/15 cases (67%). New fractures occurred in the adjacent level in 2/27 OVCF patients (7.4%).
Conclusions
Associated spinal stenosis with OVCF should not be overlooked; STIR MRI is a good predictor of deformity correction with balloon kyphoplasty. The prevalence of a new OVCF in the adjacent level is low.
Keywords: Kyphoplasty, PMMA, Osteoporotic vertebral compression fractures, Spinal stenosis, Laminectomy, STIR MRI, Biopsy
Introduction
Osteoporotic vertebral compression fractures (OVCFs) are the most common complication of osteoporosis [18]. Of the estimated 1.5 million osteoporotic fractures that occur annually in the US, 700,000 affect the spine [27, 30]. Eighty-five percent of patients with a radiological diagnosis of OVCF suffer from back pain which is often a major complaint, and may be presented as either acute and excruciating, or chronic persistent pain [26, 33].
Deformities are the most frequent cause of OVCF pain. The degree of kyphosis caused by the OVCF correlates well with the patient’s physical function (independent of pain), mental well-being, pulmonary function, increased mortality and the risk of further fractures [16, 25, 29]. Whether the fracture is painful or not, sagittal spinal deformity dramatically impairs the patient’s functions by reducing mobility and curtailing daily activities. This situation in turn leads to sleep disorders, increased anxiety, depression, lowered self-esteem, a diminished social role and increased dependency on others [25, 32].
Respiratory function (FVC, FEV1) is significantly impaired in patients with thoracic fractures and may result in an increased morbidity and mortality rate. OVCFs are associated with a 23–34% age-adjusted increase in mortality rate compared to patients without VCFs [3, 16, 24]. It has been reported that with each vertebral fracture, there is a 9% loss in predicted force vital capacity [21, 31], which may have detrimental effects particularly in patients with preexisting lung disease.
Sagittal deformity (kyphosis) caused by OVCFs may compromise the biomechanics of the spine, which in turn predisposes to increase the risk of further fractures in the adjacent vertebrae [24]. For this reason vertebroplasty and balloon kyphoplasty were introduced as a more effective management of OVCFs. Osteoporotic vertebral fractures usually occur in an advanced age group of patients that are also prone to other degenerative conditions such as spinal stenosis with leg pain. This comorbid condition has not been previously addressed.
Balloon kyphoplasty consists of percutaneously introducing an inflatable bone tamp into the vertebral body (VB). Reports claim that the bone tamp has the ability to expand and reduce the collapsed VB, improve vertebral height, restore kyphotic deformity, minimize cement leakage and allow the performance of bone biopsy [6, 10, 22].
In this study we assessed (a) the effectiveness and safety of balloon kyphoplasty in the management of pathological VCFs secondary to osteoporosis and metastatic lesions, (b) the short tau inversion recovery (STIR) MRI as a prognostic indicator for restoration of vertebral height, (c) the impact of comorbid conditions such as spinal stenosis and non-specific myofascial pain and (d) the efficacy of biopsy during kyphoplasty.
Materials and methods
Between April 2001 and December 2002, 61 consecutive balloon kyphoplasty procedures were performed in 32 patients (9 male, 23 female) with a mean age of 68.2 years (range 45–91). Patients were divided according to preoperative diagnosis into (a) the OVCF group, which included 27 patients (49 levels) and (b) the tumor group, which included 5 patients (12 levels).
The more frequently treated levels were the L1 and T12 levels in OVCF patients, and in the tumor patients the majority of the treated VBs occurred in the lumbar spine (Fig. 1). Forty-one OVCFs resulted from primary osteoporosis (24 patients) and 8 OVCFs resulted from steroid-induced secondary osteoporosis (3 patients). The diagnosis in the tumor group was (a) 2 metastatic tumors [thyroid (1 level) and breast (3 levels)], (b) 2 multiple myeloma (4 levels), and (c) 1 painful haemangioma (4 levels).
Fig. 1a,b.
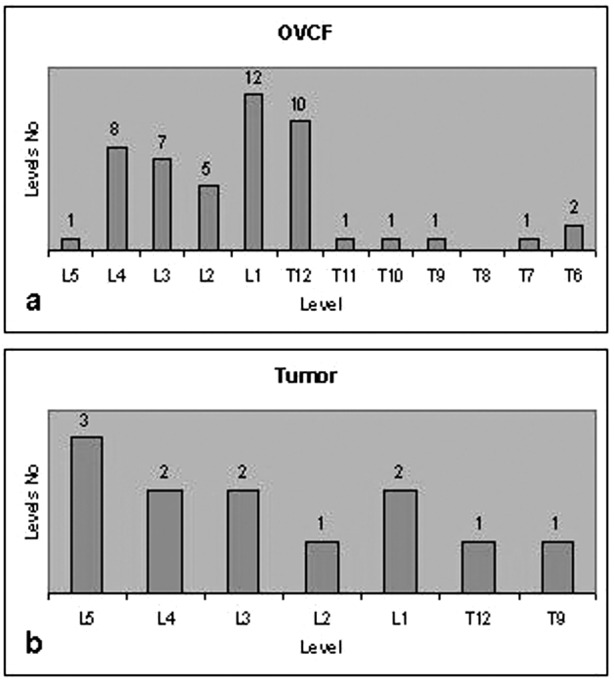
Vertebral bodies distribution treated by balloon kyphoplasty. a OVCF group; b tumor group
All patients preoperatively underwent the following imaging studies: X-rays, bone scans, and MRI [T1-, T2-weighted image sequences and STIR ], and postoperatively underwent X-rays and computed tomography scans (CTs).
Vertebral body height loss and degree of kyphosis was measured in lateral radiographs. The anterior and mid-vertebral heights were defined as the distance between the upper and lower end plates at the anterior VB wall and in the center of the VB respectively. The normal heights for the anterior vertebral wall and mid-vertebral region were considered as the sum of the measurement of the corresponding heights of the adjacent superior and inferior non-fractured vertebrae divided by two.
Vertebral deformity was observed in all of the OVCF group. There were 33 wedge fractures (67.3%) and 16 biconcave fractures (32.7%). In the tumor group, VB deformity (wedge fracture) was observed in only 1/12 levels on lateral radiographs. The remaining 11 vertebrae of the tumor group did not have any associated fractures (radiological deformity).
All patients with OVCF fractures and osteolytic lesions complained of spinal pain, in the thoracic or lumbar region, according to the level of pathology, with duration of symptoms ranging from 15 days to 19 months (mean 4.6 months) before surgery.
Patients were evaluated by means of pain visual analogue scale (VAS), pain drawing, Oswestry disability questionnaire, and detailed physical examination. Clinical examination included point pressure tenderness over the posterior spinous processes and neurological examination of the lower extremities. In the osteoporotic group, three patients also complained of concomitant leg pain and paresthesia secondary to lateral spinal stenosis (subarticular and intraforaminal) that pre-existed to OVCF. One patient in the tumor group (metastatic thyroid cancer) underwent balloon kyphoplasty for intractable low back pain and decompressive laminectomy for severe leg pain, caused by post-radiation adhesive radiculopathy. This patient’s pain failed to respond to radioiodine treatment and local radiation therapy. Eight patients (25%) were non-ambulatory due to severe spinal pain.
The procedure was performed under general anesthesia in 31 patients (59 levels) and under local anesthesia in one OVCF patient (2 levels) because of severe respiratory compromise. The technique described by Lieberman [22] and Ledlie [20] was used. The transpedicular approach was selected in 52 VBs, and the extrapedicular approach was selected in 9 VBs. The extrapedicular approach was used for all levels above T10, and in T12 in three patients. Biopsies were performed in 15 patients.
The mean balloon inflation volume was 5.04 ml (range 3.5–8 ml) and the mean amount of injected bone cement was 5.63 cc (range 3.5–8.5 cc). In the first 25 patients, Simplex-P 10% bone cement (PMMA) containing 10% barium sulfate (Stryker Howmedica osteonics, Limerick, Ireland) was used, to which another 15% sterile barium sulfate was added for a more favorable contrast condition. The setting time of this cement (at a temperature of 21°C in our operating theater) is 12 min. The bone cement used in the last seven cases was KyphX HV-R (Elmdown, London, England), which is a high viscosity polymethyl-methacrylate (PMMA) bone cement, 30.0% w/w barium sulfate for optimal opacification.
The bone cement setting time allows 0.5–1 min for mixing time, 4.5 min for filling the cement inserter, 4–5 min for holding time, 4.5–5 min for working time and 4–4.5 min for hardening time. The cement is considered to have the ideal level of viscosity when it is not runny any more, becomes doughy, does not stick to the fingers and can stand erect at the tip of the bone cement inserter when pushed out. During the insertion of the cement and until completion of surgery, the patient was monitored by recording the arterial blood pressure and blood gasses. Postoperatively, all patients underwent CT scan imaging studies to assess possible cement leakage.
All patients were assessed within the first week postoperative, and then followed up at one and three months; 32 patients were followed up for 12 months (27 OVCFs and 5 osteolytic tumors), 17 were followed up for 18 months (14 OVCFs and 3 osteolytic tumors) and 9 were followed up for 24 months (8 OVCFs and 1 osteolytic tumor) (mean follow-up 18 months).
Statistical analysis
The values of preoperative and postoperative vertebral height and kyphotic deformity were statistically compared using the paired-sample t -test. The VAS and Oswestry questionnaire were statistically compared using paired nonparametric analysis (Wilcoxon signed rank test).
Results
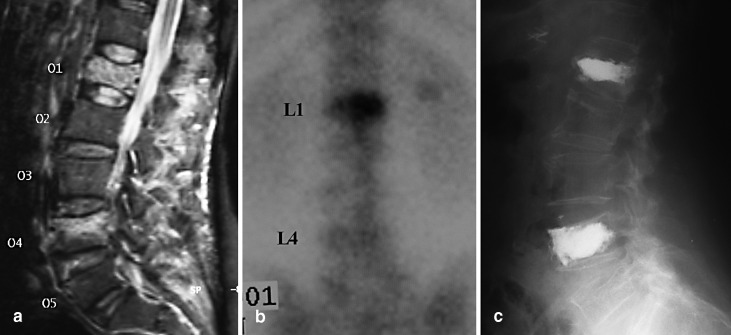
The 99Tc-MDP (methyl-diphosphonate) bone scan was positive in 45/49 VBs with osteoporotic fractures (91.8%). In the four OVCF patients with negative bone scan, duration of symptoms was less than 30 days (15–30 days) in two patients, and greater than 14 months in the other two patients (Fig. 2).
Fig. 2a–c.
A 62-year-old woman who suffered from severe back pain demonstrating a high intensity signal on L1 and L4 VBs. a Both regions were tender to point pressure over the spinal processes. b Radionuclide uptake was marked on the L1 vertebra and questionably present on the L4 vertebra. c STIR MRI showed a high signal intensity suggestive of edema due to micro-fractures. Both levels were successfully treated by means of balloon kyphoplasty
STIR MRI images revealed a hyperintense signal in 45/49 vertebrae with OVCF (91.8%). STIR MRI showed edema in all patients with fractures who had symptoms for less than nine months (Fig. 3). In the two patients with negative bone scan and duration of symptoms less than 30 days, STIR MRI was positive. In two other patients with positive bone scan and duration of symptoms over 11 months, and in the two other patients with negative bone scan and duration of symptoms more than 14 months, STIR MRI did not exhibit a high intensity signal, and balloon kyphoplasty failed to reduce the fracture and improve the kyphotic deformity.
Fig. 3a–c.
66-year-old female patient with a nine month history of back pain after lifting a grocery bag while shopping . a STIR MRI revealed a high intensity signal in the T12 collapsed vertebra, which was the only vertebra tender to point pressure over the spinal process. This finding is highly suggestive of a non-healed fracture (pseudarthrosis). Note that L1, L2, L3 and L5 vertebrae have also sustained OVCFs that have healed—spinal alignment is compromised. Note on plain X-rays improvement of vertebral height after kyphoplasty nine months after the onset of symptoms. b Preoperative X-ray. c Postoperative X-ray
Tenderness to point pressure over the spinous processes, corresponding to the level of pathology in the osteolytic tumor group in 12/12 VBs and in the OVCF group in 47/49 VBs (95.9%). No pain response to pressure applied to the spinous processes was observed in 2 VBs where the bone scan and STIR MRI was negative and the duration of symptoms was more than 14 months.
The mean normal heights in the anterior and mid-vertebral region were 30.8 mm and 31.2 mm, respectively. The mean post-fracture height was 22.4 mm at the anterior vertebral wall and 22.1 mm at the mid-vertebral region. Kyphotic deformity due to fractures was observed in all patients in the OVCF group (27/27), with a mean of 15.9° (range 4–30°). In the tumor group, kyphotic deformity (14°) due to compression fractures was observed in only one patient (multiple myeloma). Lower lumbar biconcave fractures or lytic lesions without fractures did not result in kyphotic deformity.
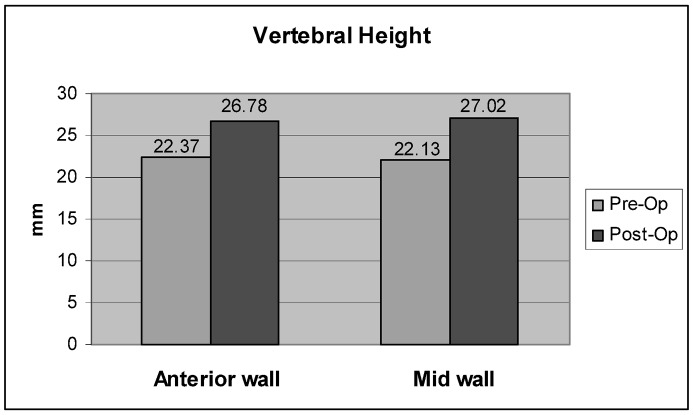
In the OVCF group, improvement of the vertebral height was achieved in 45 of the 49 fractured VBs (92%) (Fig. 4). An increase in the anterior wall height was seen in 43 of 49 VBs (87.7%), with a mean increase of 4.3 mm (range 0–17 mm) ( p =0.001), and a mean restoration of 49% (range 0–92.9%) (Fig. 5). Similarly, the mid vertebral body height was increased in 45 of 49 VBs (92%), with a mean increase of 4.9 mm (range 0–18 mm) ( p =0.001) and a mean restoration of 51.7% (range 0–91.1%) (Fig. 6).
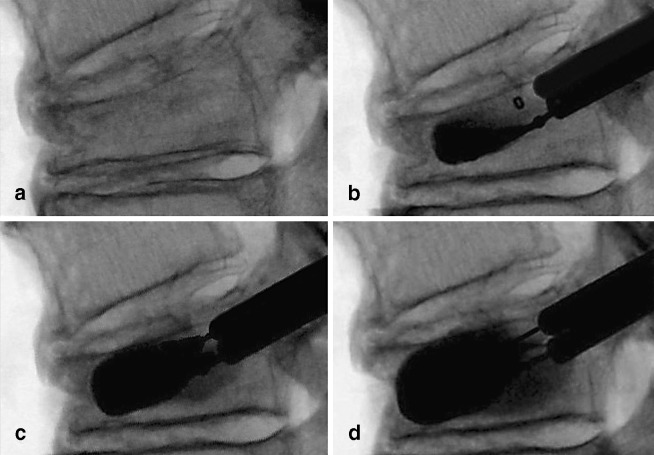
Fig. 4a–d.
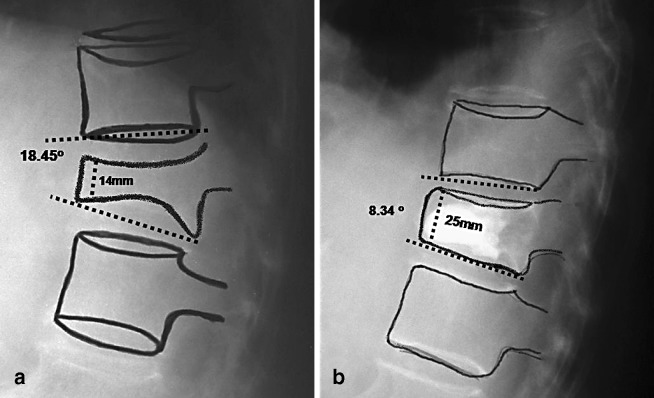
Superior wedge OVCF with a kyphotic deformity of 24.2°. a Expanding bone tamp. b–d Restoration of vertebral height and significant correction of kyphosis (9.9°) by balloon expansion
Fig. 5.
Mean restoration of vertebral body height after balloon kyphoplasty. The reduction of the mid wall was 0.48 mm greater than the anterior wall ( p =0.001)
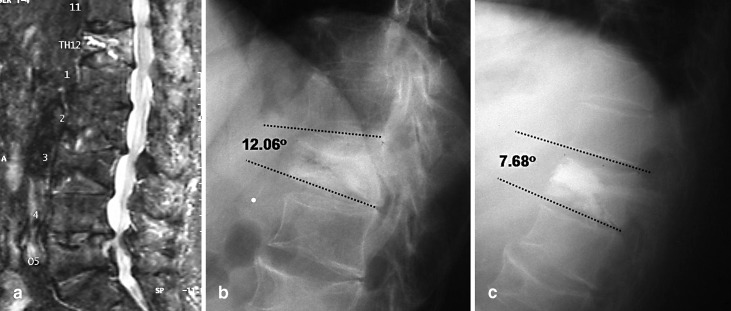
Fig. 6a–b.
Lateral radiograph demonstrating correction of kyphotic deformity. a Preoperative X-ray; b post balloon kyphoplasty restoration of vertebral body height
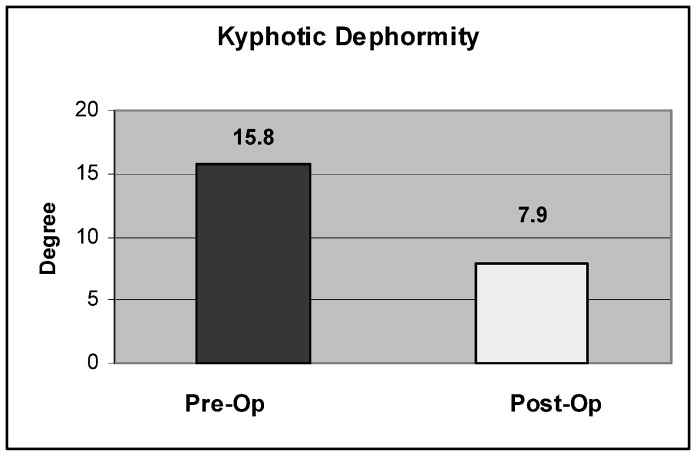
Kyphotic deformity correction was accomplished in 24/27 patients (89.6%), with a mean correction of 7.6° (0–16°) ( p =0.001) or a mean of 53% (range 0–100%) (Figs. 7, 8). In the multiple myeloma patient, the anterior and mid vertebra heights were restored by 8 mm and 12 mm, respectively. Also, kyphotic deformity in this patient was corrected by 12°.
Fig. 7.
Kyphosis correction after balloon kyphoplasty by a mean of 7.9° ( p =0.001)
Fig. 8a–e.
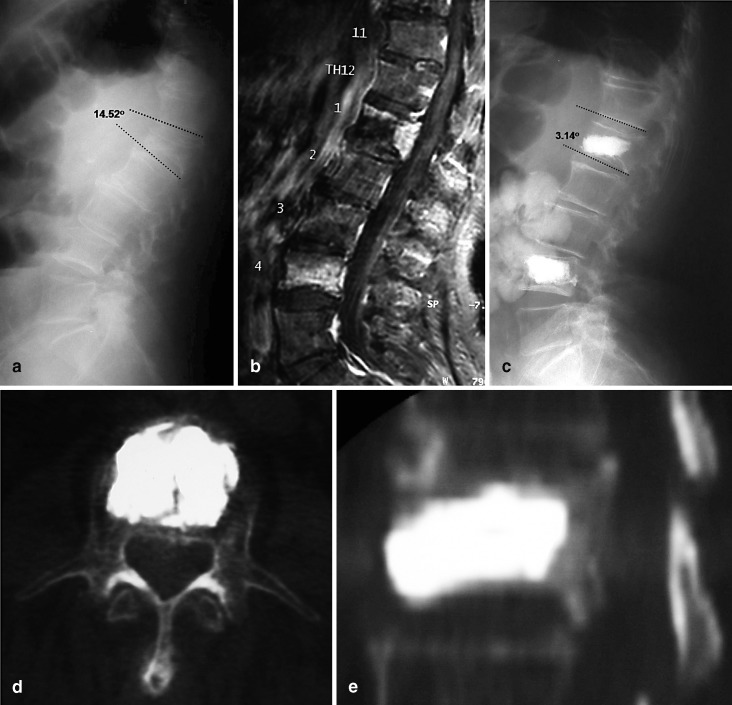
A 91-year-old man with incapacitating back pain that confined him to bed for four months. He was admitted to the hospital with the tentative diagnosis of metastatic tumor. a A lateral radiograph demonstrated a collapsed L1 vertebra. b STIR MRI was suggestive of an OVCF at L1 and L4 vertebrae. Tenderness to point pressure was present over both L1 and L4 spinal processes. The patient underwent a successful balloon kyphoplasty as seen on c lateral X-ray images; d axial view images; e lateral reformatted CT scan images
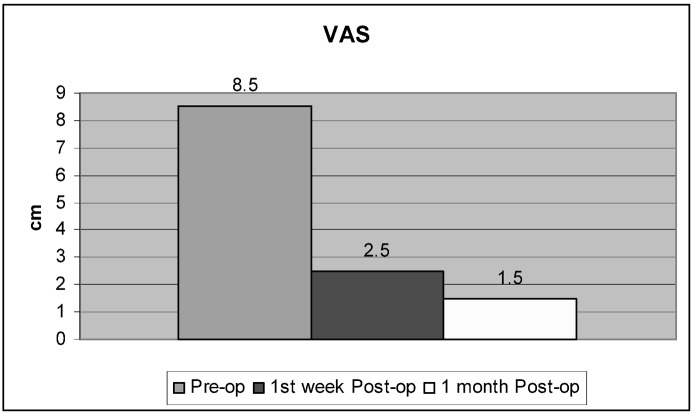
In the OVCF group, 26 of 27 patients (96.5%) ( p =0.001) exhibited excellent improvement of pain within the first 24 h. One patient, in whom no restoration of vertebral height was observed, and who had duration of symptoms of more than 11 months, did not notice significant pain improvement. In the tumor group 3/5 patients exhibited improvement of pain within the first 24 h, whereas in two patients who suffered from metastatic tumor, improvement was noticeable on the fifth postoperative day, at which time analgesic treatment (morphine) was also discontinued. According to the VAS, the mean intensity of pain before surgery in both groups was 8.5 cm and improved to 2.5 cm one week after surgery, and 1.5 cm at the one month follow-up (Fig. 9).
Fig. 9.
Mean improvement of back pain as rated on VAS before balloon kyphoplasty (8.5 cm), and one week (2.5 cm) and one month (1.5 cm) after balloon kyphoplasty ( p =0.001)
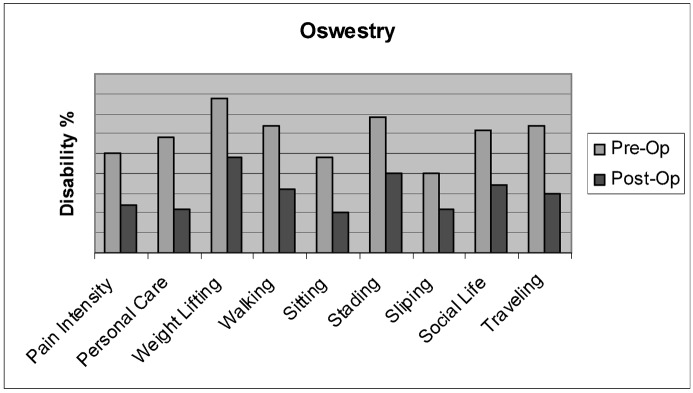
The mean limitation of daily activities before surgery was 60% (range 24–86%) ( p =0.001) on the Oswestry disability questionnaire scale. One month after surgery it improved to 28.3% (range 4–66%) ( p =0.001) (Fig. 10). Ambulation was also completely restored in all eight non-ambulatory patients.
Fig. 10.
VCF disability showed marked improvement of functions after balloon kyphoplasty as rated on the Oswestry disability questionnaire at one month after surgery
One OVCF patient developed a fever of unknown origin for 24 h on the first postoperative day. X-ray of the lungs was negative for PMMA cement embolisation. No neurological complications were encountered. The patient with metastatic thyroid tumor exhibited immediate and sustained pain relief after a combined balloon kyphoplasty and decompressive laminotomy (pain was improved after the fifth postoperative day). He remained pain-free at the follow-up, 15 months after surgery.
Two patients with secondary osteoporosis sustained a new OVCF at a lower level while engaging in rigorous activities (lifting heavy objects, moving furniture) in the fourth and sixth postoperative week respectively. Both were successfully treated with balloon kyphoplasty at the new fractured VB.
In the OVCF group patients who successfully underwent correction of the vertebral deformity, the mean time between the onset of symptoms and surgery was 3.7 months (range 3–9 months), whereas surgery in those patients in whom balloon kyphoplasty failed to correct the deformity was undertaken after 11 or more months from the onset of symptoms (range 11–19 months, mean time to surgery: 14.3 months).
Tissue samples adequate for histologic examination were retrieved in 10 out of 15 patients (67%) in whom a biopsy procedure was performed. Two inflatable bone tamps (3.2%) ruptured inside the VB, but remained attached to the main stem and were easily removed without any consequences.
PMMA cement leakage was detected in 5/49 OVCF VBs (10.2%) and in 1/12 osteolytic tumors (8.3%) (total mean 9.8%), but did not have any clinical relevance. In the OVCFs the leak was observed in the anterior epidural space in one instance (2%), and in the paravertebral region through the lateral wall in two other VBs (4%). Two cases of intradiscal leaks (4%) were also observed. Intradiscal and posterior cement leaks were picked up only on CT scans, whereas lateral leaks were evident on both plain X-rays and CT scans.
Five patients of the OVCF group still had residual paraspinal muscle spasm after balloon kyphoplasty, three of whom also suffered from bursitis of the greater trochanter. These patients’ pain was not attributed to degenerative disk disease but to non-specific myofascial pain. All five patients had their symptoms completely resolved by trigger point injection with local anesthetic (xylocain, 2% Astra company). None of these patients had any history of back pain before the outset of the vertebral fractures.
Also, three OVCF patients were diagnosed with spinal stenosis after clinical examination and imaging investigation. The symptoms of spinal stenosis were evident before the OVCF and remained unchanged after the balloon kyphoplasty. Back pain caused by OVCFs in these three patients responded well to balloon kyphoplasty, however the leg pain, which was not made worse by the procedure, was refractory to this treatment and responded only to surgical micro-foraminotomy without fusion. In two patients the symptoms resolved completely and the third remained with mild residual leg pain.
Discussion
Balloon kyphoplasty evolved from the technique of vertebroplasty, which is the percutaneous injection of PMMA bone cement into a damaged VB. This idea was born out of the excellent results obtained from the use of bone cement to fill defects created in the appendicular skeleton by giant cell tumors. This method was originally conceived to treat lytic tumors by Galibert et al. [8] (hemangioma, myelomas, and metastatic osteolytic tumors) and later by others [4, 5, 17]. Soon after, it was successfully applied to the treatment of OVCFs. Pain improvement is significant with vertebroplasty [9, 36]. However, cement leakage is of some concern and is reported to range between 30–60% [2, 4, 5, 36]. Balloon kyphoplasty is a relatively new technique designed to overcome the shortcomings of cement leakage and to reduce OVCFs [10, 22, 28]. Subsequently, it was successfully applied to the management of osteolytic tumors [6].
Reports have indicated that balloon kyphoplasty is expected to improve pain in 95% of cases [10, 20] and substantially restore the quality of life [22]. In one study, independent and complete ambulation was restored in 11 out of 12 non-ambulatory patients (97%) [20]. This was corroborated in our series that demonstrated significant improvement of mean pain intensity by 70% in 96% of patients and an improvement of physical disability by 53% (Oswestry disability scale improved to 28% from 60%). Restoration of independent walking was observed in 100% of non-ambulatory patients. There were no perioperative medical complications in our series, even in seriously debilitated elderly patients.
Lieberman et al. [22] reported a 20% balloon failure rate. The two inflatable tamp failures (3.3%) in our series might have resulted from protruding bony spicules, which had probably pierced the balloon during the procedure. This potential problem is preventable and can be avoided by tamping the drilled channel with a bone filler device (available in the instrumentation set) in order to break and dispense projecting osseous spicules.
There were no surgical approach-related complications. The transpedicular approach as a channel for intravertebral body surgery has been reported to be both safe and effective [13]. We encountered neither neurological nor vascular complications in our balloon kyphoplasty series. Whether the transient incidence of unknown fever was related to fat/bone marrow pulmonary embolism or cement toxicity is only conjectural.
Another practical advantage of balloon kyphoplasty is its ability to allow the use of a greater than 2.5 mm-gauge biopsy needle which has good chances of retrieving sufficient quantity of tissue for histopathological studies. In our series the success rate was 67%. This can be improved by using technical principles of transpedicular biopsy reported in the literature [14, 34].
The reported cement leakage during balloon kyphoplasty ranges from 8.6–10% [20, 22, 28]. The incidence of cement extravasation in our series was 9.8%, which was mostly encountered in the first few cases in the learning curve and was asymptomatic. From our data and other data [37], CT is more sensitive in depicting cement leakage than plain radiographs.
Lateral paravertebral cement extravasation and intradiscal leakage may occur as a result of existing vertebral wall fracture or by fracture caused by the expanding balloon during reduction of the collapsed vertebra. This can be prevented by avoiding placing the balloon too close to the vertebral walls and by constantly monitoring the balloon during inflation. We do not think that the effect of bone cement extravasation through the anterior or lateral wall has any clinical consequences. However, the biological and biomechanical effects of intradiscal leakage, such as disk degeneration and transfer of loads in adjacent levels, may be of some concern [1]. A recent report has indicated that leakage of cement into the disk space during vertebroplasty increases the risk of a new fracture of adjacent VBs [23].
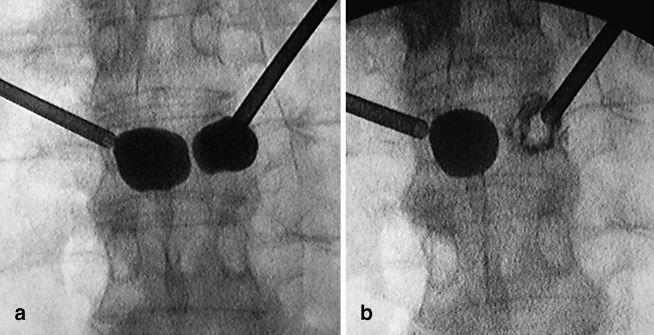
Lateral wall burst fracture can be managed effectively by the so-called “egg shell balloon cementoplasty” described by Lieberman (personal communication). After identification of lateral wall burst fracture by the expanding balloon, the surgeon removes the balloon, inserts 1 cc of thick cement, then reinserts the balloon into the cement substance and re-inflates it, in order to expand the surrounding cement until it abuts the compromised lateral wall. At this stage the cement is allowed to harden. Then the surgeon removes the balloon and proceeds with conventional kyphoplasty cement filling. The technique was tested in two cadaveric specimens by the present authors and was applied in one of our cases (Fig. 11).
Fig. 11 a.
Lateral wall burst fracture from the expanding balloon. b Egg shell cementoplasty as proposed by Lieberman. A shell of cement can be seen around the expanding balloon
Balloon kyphoplasty for OVCF in patients with prior history of vertebral fractures had a 39% incidence of new fractures, whereas for first fracture event, the incidence of new fractures was 10% [15. This suggests that previous fractures may contribute to spinal deformity predisposing to further fractures. Similarly, another report [20] indicates that balloon kyphoplasty, for first fracture event, has a low rate of 4.2% incidence of adjacent vertebral body fracture. In contrast, vertebroplasty studies report the new OVCF rate to range from 12.4% [35] to 52% [12].
A recent biomechanical study showed an increased strain in the anterior wall of the upper adjacent vertebra after a compressive fracture [19]. Another biomechanical study showed an anterior shift (20% of end plate width) of the physiologic compressive preload in the upper and lower VBs adjacent to the fractured VB [7]. Both studies suggest that post-fracture kyphosis may contribute to increasing the risk of new fractures in osteoporotic vertebrae adjacent to an uncorrected VCF deformity.
A retrospective clinical study, with at least 24 months follow-up, demonstrated that 36 new adjacent fractures occurred after vertebroplasty, the majority of which (67%) occurred within the first postoperative month and the rest of the fractures (23%) up to 10 months postoperative. In our series two secondary osteoporotic patients (7.4%), who were given chronic administration of steroids (one for respiratory disease, and the other for histiocytosis), engaged in vigorous and unrestricted activities and suffered new VCFs below the kyphoplasty levels in the 4th and 6th postoperative week. Subsequently they became asymptomatic with balloon kyphoplasty. Also, one of them has an untreated 5-year-old OVCF on the T5 VB, resulting in a severe kyphosis. We can hypothesize that the osteoporosis uncontrolled by medical treatment might have been related to bone fragility and new adjacent fracture occurrence. Therefore, secondary osteoporotic patients should be warned to avoid activities which overload the spine.
The improvement of vertebral body height ranges from 34% [6] to 68% [22]. Lieberman et al. [22] noted that in 70 consecutive levels in 30 patients treated with balloon kyphoplasty (mean duration of the symptoms 5.9 months) the mean percentage of height loss restored was 35% in 70% of the treated levels. In a large multicenter study that included 1,471 balloon kyphoplasty procedures in 944 patients [10], the kyphoplasty procedure restored 48–68% of the lost vertebral body height. In a recent study [28] two-thirds of the fractures were readily reduced (the mean treatment time since the fracture was 3.8 months). In our series, the mean improvement of anterior and mid vertebral height was 4.4 mm in 88% of the VBs (43/49) and 4.9 mm in 92% of the VBs (45/49), respectively. A mean kyphotic correction of 7.6° (mean reduction of kyphosis angle of 53%) was achieved in 89% of the patients (24/27).
Our findings tend to indicate that STIR MRI imaging is the best predictor for restoration of vertebral height and correction of kyphotic deformity by balloon kyphoplasty. Therefore, we can deduce that, when a photopositive radionuclide bone scan is associated with negative STIR MRI, the OVCF may be considered rigid and refractory to correction.
Our data suggests that OVCF deformities are amenable to balloon kyphoplasty correction when the procedure is performed within 9 months of the onset of symptoms when the STIR MRI is positive. A mechanism of correction of deformity after several months can also be assumed to be attributable either to a new fracture of the end plate by the expanding balloon, or pseudarthrosis that yield to the inflatable tamp.
Tenderness to point pressure over the spinous processes of the fractured vertebrae is a sensitive test but the reproducibility of the test has not been validated. Another study [11] reported that the pain on palpation over the fractured vertebra is not a necessary requirement in selecting patients who will benefit from percutaneous vertebroplasty. Other factors, such as MR evidence of edema, should be taken into account in the decision to treat a patient.
It is not unusual for patients in this age group to suffer from concomitant myofascial pain syndrome that easily responds to trigger point injection with local anesthetic, as was used in our cases. In this group of patients with aging spines, the co-existence of OVCF with degenerative spinal stenosis should be of no great surprise. This was encountered in three of our patients (11.1%) who needed decompressive microforaminotomy to address leg pain after successfully treated back pain with balloon kyphoplasty. In one of these three patients, MRI suggested that spinal stenosis might have been aggravated by the osteoporotic collapsed vertebrae resulting in narrowing of the L4–L5 foramen and compression of the L4 nerve root. We believe that these two comorbid conditions can be easily dealt with at the same surgical stage even in this old-age group population.
Conclusions
Balloon kyphoplasty is effective and safe in the management of pathological VCF. STIR MRI can be considered a predictor of deformity correction, which is best managed when surgery is performed within the first nine months of the onset of symptoms. This method has the added advantage of allowing a satisfactory biopsy to be performed simultaneously. Associated spinal stenosis is amenable to decompressive laminectomy. Long-term follow-up studies with large sample sizes are needed to further evaluate this technique.
References
- 1.Belkoff Bone. 1999;25:23S. doi: 10.1016/S8756-3282(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 2.ChirasJ Neuroradiol 199724459303944 [Google Scholar]
- 3.Cotten Radiographics. 1998;18:311. doi: 10.1148/radiographics.18.2.9536480. [DOI] [PubMed] [Google Scholar]
- 4.Cotten Radiology. 1996;200:525. doi: 10.1148/radiology.200.2.8685351. [DOI] [PubMed] [Google Scholar]
- 5.Deramond Radiol Clin North Am. 1998;36:533. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 6.Dudeney J Clin Oncol. 2002;20:2382. doi: 10.1200/JCO.2002.09.097. [DOI] [PubMed] [Google Scholar]
- 7.Gaitanis I, Voronov L, Ghanayem A, Carandang G, Havey R, Zindrick M, Phillips F, Hadjipavlou A, Patwardhan A (2004) Effect of balloon kyphoplasty on the restoration of spinal alignment in the treatment of vertebral compression fractures. Orthopedic Research Society, San Francisco, CA, March 7–10, 2004
- 8.GalibertNeurochirurgie 1987331663600949 [Google Scholar]
- 9.GangiAm J Neuroradiol 199415838141070 [Google Scholar]
- 10.Garfin Spine. 2001;26:1511. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Gaughen J Vasc Interv Radiol. 2002;13:1135. doi: 10.1016/s1051-0443(07)61955-1. [DOI] [PubMed] [Google Scholar]
- 12.Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P (2000) Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology (Oxford) 39(12):1410–1414 [DOI] [PubMed]
- 13.Hadjipavlou Am J Orthop. 1998;27:188. [PubMed] [Google Scholar]
- 14.Hadjipavlou J Interv Radiol. 1996;11:103. [Google Scholar]
- 15.Hyde Eur Spine J. 2002;11:S21. [Google Scholar]
- 16.Kado Arch Intern Med. 1999;159:1215. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 17.Kaemmerlen J Radiol. 1989;70:557. [PubMed] [Google Scholar]
- 18.Kanis Bone. 1992;13:S1. doi: 10.1016/8756-3282(92)90189-4. [DOI] [PubMed] [Google Scholar]
- 19.Kayanja Spine J. 2004;4:76. doi: 10.1016/j.spinee.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.LedlieJ Neurosurg 2003983612546386 [Google Scholar]
- 21.Leech Am Rev Respir Dis. 1990;141:68. doi: 10.1164/ajrccm/141.1.68. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman Spine. 2001;26:1631. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 23.Lin AJNR Am J Neuroradiol. 2004;25:175. [PMC free article] [PubMed] [Google Scholar]
- 24.Linville South Med J. 2002;95:583. [PubMed] [Google Scholar]
- 25.Lyles Am J Med. 1993;94:595. doi: 10.1016/0002-9343(93)90210-G. [DOI] [PubMed] [Google Scholar]
- 26.Lyritis Clin Rheumatol. 1989;2:66. doi: 10.1007/BF02207237. [DOI] [PubMed] [Google Scholar]
- 27.Melton Spine. 1997;22:2S. doi: 10.1097/00007632-199712151-00002. [DOI] [PubMed] [Google Scholar]
- 28.Phillips Spine. 2002;27:2173. doi: 10.1097/00007632-200210010-00018. [DOI] [PubMed] [Google Scholar]
- 29.Pluijm J Bone Miner Res. 2000;15:1564. doi: 10.1359/jbmr.2000.15.8.1564. [DOI] [PubMed] [Google Scholar]
- 30.Riggs BL, Melton LJ 3rd (1995) The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17 [Suppl 5]:505S–511S [DOI] [PubMed]
- 31.Schlaich Osteoporos Int. 1998;8:261. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 32.Silvermann SL (1992) The clinical consequences of vertebral compression fractures. Bone 13 [Suppl 1]:261–267 [DOI] [PubMed]
- 33.Sinaki M (1988) Exercise and physical therapy. In: Riggs L, Melton J (eds) Osteoporosis: etiology, diagnosis and management, Raven, NY, pp 401
- 34.Strimgham Spine. 1994;19:1985. doi: 10.1097/00007632-199409000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Uppin Radiology. 2003;226:119. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 36.Weill Radiology. 1996;199:241. doi: 10.1148/radiology.199.1.8633152. [DOI] [PubMed] [Google Scholar]
- 37.Yeom J bone Joint Surg. 2003;85:83. doi: 10.1302/0301-620X.85B1.13026. [DOI] [PubMed] [Google Scholar]