Abstract
The ligamentum flavum is considered to be one of the important causes of radiculopathy in lumbar degenerative disease. Although there have been several reports anatomically examining the positional relationship between the ligamentum flavum and nerve root, there are few reports on ventral observation. The purpose of this study is to clarify the shape of the ligamentum flavum seen ventrally, and to obtain anatomic findings related to nerve root compression. The subjects were 18 adult embalmed cadavers, with an average age of 78 years at the time of death. The ventral shapes of the ligamentum flavum were observed. The relationships between the morphological change of the ligamentum flavum and nerve root compression or radiographic findings were statistically evaluated. Among the shapes of the ligamentum flavum, bulging of the ligament was most frequently observed. Proximal bulging indicates the type with the cranial portion bulging from the subarticular zone to the foraminal zone of the ligamentum flavum. In this type associated with a decrease in disc height, nerve root compression was frequently observed. Thus, we could more realistically grasp the relationship between bulging morphology of the ligamentum flavum and nerve root compression.
Keywords: Lumbar spine, Ligamentum flavum, Anatomy, Nerve root compression, Disc height
Introduction
The ligamentum flavum attaches to the ventral side of the vertebral arch and makes up the posterior wall of the spinal canal or foramen. Thus, it is considered to be one of the important factors in the causes of radiculopathy or cauda equina symptoms in lumbar degenerative disease [4, 25, 26]. It is reported that in clinical and anatomical biomechanics, the ligamentum flavum bulges inside the spinal canal or foramen at the extension position of the lumbar spine and compresses nerve tissues [7, 10, 19, 22, 24, 31]. One of the reasons may be the change in thickness of the ligament, accompanied by distance shortening between the adjacent vertebral arches [34]. Furthermore, there is a report that foraminal stenosis occurs due to degenerative changes of the lumbar spine, such as a decrease in disc height [5, 7, 14, 19], and volume redistribution of the ligamentum flavum involves nerve compression inside of the foramen [27]. Thus, the morphological changes of the ligamentum flavum that are due to the change of lumbar spine alignment, as well as a decrease in disc height associated with degeneration, may result in compression on nerve tissues. There have been several reports anatomically examining the positional relationship between the ligamentum flavum and dura or nerve root [2, 8, 29], but there are few reports on ventral observation [11], and no reports describe the morphological changes of the ventral side of the ligament accompanied by lumbar spine degeneration. Furthermore, when the ligamentum flavum is resected via a dorsal approach [6, 9], the overall picture of the ligamentum flavum cannot be seen from the dorsal side. Consequently, some of the hypertrophied ligamentum flavum may remain un-resected, which may lead to recurrence due to lack of decompression [17].
In performing lateral fenestration for lesions in the foraminal zone, such as extreme lateral lumbar disc herniation [1, 12, 23, 28], it is difficult to determine decompression range, because the location and shape of the attachment portion of the ligamentum flavum inside of the foramen, or the structure between the transverse processes, are not clear. This area is named the “hidden zone” by Macnab [12]. Furthermore, with respect to the extraforaminal zone, the most exterior area, Wiltse [33] called this the “far-out zone” and reported that the L5 nerve root was compressed between the L5 transverse process and the superior portion of the medial sacral ala at the L5–S1 level. However, there are few detailed reports regarding the structure outside of the foramen [11, 18, 21, 33], so anatomical image in the far-out zone is difficult to grasp.
The purpose of this study was to ventrally observe the ligamentum flavum, including its attachment portion, the structure of its lateral side and the difference between levels, using anatomical cadavers. This study also aimed to examine how the shape of the ligamentum flavum changes on lumbar radiographs and whether or not the morphological changes of these ligaments involve nerve root compression.
Materials and methods
Subjects were embalmed cadavers, ten males and eight females, in the dry state after intravascular fixation. Their age at the time of death ranged 52–98 years (mean, 78 years). In order to prevent shrinkage, we took great care to keep the specimens moist. Lumbar spines were excised from L2 to the sacral spine en bloc, and frontal and lateral radiographs were taken. At that time, cadavers that had excessive deformation associated with tumorous disease or trauma in the lumbar spines were excluded from the subjects. Then, the wire saw was carefully passed through from the foramen to cut the pedicle, with care taken not to damage nerve tissues. The anterior vertebral body was removed, and positional relationships between the dura mater, nerve root, and ligamentum flavum were observed ventrally. Following the removal of the dura mater and nerve root, the shape of the ligamentum flavum was observed. The levels examined included L3–L4, L4–L5, and L5–S1 (a total of 54 levels and 108 sites).
The morphological changes of the ligamentum flavum were observed mainly from the subarticular zone to foraminal zone, according to the Macnab [13] and McCulloch [15] classification systems, using the pedicle as a standard, and normal anatomy as well as abnormal morphological changes were examined. One spine surgeon observed the ligamentum flavum from the ventral side and created classification of its morphology. According to this classification, two spine surgeons independently observed the ligamentum flavum in 108 sites and separately sorted them. As the index of agreement free from coincidence in two spine surgeons, a kappa (κ) statistic was calculated and interobserver agreement was examined.
With respect to the nerve root, we evaluated nerve root compression both in the subarticular zone and in the foraminal zone (Fig. 1). The area covering from the foraminal zone of the L3 nerve root to the subarticular zone of the S1 nerve root was investigated (a total of 144 nerve roots and 216 sites). We defined positive compression as a finding that one-third or more of the diameter of the nerve root was depressed when viewed at least from one direction. Based on this definition, we separately examined the subarticular zone (108 sites) and foraminal zone (108 sites). Two spine surgeons independently determined presence or absence of compression separately. As the index of agreement free from coincidence in two spine surgeons, a kappa (κ) statistic was calculated, and interobserver agreement in each zone was examined.
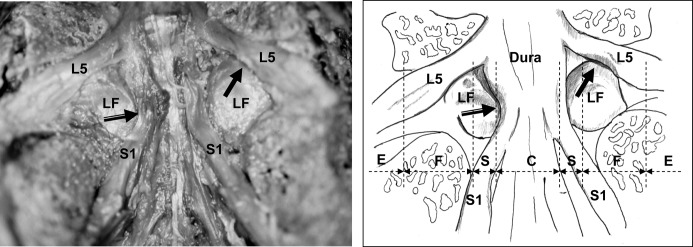
Fig. 1.
Ventral observation at the L5–S1 level showed nerve root compression by the ligamentum flavum (LF) in the subarticular zone (black arrow with white line) and foraminal zone (solid black arrow). The dotted lines of the schema show the zone classification [13, 15]. (Dura dura mater, L5 L5 nerve root, S1 S1 nerve root, E extraforaminal zone, F foraminal zone, S subarticular zone, C central canal)
The angle made by the endplate in each interbody, and disc height (anterior, central, posterior) were radiographically measured (Fig. 2). The relationships between the morphological changes of the ligamentum flavum, nerve root compression, and radiographical findings were statistically examined using a chi-square test and analysis of variance (ANOVA).
Fig. 2.
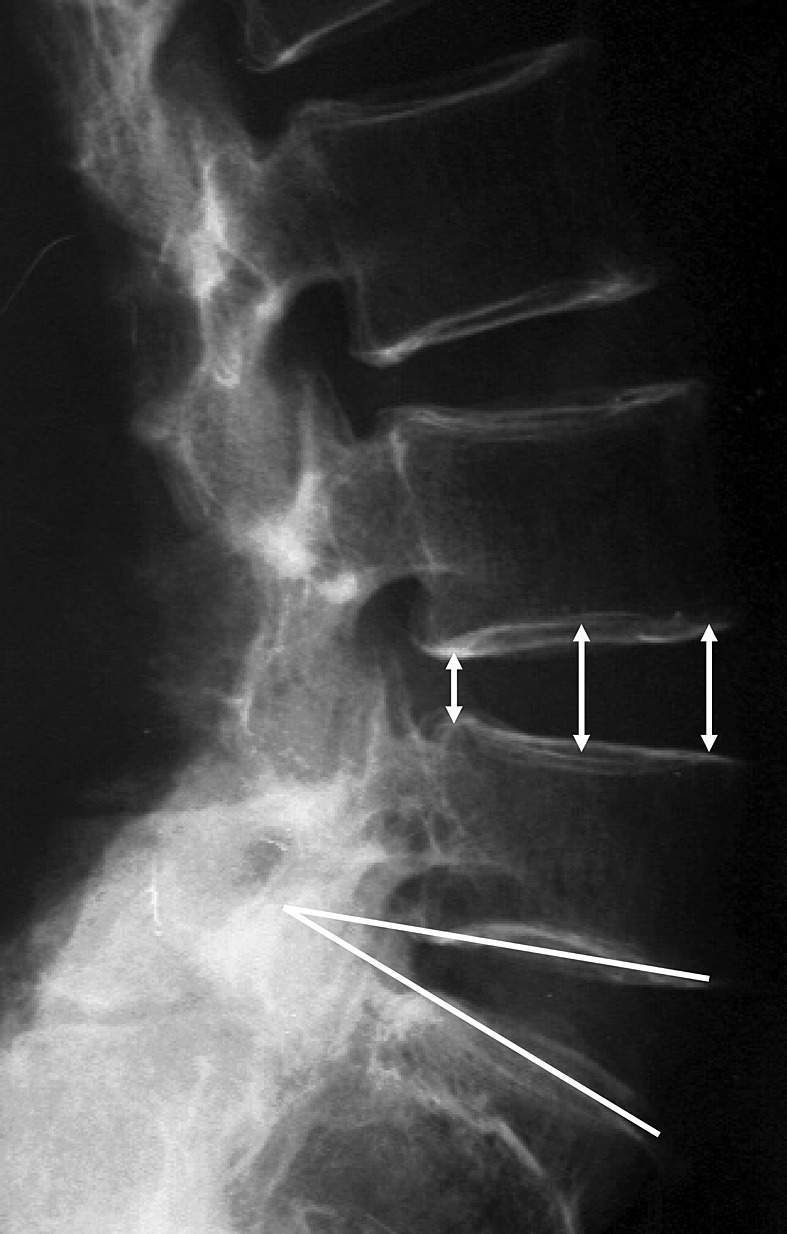
As radiographical measurements, the angle made by the endplate in each interbody and disc height were measured (anterior, central, posterior)
Results
General findings of the ligamentum flavum
Findings on the ligamentum flavum at 45 sites that were considered to be morphologically normal were described, focusing on those commonly observed at each level. When observed ventrally, inside of the central canal, the proximal and distal borders of the ligament ran along the shape of the vertebral arch and dilated laterally in a V-shape from the center. In the foraminal zones at the L3–L4 and L4–L5 levels, the proximal border of the ligament attached almost horizontally and was in contact with the lateral inferior portion of the superior pedicle. The distal border of the ligament attached gradually toward the cranial side, while advancing toward the more lateral side (Fig. 3A). On the other hand, in the foraminal zone at the L5–S1 level, the proximal border of the ligament attached almost horizontally, and the lateral portion was in contact with almost the center of L5 pedicle. The distal border of the ligament attached gradually to the cranial side, while advancing toward the lateral side similarly to other levels (Fig. 4A).
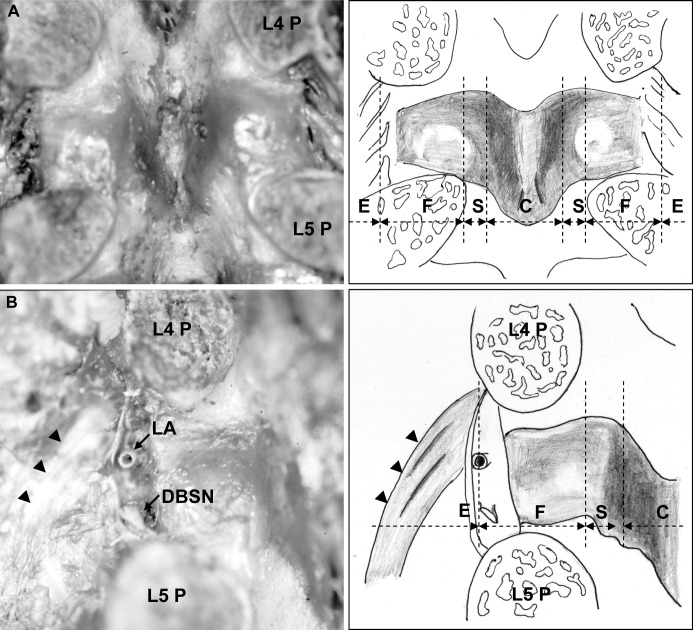
Fig. 3.
The ligamentum flavum at the L4–L5 level is shown. A Inside the spinal canal, both the cranial and caudal borders of the ligament dilated from the center to the lateral side in a V shape. In the foraminal zone, the proximal border of the ligament attached almost horizontally and was in contact with the lateral inferior portion of the superior pedicle. The distal border of the ligament attached gradually toward the cranial side, while advancing toward the lateral side. B From the intraforaminal zone to the extraforaminal zone, the fiber bundle, starting from the superior pedicle, ran as a fan toward the transverse process, forming the transverse ligament (arrowheads). Between the fiber bundle and the ligamentum flavum, the bifurcation of the lumbar artery and vein ran in the proximal side and the dorsal branch of the spinal nerve root in the distal side. The dotted lines of the schema show the zone classification. (P pedicle, LA lumbar artery, DBSN dorsal branch of the spinal nerve root, E extraforaminal zone, F foraminal zone, S subarticular zone, C central canal)
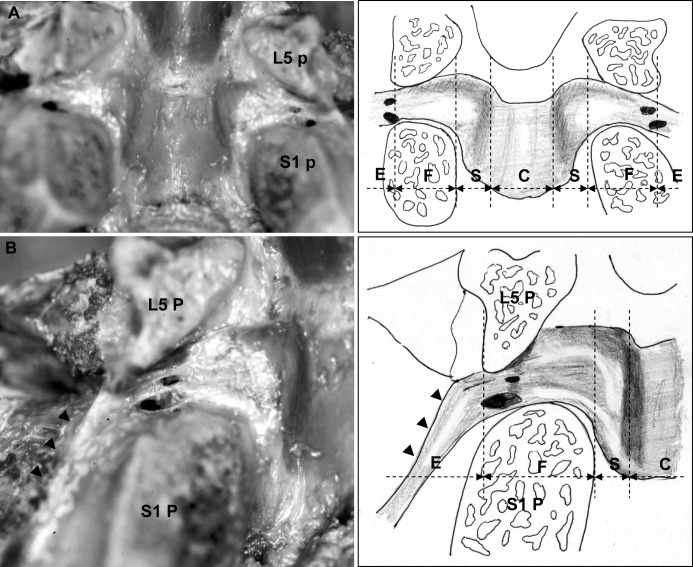
Fig. 4.
The ligamentum flavum at the L5–S1 level is shown. A In the foraminal zone, the cranial border of the ligament attached almost horizontally and the lateral portion was in contact with almost the center of the L5 pedicle. The distal border of the ligament attached gradually to the cranial side, while advancing toward the lateral side, similarly to other levels. B At the intraforaminal zone, the bifurcation of the lumbar artery and vein and the dorsal branch of the spinal nerve root passed. Also, the ligament component extending from the ligamentum flavum attached to the lateral surface of the S1 pedicle, composing the lateral side wall of the neural tube at the extraforaminal zone (arrowheads). The dotted lines of the schema show the zone classification. (P pedicle, E extraforaminal zone, F foraminal zone, S subarticular zone, C central canal)
In the area from the intraforaminal zone to the extraforaminal zone at the L3–L4 and L4–L5 levels, the fiber bundle that started from the superior pedicle ran as a fan toward the transverse process, forming the transverse ligament. Between the fiber bundle and the ligamentum flavum, the bifurcation of the lumbar artery and vein passed in the proximal side and the dorsal branch of the spinal nerve root in the distal side (Fig. 3B). The bifurcation of the lumbar artery and vein and the dorsal branch of the spinal nerve root passed through the foraminal zone at the L5–S1 level. The ligament component that extended from the ligamentum flavum attached on the lateral surface of the S1 pedicle, composing the lateral wall of the neural tube at the extraforaminal zone (Fig. 4B).
When the L5–S1 levels were compared with other levels, distinct differences were observed in the attachment pattern of the ligamentum flavum in the foraminal zone and in the ligament structure in the extraforaminal zone. These characteristic findings were commonly found in all the sites of each level by both observers.
The morphological changes of the ligamentum flavum
We defined abnormal morphology of the ligamentum flavum as follows. Bulging was defined as buckling morphology of the ligamentum flavum, and classified into two types: the whole bulging type and proximal bulging type. In the whole bulging type, the whole ligament bulged, covering from the central canal to the foraminal zone centering on the subarticular zone. In the proximal bulging type, the cranial portion covering from the subarticular zone to the foraminal zone bulged like a boomerang (Figs. 5A and 5B). Erosion was defined as the crater-like morphology on the surface of the ligamentum flavum created by the contact of the annulus fibrosus at the disc level (Fig. 5C). Fold was defined as morphology with one or two horizontal creases in the ligamentum flavum of the foraminal zone (Fig. 5D). Exposure of the superior articular process is the morphology where the cephalic ligamentum flavum of the foraminal zone disappears and the tip of the superior articular process is exposed (Fig. 5E). Furthermore, we defined normal morphology as that with a smooth surface that has none of the characteristics described above. Any abnormal morphology was considered sufficiently characteristic and easy for classification. Interobserver agreement (kappa) on the classification of the ligamentum flavum was 0.892. Since agreement ratio is high, one of the two assortments was selected randomly and used for data analysis. Of all 108 sites, the morphological changes that were considered to be abnormal ligamentum flavum included: whole bulging type (20 sites), proximal bulging type (22 sites), erosion (15 sites), fold (six sites), and exposure of superior articular process (three sites). Three sites where exposure of the superior articular process was observed had multiple other morphological changes.
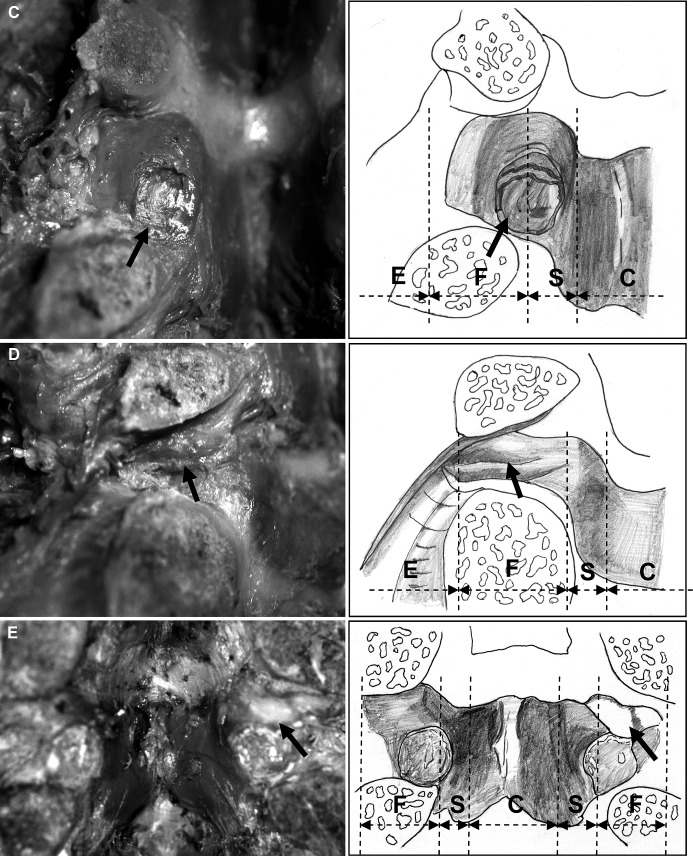
Fig. 5.
With the morphological changes of the ligamentum flavum, A in the whole bulging type, the whole ligament bulged from the central canal to the intraforaminal zone centering on the subarticular zone (20 sites). B With the proximal bulging type, the cranial portion from the subarticular zone to the foraminal zone bulged like a boomerang (22 sites); C Erosion due to contact with disc (15 sites); D Fold (six sites); E Bone exposure of the tip of the superior articular process (three sites) was observed. The dotted lines of the schema show the zone classification. ( E extraforaminal zone, F foraminal zone, S subarticular zone, C central canal)
The relationship between the morphological changes of the ligamentum flavum and radiographic findings
Radiographically, the angle made by the endplate was all dilated anteriorly, ranging 1–28° with a mean of 11°. With respect to disc height, anterior height was from 4.5 mm to 24.0 mm, with a mean of 13.7 mm, central height from 3.0 mm to 17.0 mm, with a mean of 10.9 mm, and posterior height from 1.0 mm to 11.5 mm, with a mean of 7.2 mm.
With respect to the relationship between the morphology and radiographic findings, the mean intervertebral angle of the whole bulging type was 14.3°±2.5°, showing a tendency toward anterior dilatation compared with the mean intervertebral angle for the other sites of 10.5°±0.8°, but no significant difference was observed (p=0.08). Furthermore, there were no significant relationships between the other morphological changes and intervertebral angle (data not shown).
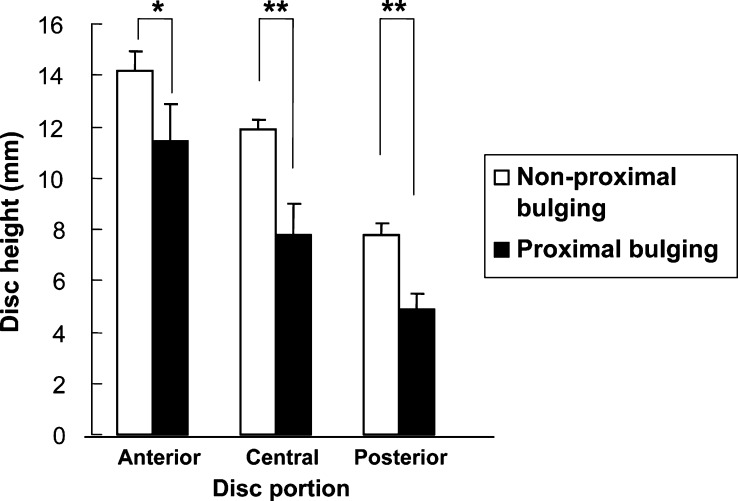
With respect to mean disc height of the proximal bulging type, anterior disc height was 11.4 mm±1.5 mm, central height 7.8 mm±1.2 mm, and posterior height 4.9 mm±0.6 mm. This showed a significant decrease in anterior (p<0.05), central (p<0.01), and posterior heights (p<0.01) when compared with mean disc height in other sites, for which anterior height was 14.2 mm±0.7 mm, central height 11.9 mm±0.4 mm, and posterior height 7.8 mm±0.4 mm (Fig. 6). There were no significant relationships between the other morphological changes and disc height (data not shown).
Fig. 6.
In the proximal bulging type, disc height was significantly decreased in the anterior, central and posterior portions. Data are mean ± SEM. (*p<0.05, **p<0.01)
In all six sites that had the fold in the ligamentum flavum of the foramen portion, lumbar spondylolysis was radiographically observed.
The relationship between the morphological changes of the ligamentum flavum and nerve root compression
Interobserver agreement (kappa) between the two observers regarding nerve root compression was 0.860 for the subarticular zone, and 0.911 for the foraminal zone. Since agreement ratios are high, one of the two evaluations of nerve root compression was selected randomly and used for data analysis.
For all 144 nerve roots and 216 sites, nerve root compression was observed in 35 sites in the subarticular zone and 40 sites in the foraminal zone.
With respect to the relationship between the morphological changes of the ligamentum flavum and nerve root compression, in the proximal bulging type, nerve root compression was observed in 17 of 22 sites in the subarticular zone and 14 of 22 sites in the foraminal zone, showing significant frequency when compared with other sites (p<0.01) (Tables 1 and 2). Furthermore, fold was observed at six sites. Nerve root compression was found, at a higher rate—at four sites in the foraminal zone, showing a statistically significant difference (p<0.01). With regard to other morphology including the normal type, a significant relationship with nerve root compression was not observed (data not shown).
Table 1.
The relationship between the proximal bulging type and nerve root compression in the subarticular zone (Pearson χ2=25.388, p<0.01)
| No. of non-compression sites | No. of compression sites | Total | |
|---|---|---|---|
| Non-proximal bulging | 68 | 18 | 86 |
| Proximal bulging | 5 | 17 | 22 |
| Total | 73 | 35 | 108 |
Table 2.
The relationship between the proximal bulging type and nerve root compression in the foraminal zone (Pearson χ2=8.382, p<0.01)
| No. of non-compression sites | No. of compression sites | Total | |
|---|---|---|---|
| Non-proximal bulging | 60 | 26 | 86 |
| Proximal bulging | 8 | 14 | 22 |
| Total | 68 | 40 | 108 |
Discussion
In the present study, ventral observation of the normal ligamentum flavum revealed that the shape of the ligament is slightly different between the L3–L4 or L4–L5 level and the L5–S1 level. In particular, the most lateral portion of the ligamentum flavum proximal border inside the foramen at the L3–L4 or L4–L5 level is in contact with the lateral inferior portion of the superior pedicle, whereas that at the L5–S1 level is in contact with almost the center of the L5 pedicle. Thus, the nerve root in the foraminal zone at the L5–S1 level may be more vulnerable to compression by the ligamentum flavum compared with that at the L3–L4 or L4–L5 level. Furthermore, from the foraminal zone to the extraforaminal zone at L3–L4 or L4–L5 level, the fiber bundle that starts from the superior pedicle runs as a fan toward the transverse process, forming the transverse ligament. On the other hand, at the L5–S1 level, the lumbosacral ligament that extends to the lateral side of the ligamentum flavum is observed between the L5 transverse process and sacral ala. More lateral inferiorly, the ligament component that is considered a part of the lumbosacral hood [11] or lumbosacral tunnel [18] attaches the lateral surface of the S1 pedicle, composing the lateral wall of the neural tube outside of the foramen. This structure specific to the L5–S1 level may be used as the anatomical base that may cause a stenosis condition in the extraforaminal zone. To precisely grasp the shape of the ligamentum flavum and its positional relationship with surrounding tissues provides a precise prediction of the resection range of the ligamentum flavum in performing surgery for lumbar spine degenerative disease. It also prevents the possibility of leaving a ligament un-resected, and enables safer treatment for lesions in the foraminal zone or extraforaminal zone.
The shape of the ligamentum flavum seen ventrally was not uniform and had changes including bulge, erosion, fold, and bone exposure. The relationship between these morphological changes of the ligamentum flavum and radiographic findings was examined, and a significant correlation between the proximal bulging type and a decrease in disc height was found. A possibility was reported that disc collapse associated with disc degeneration led to a decrease in intervertebral height and frequently buckled the thickened ligamentum flavum [24, 30]. This was consistent with our results. There are two reasons why proximal bulging occurs: (1) A decrease in disc height leads to shortening of the distance between the vertebral arches and thickening of the interlaminar portion of the ligament, while the superior articular process moves to the cranial side, causing infolding in the capsular portion of the ligament [5, 7, 14, 19]. As this mechanism, Rauschning [27] mentioned involvement of a secondary volume redistribution of the ligamentum flavum, due to a decrease in disc height. (2) In the ligamentum flavum of the proximal bulging type, the distal side of the ligament is in contact with the disc and forms depression at that site. Thus, the annulus fibrosus bulges posteriorly in association with a decrease in disc height and compresses the distal side of the ligamentum flavum facing the annulus fibrosus. This causes the cranial portion covering from the subarticular zone to the foraminal zone to bulge like a boomerang.
With the whole bulging type, the intervertebral angle tends to be great, which may be caused mainly by shortening of the interlaminar portion of the ligament, due to a large amount of anterior dilatation of the interbody [7, 10, 19, 22, 24, 31, 34]. In the whole bulging type, disc height was maintained, and compression on the ligamentum flavum due to posterior bulge of the disc was not observed. Thus, the whole ligament centering on the subarticular zone may bulge anteriorly. With other morphological changes, erosion occurred at disc level in the foraminal zone, and the ligament surface was not smooth. This may have developed due to strong contact with the disc. The fold was mainly formed in the cranial side of the foraminal zone, and lumbar spondylolysis was radiographically observed in six sites, which may be the influence by the bone shape of the isthmic spondylolysis. In the fold, nerve root compression was observed at a high frequency in the foraminal zone, so the fold may be the cause of radiculopathy in lumbar spondylolysis. With bone exposure, the ligamentum flavum at the capsular portion disappeared, exposing the tip of the superior articular process. As the cause of this, involvement of not only cranial movement of the superior articular process, due to a decrease in disc height, but also a dynamic element, such as rotation or instability, was suggested.
With respect to nerve root compression, in the proximal bulging type, compression was frequently observed in both the subarticular zone and foraminal zone. As mentioned above regarding the morphological changes of the ligamentum flavum, this result was consistent with the report that secondary hypertrophied ligamentum flavum due to a decrease in disc height was involved in nerve compression [27] or with the report that the foramen area was reduced, accompanied by a decrease in disc height, so the nerve roots in the foraminal zone became vulnerable to compression [7]. Therefore, in patients who clinically complain of lumbar radiculopathy symptoms, with a decrease in disc height on lumbar radiographs, the ligamentum flavum that bulges in the proximal portion from the subarticular zone to the foraminal zone is potentially compressing the nerve root. Thus, surgeons should remember that the nerve root may be compressed by bulging ligament in these zones.
A discussion of our study is limited by the unknown disease history of the cadavers in our clinical setting. Consequently, it was considered impossible to demonstrate clinical symptoms by abnormal morphologies of the ligamentum flavum or nerve root. Considering this limitation, we reviewed, paying attention to the following points. About half of the ligamentum flavum observed in this study had seemingly abnormal morphological changes, and nerve root compression was observed in 75 of 216 sites, comparatively frequently. However, this may reflect the highly degenerative findings in cadavers, many of which were aged 70 years or older [3, 16, 32]. In addition, one study found that compressions of the nerve roots were correlated with histopathological alternations of the nerve roots themselves [20]. Although the posture of a cadaver in the process of embalming may affect nerve root compression, the cadavers that we reviewed in this study were fixed in the neutral position, and those with increased flexion, extension, and lateral flexion were excluded. Therefore, it appeared that the posture of embalming had little effect on nerve root compression.
From the ventral side, we were successful in observing the surface where the ligamentum flavum actually contacts the nerves three-dimensionally. Therefore, we could more realistically grasp the relationship between the swollen morphology of the ligamentum flavum and nerve root compression, which has until now been difficult to imagine.
References
- 1.AbdullahJ Neurosurg 1974412294841878 [Google Scholar]
- 2.AvrahamiSpine 199015212139237 [Google Scholar]
- 3.Boden J Bone Joint Surg Am. 1990;72:403. [PubMed] [Google Scholar]
- 4.Brown J Bone Joint Surg. 1938;20:325. [Google Scholar]
- 5.Dunlop J Bone Joint Surg Br. 1984;66:706. doi: 10.1302/0301-620X.66B5.6501365. [DOI] [PubMed] [Google Scholar]
- 6.Epstein J Neurol Neurosurg Psychiatry. 1962;25:165. doi: 10.1136/jnnp.25.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasegawa J Bone Joint Surg Am. 1995;77:32. [PubMed] [Google Scholar]
- 8.Hasue Spine. 1983;8:50. doi: 10.1097/00007632-198301000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Kirkaldy-Willis Clin Orthop. 1974;99:30. doi: 10.1097/00003086-197403000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kirkaldy-Willis Spine. 1978;3:319. doi: 10.1097/00007632-197812000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi S, Hasue M (1996) Radicular symptoms of lumbo-sacral spine—anatomic consideration. Kanehara, Tokyo pp 32–40
- 12.Macnab J Bone Joint Surg Am. 1971;53:891. [PubMed] [Google Scholar]
- 13.Macnab I, McCulloch JA (1990) Backache, 2nd edn. Williams & Wilkins, Baltimore, pp 1–21
- 14.Mayoux-Benhamou Surg Radiol Anat. 1989;11:97. doi: 10.1007/BF02096463. [DOI] [PubMed] [Google Scholar]
- 15.McCulloch J (1990) Microsurgical approach to the foraminal herniated nucleus pulposus. In: Watkins RG (ed) Microsurgery of the lumbar spine. Raven Press, New York, pp 187–198
- 16.Miller Spine. 1988;13:173. [PubMed] [Google Scholar]
- 17.Nakai J Bone Joint Surg Am. 1991;73:1184. [PubMed] [Google Scholar]
- 18.Nathan Int Orthop. 1982;6:197. doi: 10.1007/BF00267730. [DOI] [PubMed] [Google Scholar]
- 19.Nowiki AJNR Am J Neuroradiol. 1996;17:1605. [PMC free article] [PubMed] [Google Scholar]
- 20.Oishi J Neurosurg. 1995;83:342. doi: 10.3171/jns.1995.83.2.0342. [DOI] [PubMed] [Google Scholar]
- 21.Olsewski Spine. 1991;16:336. doi: 10.1097/00007632-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 22.PanjabiSpine 198383486635782 [Google Scholar]
- 23.Postacchini Clin Orthop. 1979;138:222. [PubMed] [Google Scholar]
- 24.Postacchini Spine. 1994;19:917. doi: 10.1097/00007632-199404150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ramani J Neurol Neurosurg Psychiatry. 1975;38:550. doi: 10.1136/jnnp.38.6.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey Clin Orthop. 1966;44:129. [PubMed] [Google Scholar]
- 27.Rauschning Spine. 1987;12:1008. doi: 10.1097/00007632-198712000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Ray CD (1987) Far lateral decompression for stenosis: the paralateral approach to the lumbar spine. In: White AH, Rothman RH, Ray CD (eds) CV Mosby, St Louis, pp 175–186
- 29.Sato Spine. 1993;18:2246. doi: 10.1097/00007632-199311000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Schönström NR, Hansson TH (1991) Thickness of the human ligamentum flavum as a function of load: an in vitro experimental study. Clin Biomech (Bristol, Avon) 6:19–24 [DOI] [PubMed]
- 31.Sortland Acta Radiol Suppl. 1977;355:42. [PubMed] [Google Scholar]
- 32.VidemanSpine 1990157282146754 [Google Scholar]
- 33.WiltseSpine 19849316719255 [Google Scholar]
- 34.Yong-Hing Spine. 1976;1:226. [Google Scholar]