Abstract
Several clinical and experimental reports have evaluated the spinal application of bioabsorbable material for plating the anterior lumbar and cervical spine, and in anterior and posterior lumbar interbody spinal fusion. Nevertheless, the use of these materials in posterolateral interlaminar fusion has yet to be elucidated in the literature. The effects of bioabsorbable self-reinforced polylactide rod (SR-PLLA) implantation, rigid fixation (K-wire) and non-implantation with posterior interlaminar fusion were compared using a rabbit model. Twenty-four mature domestic rabbits were divided into three groups. Eight received implantation with SR-PLLA, eight with K-wire, and eight were fused without instrumentation. The animals were killed at 12 weeks and evaluated by posteroanterior radiography, manual palpation and histological examination for the presence of fusion. Successful fusion was achieved in all of the animals in both implanted groups (SR-PLLA and K-wire), whereas solid fusion was not detected in any of the specimens in the non-implanted group. Computed tomography (CT) scans were used to detect fusion mass volume. The fusion mass in the SR-PLLA implanted group had a mean volume of 1,196 mm3±167 mm3 vs 1,061 mm3±181 mm3 for the K-wire implanted group (not significant) and 711 mm3±407 mm3 (p<0.05) for the non-implanted group. The results of this study suggest that the stabilization properties of both SR-PLLA rods and K-wire seem to be sufficient for spinal fusion, but using SR-PLLA is especially advantageous, since they do not require a removal operation and do not interfere with magnetic resonance imaging (MRI).
Keywords: Polylactide (PLLA) rod, Spine fusion, Experimental
Introduction
Spinal instrumentation is commonly used with posterolateral spinal arthrodesis to stabilize the treated spine segment until a solid osseous union is achieved [23]. Commonly used metal implants (i.e., titanium alloy and stainless steel) have some drawbacks in regard to stress shielding, due to excessive rigidity and permanence of constructs that can lead to bone resorption and osteopenia [1, 5, 19]. Other disadvantages of the rigid implant systems include the occasional need for removal and interference with magnetic resonance imaging during postoperative follow-up. Although the ideal degree of implant rigidity is unknown, load sharing is necessary for the healing bone to form a trabecular structure. Theoretically, the ideal rigidity is a range of rigidity that varies during different stages of healing [13]. This may be possible with semi-rigid stabilization systems. Bioabsorbable materials are a potential alternative to rigid fixation, since they are able to gradually transmit the load to the fusion mass during the healing phase. These materials do not require a removal operation and do not interfere with MRI. Additionally, since bioabsorbable materials are radiolucent, better visualization of the fusion mass is achieved on plain radiographs.
Bioabsorbable materials have been used successfully in various applications including sutures, repair of craniofacial defects, appendicular fracture fixation and soft-tissue repair [6, 7, 8, 9, 10, 18, 20, 21, 27, 31, 32] Their use in spinal surgery has been advocated recently. Johsson et al. [22], Lowe et al. [24] and Alexander et al. [1] used bioabsorbable polymer implants for facet joint fixation and lumbar interbody fusion in clinical studies. There are in vivo and in vitro animal and cadaver studies in which bioabsorbable materials are used as spinal implants [13, 14, 24, 26, 29, 30, 33]; however, their application as spinal rods for multilevel posterolateral interlaminar fusion has not been documented.
The purpose of this study was to compare the posterolateral healing and fusion process of bioabsorbable self-reinforced polylactide rod (SR-PLLA) implantation, rigid fixation (K-wire) and non-implantation in posterior interlaminar fusion in a rabbit model.
Materials and methods
Twenty-four adult male skeletally mature domestic rabbits weighing 2–2.5 kg underwent a unilateral L4–L6 posterior midline interlaminar fusion [17] using autogenous iliac crest bone graft (ICBG) with and without internal fixation. Internal fixation consisted of instrumentation with SR-polylactide or stainless steel rod.
The experimental groups consisted of:
Group 1: ICBG, SR-polylactide rod instrumentation group (n=8)
Group 2: ICBG, stainless steel instrumentation group (n=8)
Group 3: ICBG, non-instrumented group (n=8) (control)
Surgical technique
Animals were anesthetized with xylazine hydrochloride (8 mg/kg) and ketamine hydrochloride (80 mg/kg) by intramuscular injection. The skin and subcutaneous tissue were infiltrated with 6 ml 0.5% bupivacaine to assist with intraoperative and postoperative pain control. After removal of hair at the operation site and sterile draping, a midline skin incision from L3 to the L6 spinous process was performed. Subperiosteal dissection was used to elevate all muscles from the spinous processes, laminae and superior facets of the right side. A destabilization procedure was done from L4 to L6 with a rongeur, by removing the interspinous ligaments and right-sided facet joint excisions. The removed bones were set aside for grafting. Autogenous iliac crest bone graft of 2 ml (0.8 g) was harvested from the single iliac crest in all animals. The resultant bone graft from the spinous processes and iliac crest were morselized with a rongeur. The laminae of L4, L5, L6 on the right side were decorticated as a fusion bed. The morselized bone graft was implanted along the decorticated laminae from L4 to L6.
In animals receiving internal fixation, transverse holes were made at the spinous process base of L4, L5 and L6 using a towel clamp allowing passage of 26-gauge wire. SR-PLLA rods (Bioscience,Tampere, Finland) with a diameter of 3.2 mm in group 1 and Kirschner wires (Evrenler, Istanbul, Turkey) with a diameter of 3 mm in group 2 were placed on the fusion bed and 26-gauge wires that passed at the base of the spinous processes were tightened to them. The 3.2 mm SR-PLLA rod had initial shear strength of 170–220 MPa, whereas 3 mm stainless steel K-wire had an initial shear strength of 1,450–1,500 MPa. In the control group (group 3) no rod was placed over the fusion bed.
The surgical sites were closed in layers after homeostasis was ensured to obviate the need for a suction drain. Cefazolin (80 mg/kg) was injected intramuscularly immediately before surgery and 1 day after surgery. There were no ambulatory or dietary restrictions, and all wounds healed without complication. Daily, general and neurological examinations were performed on the rabbits.
Specimen harvest
All rabbits were killed 12 weeks postoperatively via intraperitoneal sodium-pentothal injection. Vertebrae L4 to L6 with adjacent paravertebral musculature were removed en bloc after radiological examination. The specimens were also subjected to manual palpation and histological analysis.
Radiological evaluation
Radiological evaluation consisted of posteroanterior radiographs to assess fusion and computed tomography to quantify bone formation. The posteroanterior radiographs were used to grade fusions as “fused” or “not fused,” based on the presence of a continuous trabecular pattern in the fusion mass by two blinded radiologists. The specimens were defined as fused only when both radiologists agreed. Axial CT, 2 mm images (with 1 mm overlap) were produced with a CT scanner (General Electric, USA). Each CT section was digitized and the fusion mass area was manually delineated with a cursor. The surface area of all cuts encompassing the spinal fusion was then summated and multiplied by the average cut thickness to yield a fusion mass volume [12].
Manual palpation of spine fusions
The rods and wires, if present, were carefully removed from all specimens before manual palpation. The lumbar spines excised en bloc were manually palpated by flexion, extension and rotation by two blinded observers. The results were graded as fused when no motion was present and as not fused when any motion was detected. The specimens were defined as fused only when both observers agreed.
Histological examination
After radiographic evaluation and manual palpation, the specimens were fixed by using 10% buffered formol solution and decalcified with Shandon decalcifier (formic acid + sodium citrate). Horizontally dissected segments were dehydrated using 70% and 100% alcohol solutions. The sections that were embedded in paraffin were stained with hematoxylin-eosin and Gomon trichrome. Examination was performed under light microscope. Histopathological analysis was performed by two different pathologists using a semi-quantitative scoring system [15] (Table 1). Fusion scoring was based on the predominant tissue type of the fusion mass located over the interlaminar space. The results were divided into three main groups according to the histopathological scores, as fibrous fusion, fibrocartilaginous fusion and bony fusion.
Table 1.
Histological scoring system and fusion types according to scores
| Score | Histologic appearance |
|---|---|
| 7 | Only bone |
| 6 | Bone > fibrocartilage |
| 5 | Fibrocartilage > bone |
| 4 | Only fibrocartilage |
| 3 | Fibrocartilage > fibrous tissue |
| 2 | Fibrous tissue > fibrocartilage |
| 1 | Only fibrous tissue |
| 0 | Non-union |
| Fusion type | Fusion score |
| Fibrous | 0–2 |
| Fibrocartilaginous | 3–5 |
| Bony | 6–7 |
Statistical method
Fusion rates in different instrumentation groups were compared using Fisher’s exact probability test. A P value of less than 0.05 was considered significant for a statistical test.
Results
Radiographic results
According to the qualitative radiographic assessment by the two independent observers, all of the 12-week fusion masses were solid osseous unions in the implanted groups (groups 1 and 2) (Fig. 1), whereas no solid union was reported radiographically in any of the specimens in the non-implanted group (group 3).
Fig. 1.
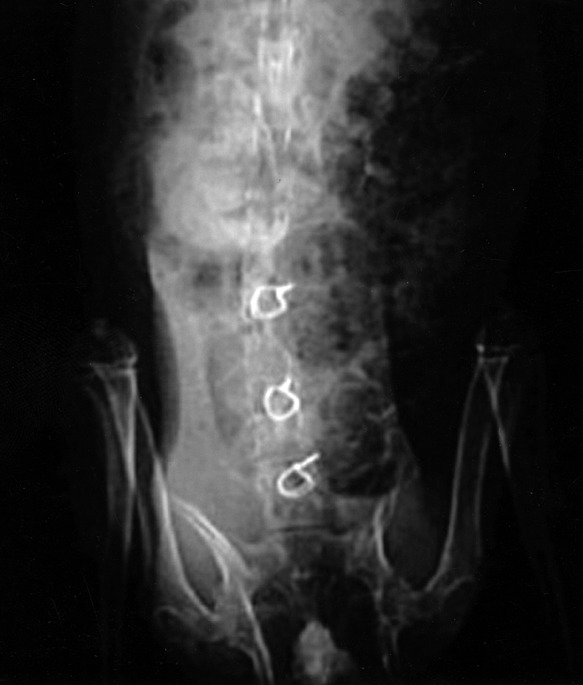
Solid osseous union was present on radiographic AP views over the right interlaminar space in the implanted group. (Left side to the reader)
The fusion mass volumes were measured using CT images (Fig. 2). The mean volume of the fusion mass was 1,196 mm3±167 mm3 in group 1, 1,061 mm3±181 mm3 in group 2 and 711 mm3±407 mm3 in the control group 3. Although the mean fusion mass volume of group 1 was greater than group 2’s, there was no statistically significant difference. However, a statistically significant difference was found between the control group and both group 1 and 2 (p<0.05).
Fig. 2.
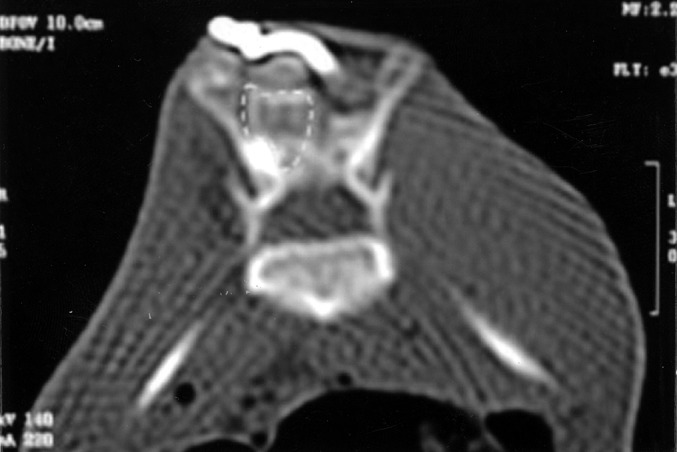
The fusion mass volume was measured with computed tomography (marked with dotted lines)
Manual palpation
Fusion success or failure determination by manual palpation of both observers revealed that solid fusion was achieved in all of the rabbits in groups 1 and 2. Solid fusion was not achieved in any of the specimens in the control group.
Histological results
Histological analysis of the fusion sites of all the specimens showed bony fusion over the interlaminar space in groups 1 and 2 (Fig. 3a and b). Only one fusion occurred over the surface of the laminae and interlaminar space of group 3 (the control group). In this control specimen, the fusion type was mainly fibrocartilaginous. No significant difference was found on statistical analysis of the histologic fusion scores between groups 1 and 2, whereas a significant difference was found between groups 1 and 2 and the control group (p<0.05).
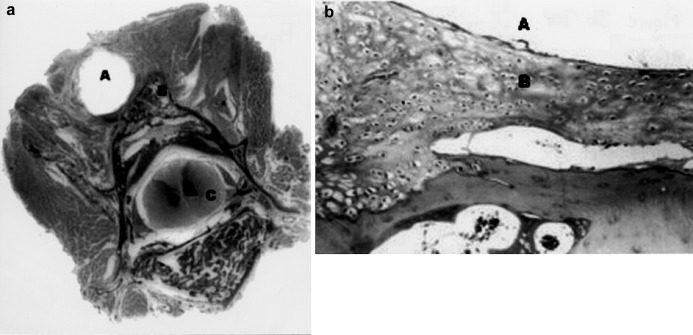
Fig. 3 a, b.
Sections of the paravertebral structures and vertebral body from an implanted animal from group 1 (a gross section original magnification; b hematoxylin-eosin stain, original magnification 40× ). A indicates the rod space; B indicates the fusion mass and C indicates the medulla spinalis
Discussion
The purposes of spinal implants are to maintain spinal alignment, improve the rate of bone fusion healing and decrease the need for external immobilization [27]. Bioabsorbable polymers have significant advantages, including clear imaging without artifact, reduction of stress shielding due to changing mechanical properties over time and elimination of the need for second surgery to remove permanent implants [14]. Some experience has been gained in the use of bioabsorbable materials in spinal fusion. The majority of clinical and experimental studies for these polymers have involved tension band plating in the anterior lumbar and cervical spine [2, 14, 28], anterior and posterior lumbar interbody fusion [22, 24, 26, 29, 30, 33] posterior bone graft containment [2, 25] and bone graft harvest site reconstruction [11]. However, to our knowledge there is no previous report that evaluated the convenience of bioabsorbable materials on posterolateral interlaminar fusion, where polymers were utilized as rods.
In the current study bioabsorbable 3.2 mm diameter SR-PLLA rods were compared with 3 mm diameter metallic rods in the formation of multilevel posterolateral lumbar fusion in a rabbit model. The performance of the bioabsorbable rods was found to be equal to that of the metal rods in terms of fusion after 12 weeks. There are other in vivo animal studies in which bioabsorbable materials are used for spinal fusion. In a study by Van Dijk et al., PLLA cages were compared with titanium cages on the rate of monosegmental lumbar interbody fusion in a goat model [29]. At 3 months, bone ingrowth was observed in PLLA cages but with a radiolucency in the fusion mass. At 6 months, this group observed solid arthrodesis in four of six (67%) of the PLLA implants, whereas none of the three titanium cages of similar design had full fusion. In a similar study with a longer follow-up period (36 months), two resorbable polymer cages with different stiffness and a titanium cage were compared in a goat model [33]. Radiographic evaluation of fusion at 3 months showed ingrowth of trabecular bone within PLLA cages without fusion. Interbody fusion with bridging trabecular bone was observed at 6 months in 80% of the PLLA specimens. Subsequent retrievals at 12, 24 and 36 months showed 88% of PLLA specimens showed fusion, compared with only 66% for the titanium specimens.
In the current study, the fusion mass volume was greater in the SR-PLLA group than in the rigid-fixation group, although it did not reach a statistical significance level. PLLA retains an excess of 90% of its strength for 6–9 months and is resorbed for 18–36 months [24]. As degradation of the PLLA is slow, the rods remained intact during the fusion and thus performed similar mass volume to the metal K-wires. The reduced stiffness of the PLAA, therefore, did not help the fusion process, yet it appeared stiff enough to provide stabilization. Nevertheless, regarding the previous study, [33] in which the newly formed trabecular bone showed a steady increase during the first 12 months within the PLLA cages, it can be assumed that the fusion mass volume could have been greater if the follow up had been longer in the current study.
The PLLA cages used for interbody fusion in the previous studies were full-load bearing. However, the rods in the current study only served for the alignment and were partially unloaded. The fusion was monosegmental in the previous studies, but multisegmental in the current study. Together with the follow-up time, these differences between the studies might be influential for the irrelevancy of the results, where bioabsorbable materials were advantageous in the former studies and non-significant in the current one in the formation of lumbar fusion. In the present study, although the superiority of bioabsorbable polymers was not documented for formation of posterolateral lumbar fusion, their utilization as rods was found to be convenient for fusion formation. This study shows bio-capability, not the biomechanical appropriateness of bioabsorbable rods. For this reason, the comparison of average loads in the rabbit spine with those in the human spine was not performed. For biomechanical evaluation, larger animal models for which the average loads are comparable with human spine (sheep or similar) could be more appropriate for further studies.
In this experiment, SR-PLLA rods with 3.2 mm were sufficient for stabilization of the spine during the formation of fusion mass, but in clinical practice, certainly, stronger absorbable rod systems are required. A 4.5 mm stainless steel rod and SR-PLLA rod with 6.4 mm diameter has strengths of 1,800–2,000 MPa and 1,150–1,760 MPa, respectively. For this reason, a SR-PLLA rod with 6.4 mm or more diameters could be appropriate for the human spine. Moreover, the strength of SR-PLLA rods could be reinforced with the use of co-polymers in the near future.
A bioabsorbable polymeric implant must be absolutely biocompatible and have sufficient biomechanical strength to permit bone healing [16]. The possible disadvantages are loss of the stability due to mechanical weakness and the biological reaction to the host tissues. The absorbable rods are rigid and brittle so that contouring them is usually difficult, which makes them impractical to be used for deformity correction in clinical settings. They can, however, be employed in spinal surgery for in situ fusion that does not require bending of the rods.
In this study, follow-up was terminated at the 12th week, because in previous reports the postoperative fifth week has been shown as the end-point for the strength of fusion mass to maturate, and longer healing times were found not to change the fusion success rate [3, 4].
The strength and maturation of fusion mass were determined by radiographic examination (including volume of the fusion mass), manual palpation of fusion and histologic evaluation. The validity of manual palpation was confirmed by Boden et al. [4] in a previous study. Since there is a close correlation between radiographic evaluation and the biomechanical results of the fusion mass stiffness, [12] we did not perform biomechanical testing over the specimens.
In this study, implantation was done unilaterally, since bilateral application of the implants and their fixation with spinous wiring was impractical on domestic rabbits, due to the small size of the animals. Although there is no scientific data, application of the system unilaterally or bilaterally in our opinion will not change the results on group comparisons.
Conclusion
The effect of SR-PLLA rod application on spinal fusion was evaluated and the results were compared with rigid fixation in a rabbit model. Semi-rigid bioabsorbable PLLA rods were found to be comparable to rigid K-wire fixation for the formation of solid spinal fusion. Although statistically not significant, fusion mass volume was greater in the PLLA rod group. Advantages of eliminating a second removal operation, not interfering with MRI and gradual transmission of load to the fusion mass are the leading reasons for PLLA rods to be used in clinical settings after consistent human data.
References
- 1.Alexander JT, Branch CL, Subach BR, Haid R (2002) Applications of a resorbable interbody spacer via a posterior lumbar interbody fusion technique. Orthopedics Oct 25 [Suppl 10]:1,185–1,189 [DOI] [PubMed]
- 2.Ames CP, Cornwall B, Crawford NR, Nottmeier E, Chamberlain RH, Sonntag VKH (2002) Feasibility of a resorbable anterior cervical graft containment plate. Orthopedics Oct 25 [Suppl 10]:1,149–1,155 [DOI] [PubMed]
- 3.Boden Spine. 1995;20:2. [Google Scholar]
- 4.Boden Spine. 1999;24:320. doi: 10.1097/00007632-199902150-00003. [DOI] [PubMed] [Google Scholar]
- 5.B Biomaterials. 2000;21:2. [Google Scholar]
- 6.Böstman O, Paivarinta U, Partio E, Vasenius J, Manninen M, Prokkanen P (1992) Degradation and tissue replacement of an absorbable polyglycolide screw in the fixation of rabbit femoral osteotomies. 74:1,021–1,031 [PubMed]
- 7.B J Bone Joint Surg Am. 1991;73:148. [PubMed] [Google Scholar]
- 8.B Bone Joint Surg Br. 1991;73:679. doi: 10.1302/0301-620X.73B4.1649195. [DOI] [PubMed] [Google Scholar]
- 9.B J Bone Joint Surg Br. 1989;71:706. doi: 10.1302/0301-620X.71B4.2549074. [DOI] [PubMed] [Google Scholar]
- 10.Bucholz J Bone Joint Surg Am. 1994;76:319. doi: 10.2106/00004623-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Cornwall GB, Thomas KA, Turner AS, Wheeler DL, Taylor WR (2002) Use of a resorbable sheet in iliac crest reconstruction in a sheep model. Orthopedics Oct 25 [Suppl 10]:1,167–1,171 [DOI] [PubMed]
- 12.Curylo Spine. 1999;24:434. doi: 10.1097/00007632-199903010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Deguchi Spine. 1998;23:1. doi: 10.1097/00007632-199806150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Diangelo DJ, Scifert JL, Kitchel S, Cornwall B, Mcvay BJ (2002) Bioabsorbable anterior lumbar plate fixation in conjunction with anterior interbody fusion cages. Orthopedics Oct 25 [Suppl 10]:1,157–1,165 [DOI] [PubMed]
- 15.Emery J Bone Joint Surg Am. 1994;76:540. doi: 10.2106/00004623-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Eppley Plast Reconst Surg. 1995;96:316. doi: 10.1097/00006534-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 17.FeighanSpine 19952017709266 [Google Scholar]
- 18.GillJ Bone Joint Surg Am 1997791. 10.1302/0301-620X.79B1.70209010181 [DOI] [Google Scholar]
- 19.Gogolewski Injury. 2000;31:28. doi: 10.1016/S0020-1383(00)80020-0. [DOI] [PubMed] [Google Scholar]
- 20.Hirvensalo Acta Orthop Scand. 1989;60:601. doi: 10.3109/17453678909150131. [DOI] [PubMed] [Google Scholar]
- 21.Hirvensalo Arch Orthop Trauma Surg. 1990;109:258. doi: 10.1007/BF00419939. [DOI] [PubMed] [Google Scholar]
- 22.Johnsson Eur Spine J. 1997;6:144. doi: 10.1007/BF01358748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KanayamaJ Bone Joint Surg Am 1997791. 10.1302/0301-620X.79B1.70209010181 [DOI] [Google Scholar]
- 24.Lowe TG, Coe JD (2002) Bioresorbable polymer implants in the unilateral transforaminal lumbar interbody fusion procedure. Orthopedics Oct 25 [Suppl 10]:1,179–1,183 [DOI] [PubMed]
- 25.Poynton AR, Zheng F, Tomin E, Lane JM, Cornwall B (2002) Resorbable posterolateral graft containment in a rabbit fusion model. Orthopedics Oct 25 [Suppl 10]:1,173–1,117 [DOI] [PubMed]
- 26.Toth JM, Estes BT, Wang M, Seim HB 3rd, Scifert JL, Turner AS, Cornwall GB (2002) Evaluation of 70/30 D,L-PLa for use as a resorbable interbody fusion cage. Orthopedics Oct 25 [Suppl 10]:1,131–1,140 [DOI] [PubMed]
- 27.Vaccaro AR, Madigan L (2002) Spinal applications of bioabsorbable implants. Orthopedics Oct 25 [Suppl 10]:1,115–1,120 [DOI] [PubMed]
- 28.Vaccaro AR, Carrino JA, Venger BH, Albert T, Kelleher PM, Hilibrand A, Singh K (2002) Use of a bioabsorbable anterior cervical plate in the treatment of cervical degenerative and traumatic disc disruption. J Neurosurg 97 [Suppl 4]:473–480 [DOI] [PubMed]
- 29.Van Spine. 2002;27:682. doi: 10.1097/00007632-200204010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Van Spine. 2002;27:2. doi: 10.1097/00007632-200212010-00002. [DOI] [Google Scholar]
- 31.Viljanen J, Kinnunen J, Bondestam S, Majola A, Rokkanen P, Tormala P (1995) Bone changes after experimental osteotomies fixed with absorbable self-reinforced poly-L-lactide screws or metallic screws studied by plain radiographs, quantitative computed tomography and magnetic resonance imaging. Biomaterials. 16:1,353–1,358 [DOI] [PubMed]
- 32.Viljanen Ann Chir Gynaecol. 1997;86:66. [PubMed] [Google Scholar]
- 33.Wuisman PIJM, Van Dijk M, Smit TH (2002) Resorbable cages for spinal fusion: an experimental goat model. Orthopedics Oct 25 [Suppl 10]:1,141–1,148 [DOI] [PubMed]