Abstract
Objective
Many components of the immune system undergo adverse changes during intense physical activity in athletes, leading to a heightened risk of respiratory tract infections. This study evaluated the reduction in infectious processes in athletes due to intensive training with anapsos.
Methods
The study compared athletes who took 480 mg Polypodium leucotomos Extract (Armaya fuerte; Centrum laboratories, Alicante, Spain) twice daily for 3 months (n = 50) with a control group (n = 50) in the evaluation of the onset of infectious processes and relapses during an 8-month period (June 2010 to January 2011).
Results
The onset of infectious processes in the Polypodium leucotomos Extract group was lower when compared to the control group (14% versus 56%). Relapse in the Polypodium leucotomos Extract group was seen in just one athlete (14.2%) compared to ten athletes (37.5%) in the control group.
Conclusion
Polypodium leucotomos Extract has been shown to be useful in the prevention of infectious processes, as well as reducing recurring episodes in athletes.
Keywords: immunological stress, recurrent infections, polypodium leucotomos extract
Introduction
The concept of the “open window,” first introduced by Neiman,1 refers to a period of time when different components of the immune system present adverse effects in the athlete during intense physical activity.1,2 Moderate physical activity may improve the immune function, whilst high-intensity exercise has a temporary effect on immune response.3–5 During this period of immunosuppression, there is an added risk of contracting infections caused by viruses and bacteria,2,6,7 with the onset of symptoms characteristic of the upper respiratory tract,6,8 which increase relative to the intensity and duration of the exercise undertaken.9–11 Nevertheless, in those who do not practise sport, increased physical activity is associated with a lower risk of respiratory infections.8,12,13 The changes related to a series of components of the immune system, such as natural killers, macrophages, and lymphocytes,4,5 affect different parts of the body including the skin, mucus membranes, lung tissue, blood, or muscles.14 Lack of sleep, stress, or unsuitable diet during periods of competition are additional factors for the immunosuppressant effect in athletes.15,16 Studies have shown that this immunosuppression normally lasts for 3–72 hours after intense physical activity,5 although the possibility of acquiring infection may extend to 1–2 weeks for athletes taking part in competitions.15 Diverse studies have been carried out with supplements such as zinc, vitamin C, other antioxidants, and glutamine, but no conclusive data has emerged on these substances to prove their positive effect on the immune system.8,13,15
It would seem that carbohydrate drinks contribute to tackling this effect to some extent, although the period of effect is very short. For a number of years, use has been made of drugs known as biological response modifiers, which have been seen to directly modify the specific immune function or to have a positive or negative effect in the activity of the immune system. Immunomodulators, such as glychophosphopeptical19– 21 and Polypodium leucotomos Extract (Armaya fuerte, Centrum laboratories, Alicante, Spain),21–34 have been shown to influence the components of the immune system. In vitro studies carried out on humans show that Polypodium leucotomos Extract stimulates the proliferation of peripheral blood mononuclear cells in vitro and increases interleukin- 2 (IL-2) and interferon-γ secretion. It also enhances the stimulant effect of other mitogens on cytokines such as IL-10.22 Moreover, it is capable of significantly reducing and delaying IL-1β secretion in the same way it tends to reduce tumor necrosis factor-α levels. Several of the correlations obtained in the reference studies for different cytokines lead to the conclusion that Polypodium leucotomos Extract actually produces an opposing and independent effect on IL-1β and IL-2 and IL-1β and IL-10.21,22 These results indicate a pleiotropic effect of Polypodium leucotomos Extract for different cytokines, probably due to a different way of acting on distinct areas of the immune system.
In vitro and in vivo studies also performed on humans clearly show an increase in the percentage of cells with membrane antigen expression, characteristic of T-lymphocytes and natural killer cells,27 as well as certain activation markers (CD69, CD25).27 The action of Polypodium leucotomos Extract on these lymphocyte populations makes it an interesting product for evaluation in the treatment of certain infectious diseases (especially those caused by a virus). At 24–48 hours, Polypodium leucotomos Extract produces a significant decrease in the expression of alpha (CD11a and CD11b) and beta chains (CD18) of the β2 integrins ( adhesion molecules) in both monocytes and in lymphocytes.24 The action of Polypodium leucotomos Extract, as a regulator of the expression of various adhesion molecules, suggests that it is potentially of interest for use as a coadjuvant in the treatment of systemic or organ-specific inflammatory and/or autoimmune diseases.21,25,26,31 Polypodium leucotomos Extract has been shown to be efficient in recurrent nonbacterial pharyngoamygdalitis28,29,33,34 and in papillomavirus infections,30–32 leading to a reduction in relapse as well as improved clinical symptomatology in the lesions caused by viral infection. These results suggest that Polypodium leucotomos Extract focuses its action on cell immunity.21,22
Therefore, Polypodium leucotomos Extract may be effective in reducing infections in athletes during the open window. The aim of this study was to evaluate the activity of Polypodium leucotomos Extract in the onset/reduction of infectious processes in the respiratory tract, from a clinical point of view, in a group of athletes undergoing intense training and participating in official competitions, compared to the results obtained in a control group which did not receive treatment. Neither of the groups received vitamin or mineral supplements during the period of study.
Materials and methods
An observational study was undertaken at sports medicine clinics of the Department of Sports Medicine de Mallorca (Spain), following normal clinical practice. The appearance of infections due to viral, bacterial, or fungal processes, as well as the immunosuppressant effect during intense physical activity, were noted.
The study group was made up of athletes who took part in competitive activity (any sport), trained or competed > 20 hours per week, were aged ≥ 18 years, and were periodically monitored in sports medicine clinics. A total of 116 athletes were included (63 in the Polypodium leucotomos Extract-treated group and 53 in the control group), with 58 males and 58 females aged 18–30 years. The average age of the sample was 24.6 years and 23.2 years for the males and females, respectively.
Exclusion from the study for all patients was based on a history of autoimmune or chronic disease – as when such a disease was clearly detected, completion criteria for the drug could not be sufficiently guaranteed. During the study, 13 athletes from the Polypodium leucotomos Extract-treated group were excluded due to poor compliance with the treatment or because they left voluntarily, and three athletes from the control group chose to leave the study.
The distribution of athletes by sport in the study group (Polypodium leucotomos Extract-treated) and the control group was similar, with preference for the practice of volleyball, football, athletics, and cycling. This allowed the examination of Polypodium leucotomos Extract performance in both individual and team sports. The final number of athletes in the study group (Polypodium leucotomos Extract-treated) (n = 50) took 480 mg Polypodium leucotomos Extract daily in two 240 mg doses, one in the morning and one at night, over a period of 3 months (beginning in June 2010); the control group (n = 50) did not take Polypodium leucotomos Extract. A record was made of all the incidences and effects noted over the entire study period for both groups, making a final comparison in the incidence of the appearance of viral and bacterial pathologies between the two groups. The observation period was 8 months (June 2010 to January 2011), which included part of the summer and autumn in order to obtain an overall view of the pathologies presented at different stages of the summer–autumn, with later reviews at 5 and 8 months. Clinical variables were correlated – including the duration over time of the symptomatology, onset of fever, headaches, sore throat, general malaise, and relapse of the same pathology – and compared between the two groups.
Before starting the study, a complete examination and clinical history was taken. It was also established that the athletes would report, at the very first opportunity, the onset of any symptom compatible with bacterial or viral pathologies as well as the onset of fever, febricula, or any other significant events that might be related to an infectious disease.
In none of the selected cases were antiinfluenza or any other type of vaccination applied.
Results
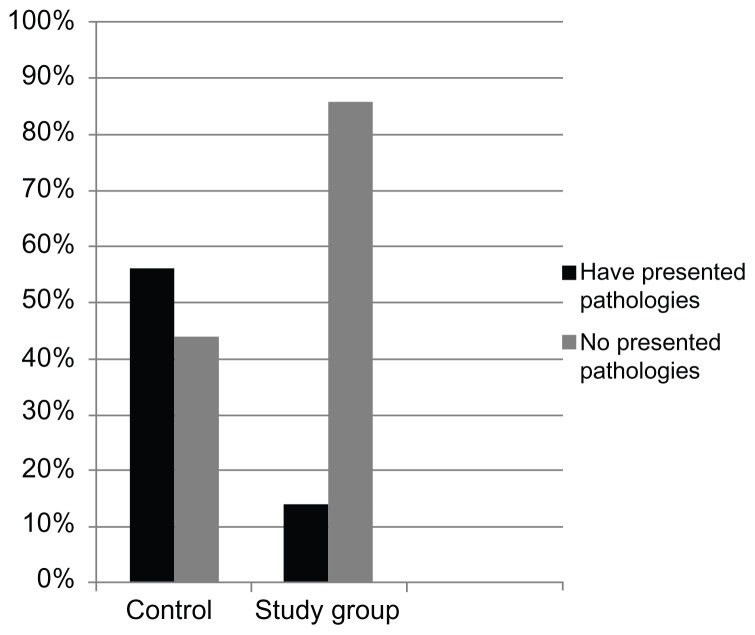
In the control group, the following diagnoses were made: 12 cases of pharyngoamygdalitis (ten nonbacterial and two bacterial), four cases of rhinopharyngitis, six cases of otitis (three bacterial and three nonbacterial), five cases of respiratory tract infection, and one case of influenza syndrome. In the study group, the following diagnoses were made: three cases of pharyngoamygdalitis (two nonbacterial and one bacterial), two cases of conjunctivitis, one case of acute gastroenteritis, and one dental abscess (Table 1). In regard to the onset of infectious processes, a total of 28 cases (56%) were detected in the control group compared to 7 cases (14%) in the study group (Polypodium leucotomos Extract-treated) (Figure 1).
Table 1.
Prevalence of infectious processes in the control group and study group (Polypodium leucotomos Extract-treated)
| Infectious processes: control group | Infectious processes: study group |
|---|---|
| Nonbacterial pharyngoamygdalitis (n = 10) | Nonbacterial pharyngoamygdalitis (n = 2) |
| Bacterial pharyngoamygdalitis (n = 2) | Bacterial pharyngoamygdalitis (n = 1) |
| Rhinopharyngitis (n = 4) | Conjunctivitis (n = 2) |
| Viral otitis (n = 3) | Acute gastroenteritis (n = 1) |
| Bacterial otitis of the lower tract (n = 3) | Dental abscess (n = 1) |
| Respiratory tract infection (n = 5) | |
| Influenza syndrome (n = 1) | |
| Total: 28 cases | Total: 7 cases |
Figure 1.
Percentage of athletes who presented pathologies in the control group and the study group (Polypodium leucotomos Extract-treated).
All subjects were administered with conventional treatment (ibuprofen, paracetamol, and antibiotics) in the cases that were clearly the result of bacterial pathology.
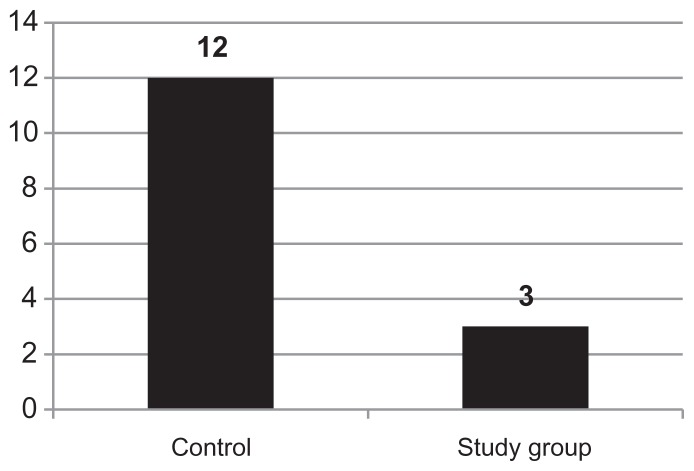
Of the total number of infectious processes, the cases of pharyngoamygdalitis were the most noteworthy – 12 patients (24%) in the control group compared to three patients (6%) in the Polypodium leucotomos Extract-treated group (Figure 2).
Figure 2.
Number of athletes with conditions of viral/bacterial pharyngoamygdalitis in the control group and study group (Polypodium leucotomos Extract-treated).
No correlation was seen between the different age groups since these were very homogeneous and there was no significant difference in the average ages.
Intercurrent infectious processes were present in 58% of males and in 42% of the females. In the group treated with Polypodium leucotomos Extract, the incidence of infection was 56% for males and 44% for females.
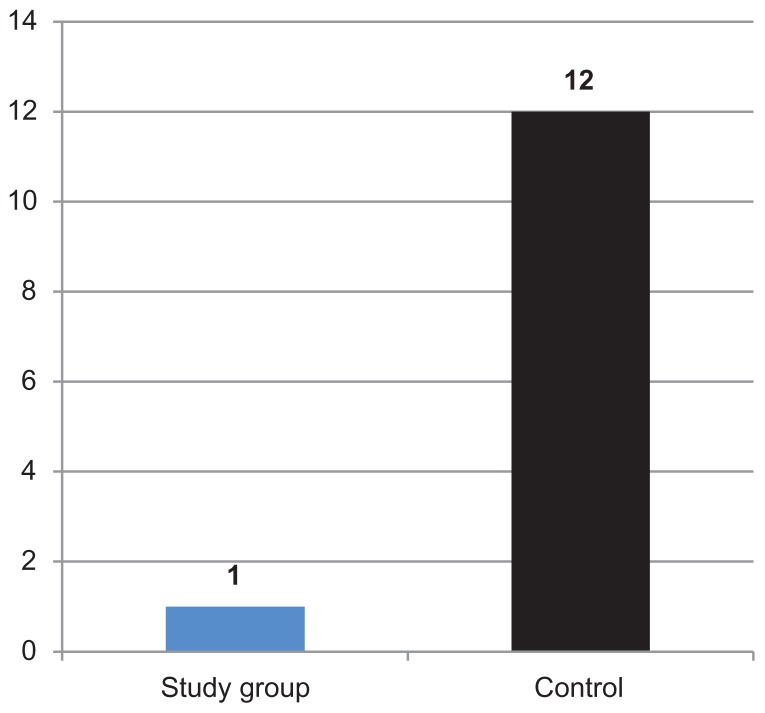
While monitoring infectious processes, it was observed that symptomatic improvement was more favorable in patients from the study (Polypodium leucotomos Extract-treated) group – an asymptomatic state was reached in a significantly shorter timeframe and accompanied with a lower number of cases of relapse. In the Polypodium leucotomos Extract-treated group, one athlete presented a state of relapse for nonbacterial pharyngoamygdalitis. In the control group, 12 athletes presented relapse states of viral processes (nonbacterial pharyngoamygdalitis, viral otitis, poor respiratory response) Figure 3.
Figure 3.
Number of athletes with relapses in the control group and study group (Polypodium leucotomos Extract-treated).
Discussion
In regard to etiopathology and symptomatology, the majority of intercurrent infections in athletes are similar to those of the general population,6 with a greater incidence in infectious processes in the upper respiratory tract.3,7,15,18 Moderate physical activity may improve the working of the immune system, whilst prolonged physical activity over time may temporarily affect the capacity of the immune system.2,8,13 Competitive athletes, compared to less physically active people, are more prone to contracting infections.5,9,10,17 In normal individuals, physical activity is associated with greater protection against infections of the upper respiratory tract.6 The duration of the immunosuppressant effect depends on the intensity of the exercise and on the prevailing state of the athlete’s immune system.
In the present study, 50 patients were included in the study group (Polypodium leucotomos Extract-treated) and 50 patients took part in the control group during the 8-month period of June 2010 to January 2011. In the control group, 28 athletes (56%) presented infectious conditions during the study, whilst just seven cases (14%) were detected in the Polypodium leucotomos Extract-treated group. It is important to point out that the relapses seen in different infectious processes, especially the viral infections, occurred in one athlete from the study (Polypodium leucotomos Extract-treated) group and 12 athletes from the control group.
Conclusion
Different studies have demonstrated that Polypodium leucotomos Extract possesses antiinflammatory and immunomodulatory activity, producing different effects on cell immunity – both natural and specific. The stimulant effect caused by in vivo or in vitro Polypodium leucotomos Extract on immune system cells (T lymphocytes and natural killer cells) and its effect on diverse cytokines gives it a significant immunomodulatory capacity and suggests antiinflammatory activity, improved clinical symptomatology, and reduced relapse process in recurrent infections. Polypodium leucotomos Extract offers an optimum security profile alone or when used with other medications, thus offering a suitable alternative for the prevention of the open window effect in athletes who might be vulnerable to immunological stress due to intense physical exercise.
During the treatment study period and later evaluation, the athletes treated with Polypodium leucotomos Extract demonstrated a lower incidence of infectious diseases – related to the degree of physical activity, their symptomatic processes – when infected – were shorter than those athletes in the control group, and they were much less prone to relapses. Polypodium leucotomos Extract has been shown to be useful in the prevention of intercurrent infectious processes when an appropriate dose is prescribed and compliance is adequate. Further studies are required to confirm the results of the present study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nieman DC. Upper respiratory tract infections and exercise. Thorax. 1995;50(12):1229–1231. doi: 10.1136/thx.50.12.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieman DC. Exercise, infection, and immunity. Int J Sports Med. 1994;15(Suppl 3):S131–S141. doi: 10.1055/s-2007-1021128. [DOI] [PubMed] [Google Scholar]
- 3.Nieman DC. Risk of upper respiratory tract infection in athletes: an epidemiologic and immunologic perspective. J Athl Train. 1997;32(4):344–349. [PMC free article] [PubMed] [Google Scholar]
- 4.Nieman DC, Pedersen BK. Exercise and immune function. Recent developments. Sports Med. 1999;27(2):73–80. doi: 10.2165/00007256-199927020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kakanis MW, Peake J, Brenu EW, et al. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. 2010;16:119–137. [PubMed] [Google Scholar]
- 6.Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep. 2003;2(5):239–242. doi: 10.1249/00149619-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Moreira A, Delgado L, Moreira P, Haahtela T. Does exercise increase the risk of upper respiratory tract infections? Br Med Bull. 2009;90:111–131. doi: 10.1093/bmb/ldp010. [DOI] [PubMed] [Google Scholar]
- 8.Nieman DC. Immunonutrition support for athletes. Nutr Rev. 2008;66(6):310–320. doi: 10.1111/j.1753-4887.2008.00038.x. [DOI] [PubMed] [Google Scholar]
- 9.Akerstrom TC, Pedersen BK. Strategies to enhance immune function for marathon runners: what can be done? Sports Med. 2007;37(4–5):416–419. doi: 10.2165/00007256-200737040-00037. [DOI] [PubMed] [Google Scholar]
- 10.Nieman DC. Marathon training and immune function. Sports Med. 2007;37(4–5):412–415. doi: 10.2165/00007256-200737040-00036. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen HG, Lyberg T. Long-distance running modulates the expression of leucocyte and endothelial adhesion molecules. Scand J Immunol. 2004;60(4):356–362. doi: 10.1111/j.0300-9475.2004.01486.x. [DOI] [PubMed] [Google Scholar]
- 12.Moreira A, Kekkonen RA, Delgado L, Fonseca J, Korpela R, Haahtela T. Nutritional modulation of exercise-induced immunodepression in athletes: a systematic review and meta-analysis. Eur J Clin Nutr. 2007;61(4):443–460. doi: 10.1038/sj.ejcn.1602549. [DOI] [PubMed] [Google Scholar]
- 13.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 14.Walsh NP, Gleeson M, Pyne DB, et al. Position statement. Part two: maintaining immune health. Exerc Immunol Rev. 2011;17:64–103. [PubMed] [Google Scholar]
- 15.Boffi El, Amari E. Upper respiratory tract infections and sports. Rev Med Suisse. 2010;6(258):1499–1503. French. [PubMed] [Google Scholar]
- 16.Romeo J, Warnberg J, Pozo T, Marcos A. Physical activity, immunity and infection. Proc Nutr Soc. 2010;69(3):390–399. doi: 10.1017/S0029665110001795. [DOI] [PubMed] [Google Scholar]
- 17.Prieto A, Reyes E, Bernstein ED, et al. Defective natural killer and phagocytic activities in chronic obstructive pulmonary disease are restored by glycophosphopeptical (Inmunoferon) Am J Respir Crit Care Med. 2001;163(7):1578–1583. doi: 10.1164/ajrccm.163.7.2002015. [DOI] [PubMed] [Google Scholar]
- 18.Dias R, Frollini AB, Brunelli DT, et al. Immune parameters, symptoms of upper respiratory tract infections, and training-load indicators in volleyball athletes. Int J Gen Med. 2011;4:837–844. doi: 10.2147/IJGM.S24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordova A, Martin JF, Reyes E, Alvarez-Mon M. Protection against muscle damage in competitive sports players: the effect of the immunomodulator AM3. J Sports Sci. 2004;22(9):827–833. doi: 10.1080/02640410410001716742. [DOI] [PubMed] [Google Scholar]
- 20.Cordova A, Monserrat J, Villa G, Reyes E, Soto MA. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J Sports Sci. 2006;24(6):565–573. doi: 10.1080/02640410500141158. [DOI] [PubMed] [Google Scholar]
- 21.Sempere JM, Rodrigo C, Campos A, Villalba JF, Diaz J. Effects of anapsos (Polypodium leucotomos extract) on in vitro production of cytokines. Br J Clin Pharmacol. 1997;43(1):85–89. doi: 10.1111/j.1365-2125.1997.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernd A, Ramirez-Bosca A, Huber H, et al. In vitro studies on the immunomodulating effects of Polypodium leucotomos extract on human leucocyte fractions. Arzneimittelforschung. 1995;45(2):901–904. [PubMed] [Google Scholar]
- 23.Navarro-Blasco FJ, Sempere JM. Modification of the inflammatory activity of psoriatic arthritis in patients treated with extract of Polypodium leucotomos (anapsos) Br J Rheumatol. 1998;37(8):912–917. doi: 10.1093/rheumatology/37.8.912a. [DOI] [PubMed] [Google Scholar]
- 24.Sempere JM, Campos A, Velasco I, et al. Anapsos (Polypodium leucotomos) modulates lymphoid cells and the expression of adhesion molecules. Pharmacol Res. 2002;46(2):185–190. doi: 10.1016/s1043-6618(02)00091-9. [DOI] [PubMed] [Google Scholar]
- 25.Carreno MM, De Castro P. Immune Phenotype and Polypodium leucotomos treatment in patients with MS. Neurologia. 1994;10:509. [Google Scholar]
- 26.De Castro P, Carreno MM, Sempere JM. Effects of anapsos in the treatment of multiple sclerosis patients. J Neurol. 1999;246(1):111. [Google Scholar]
- 27.Sempere JM, Campos A, Rodrigo C, Velasco MF, Carrion MA. Induction of T lymphocytes and NK cells by anapsos. Inmunologia. 1999;18(Suppl 1) [Google Scholar]
- 28.Martinez FO, Rodriquez JH. Treatment of adult non-bacterial tonsillitis with anapsos. Elsevier Doyma; 2004. [Google Scholar]
- 29.Martinez FO. 2007 Monitoring in patients with non-bacterial tonsillitis treated anapso. Elsevier Doyma; 2007. [Google Scholar]
- 30.Ordonez JE. Anapsos utilization in mollusc injuries. Elsevier Doyma; 2008. [Google Scholar]
- 31.Ramirez A, Martinez A, Gonzalez M, Gosalbez J, Ballester J, Campos SJ. Reduced consumption of topical steroids in patients with atopic dermatitis treated with anapsos. Elsevier Doyma; 2008. [Google Scholar]
- 32.Del Prado ME. Treatment of common warts with anapsos recaltritantes. Elsevier Doyma; 2009. [Google Scholar]
- 33.Arruti I, Rodriguez P, Casall P. Rating anapsos activity in children with pharyngitis reduction mediantes number of tonsillectomies. Elsevier Doyma; 2011. Spanish. [Google Scholar]
- 34.Aguila A, Bargues R, Saiz JM, et al. Anapsos use in the treatment of chronic recurrent tonsillitis pharyngocutaneous. ORL Aragon. 2011;14(2):25–27. Spanish. [Google Scholar]