Abstract
Macrophages are critical players in the innate immune response to infectious challenge or injury, initiating the innate immune response and directing the acquired immune response. Macrophage dysfunction can lead to an inability to mount an appropriate immune response and as such, has been implicated in many disease processes, including inflammatory bowel diseases. Macrophages display polarized phenotypes that are broadly divided into two categories. Classically activated macrophages, activated by stimulation with IFNγ or LPS, play an essential role in response to bacterial challenge whereas alternatively activated macrophages, activated by IL-4 or IL-13, participate in debris scavenging and tissue remodeling and have been implicated in the resolution phase of inflammation. During an inflammatory response in vivo, macrophages are found amid a complex mixture of infiltrating immune cells and may participate by exacerbating or resolving inflammation. To define the role of macrophages in situ in a whole animal model, it is necessary to examine the effect of depleting macrophages from the complex environment. To ask questions about the role of macrophage phenotype in situ, phenotypically defined polarized macrophages can be derived ex vivo, from bone marrow aspirates and added back to mice, with or without prior depletion of macrophages. In the protocol presented here clodronate-containing liposomes, versus PBS injected controls, were used to deplete colonic macrophages during dextran sodium sulfate (DSS)-induced colitis in mice. In addition, polarized macrophages were derived ex vivo and transferred to mice by intravenous injection. A caveat to this approach is that clodronate-containing liposomes deplete all professional phagocytes, including both dendritic cells and macrophages so to ensure the effect observed by depletion is macrophage-specific, reconstitution of phenotype by adoptive transfer of macrophages is necessary. Systemic macrophage depletion in mice can also be achieved by backcrossing mice onto a CD11b-DTR background, which is an excellent complementary approach. The advantage of clodronate-containing liposome-mediated depletion is that it does not require the time and expense involved in backcrossing mice and it can be used in mice regardless of the background of the mice (C57BL/6, BALB/c, or mixed background).
Keywords: Immunology, Issue 66, Molecular Biology, macrophages, clodronate-containing liposomes, macrophage depletion, macrophage derivation, macrophage reconstitution
Protocol
1. Depleting Macrophages Using Clodronate-containing Liposomes
Liposomes are stored at 4 °C. Two hours prior to injection, remove clodronate-containing liposomes, PBS-containing liposomes, or sterile PBS (injection control) from the refrigerator to allow them to acclimate to room temperature (18 °C).
Invert the tubes containing liposomes 8-10 times to ensure an even distribution before loading 200 μl into a 1 ml syringe. Attach a 26 gauge needle to the top of the syringe.
Using your left hand, scruff the mouse just below the ears holding enough skin to immobilize its head and limbs.
Tilt the mouse so its head is slightly towards the ground so internal organs move away from the injection site, which is located in the lower right quadrant of the abdomen.
Roll the syringe between your palms and invert it 6 times to distribute the liposome solution evenly prior to injection.
Insert the needle in the lower right side of the mouse abdomen at a 30-40 degree angle. Inject 200 μl of liposome solution or PBS.
During an induced inflammation model, like DSS-induced colitis, inject mice 4 days prior to onset of experimental inflammation to ensure depletion of resident macrophages and every 2 days during the protocol to deplete new infiltrating macrophages.
At the end of the experiment, along with planned experimental analyses, remove tissues from the site of interest and fix with paraformaldehyde to confirm macrophage depletion by immunohistochemical staining.
2. Derivation of Polarized Macrophages from Bone Marrow Aspirates
Bone marrow from mice between 8 and 12 weeks of age provides the highest yield of bone marrow-derived macrophages. Euthanize the mouse, lay it on its back, and pin down its paws, stretching its limbs as far as possible.
Working in a sterile hood, remove femurs and tibias, cutting away as much muscle as possible.
Flush marrow from bones using IMDM with 10% FCS loaded into a 5 ml syringe fitted with a 26 gauge needle.
Dilute bone marrow aspirates to 40 ml in IMDM with 10% FCS, place cells in a 75 cm2 tissue culture flask, and incubate at 37 °C, 5% CO2 for 4 hours. This step removes adherent mesenchymal cells or mature macrophages from hematopoietic progenitors.
Transfer culture supernatant containing non-adherent progenitors to a 50 ml conical Falcon tube and centrifuge for 5 min at 1200 rpm.
Resuspend cell pellet in 5 ml IMDM, 10% FCS and count nucleated cells by diluting cell suspension 1 in 20 in acetic acid. This procedure lyses all cells including red blood cells and the remaining nuclei can be counted on a hemocytometer.
Resuspend cells at a concentration of 0.5×106 cells/ml in complete medium; IMDM, 10% FCS, 150 μM monothioglycerol (MTG), and 10 ng/ml of either MCSF, GM-CSF, or IL-3. MCSF-derived macrophages are classically activated macrophages whereas GM-CSF- or IL-3-derived macrophages are alternatively activated.
Replace medium at day 4, spinning down non-adherent cells and returning them to the flask, and at day 7, discarding non-adherent cells.
In addition, IFNγ (10 ng/ml) or IL-4 (10 ng/ml) can be added to flasks containing MCSF-derived cells at day 7 to skew macrophages to a classically activated or an alternatively activated phenotype, respectively. Incubate cells for 3 more days.
At day 10, remove media and lift cells off of the tissue culture flask using Cell Dissociation Buffer (Gibco-BRL). Place 5 ml of buffer on cells for 5 min at room temperature (18 °C) and then bang the side of the flask with the heel of your palm firmly several times. Ensure that cells have lifted off of the flask by examining the flask under a microscope. Pipet resuspended cells into a 15 ml conical Falcon tube and wash the flask with an additional 10 ml of IMDM, 10% FBS. Pool and spin down the cells for 5 min at 300×g.
Count viable cells using a hemocytometer and resuspend at a concentration of 107 cells/ml in sterile PBS pH7.4. Macrophages are ready for injection into mice.
Reserve 1.0×106 macrophages for assessment of macrophage phenotype. Classically activated macrophages stimulated with lipopolysaccharide secrete high levels of nitric oxide and IL-12p70 and low levels of IL-10 compared to alternatively activated macrophages. Alternatively activated macrophages constitutively express YM1 and arginase I (ArgI), which can be detected by Western immunoblotting.
3. Tranferring Macrophages into Mice
Resuspend macrophages in sterile PBS at a concentration of 107 cells/ml. This allows for an intravenous injection of 106 macrophages in a total volume of 100 μl, which is a volume that can be safely and comfortably injected into mice by the tail vein.
Warm mice using a heating pad or heat lap for 5-10 min to dilate the tail vein. Monitor the mouse at all times for signs of hyperthermia.
Transfer the mouse to a restraining device that permits access to the tail veins.
Rotate the tail 90° so that the vein is facing upward. Veins run laterally down both sides of the tail and either vein can be used.
Clean the injection site with an alcohol swab and slowly inject 100 μl ( 106 macrophages) using a 1 ml syringe with a 26 or 28 gauge needle. Insert the needle with the bevel side facing up and at a slight angle, almost parallel to the vein.
If the needle is inserted properly no resistance should be felt and the vein should become clear after injection. If the needle is not in the vein, the tail will bulge. If this happens, remove the needle and try again proximal to the last attempt or in the other vein. A new needle must be used for each injection as the tail is tough and the needles become dull quickly during tail vein injection.
Following injection, remove the needle, apply gentle pressure to the injection site until the bleeding stops, and monitor the mouse for an additional 5min to ensure that bleeding has stopped.
Repeat macrophage injections every 4 days to ensure a continuous delivery of polarized macrophages during the experimental procedure.
At the end of the experiment, along with experimental analyses, remove tissues from the site of interest and fix with paraformaldehyde or formalin to confirm effective delivery of polarized macrophages by immunohistochemical staining.
4. Representative Results
The number and phenotype of resident macrophages in any given tissue depends on the tissue being examined and will change during infection or inflammation. Similarly, the ability of clodronate-containing liposomes to deplete macrophages from a tissue will depend on the route of administration and effective delivery to the target tissue. Intraperitoneal injection of clodronate-containing liposomes results in a decrease in F4/80+ macrophages in the mouse colon during DSS-induced colitis evident by immunohistochemical staining for the macrophage marker, F4/80 (Figure 1A). Histological staining can also be used to determine quantitative differences in macrophage number and phenotype in tissue sections. Figure 1B shows that an average of 55% of macrophages is depleted by clodronate-containing liposomes compared to no depletion detected in mouse colons when mice are injected with PBS alone as an injection control. Each value is determined by counting F4/80 and ArgI stained positive cells in serial tissue sections from 6 mice at 3 points, in 3 sections per mouse with counting being performed by two individuals blinded to experimental condition. These results demonstrate that intraperitoneal injections of clodronate liposomes deplete colonic macrophages in mice.
Derivation of macrophages from bone marrow in the presence of different growth factors yields macrophages that are classically activated (MCSF-derived or MCSF-derived and treated with IFNγ) or alternatively activated (GM-CSF or IL-3-derived or MCSF-derived and treated with IL-4). Flushing bone marrow from 2 femurs and 2 tibias from an 8 week old mouse typically yields 6×107 nucleated cells per mouse and yields 8×106 MCSF-derived macrophages, 10×106 GM-CSF-derived macrophages, or 12×106 IL-3-derived macrophages. Figure 2A shows nitrite and the IL-12p70/IL-10 ratio in macrophage supernatants treated with lipopolysaccharide for 24 hours. Figure 2B shows a typical Western blot analysis of 0.5×106 macrophages from MCSF +/- IL-4 derivation conditions probed with antibodies for alternatively activated macrophage markers, ArgI, YM1, or with GAPDH as a loading control. These data demonstrate that bone marrow-derived macrophages can be skewed to a polarized phenotype during derivation.
Effective transfer of ex vivo derived polarized macrophages to a tissue is dependent on the route of administration and access to the tissue. During DSS-induced colitis, macrophages injected intravenously travel to the site of inflammation and alternatively activated macrophages reduce disease severity. Figure 3A shows the total number of macrophages and the number of ArgI+ (alternatively activated) macrophages counted in histological sections from mice injected with polarized bone marrow-derived macrophages. The impact of transfer of polarized macrophages to the colon during DSS-induced colitis is shown by disease activity index, an additive score based on weight loss, rectal bleeding, and decreased stool consistency (Figure 3B). These results demonstrate that ex vivo derived, adoptively transferred macrophages can traffic to the colon and impact induced inflammation. M1 macrophages do not impact inflammation whereas M2 macrophages significantly reduce disease activity index.
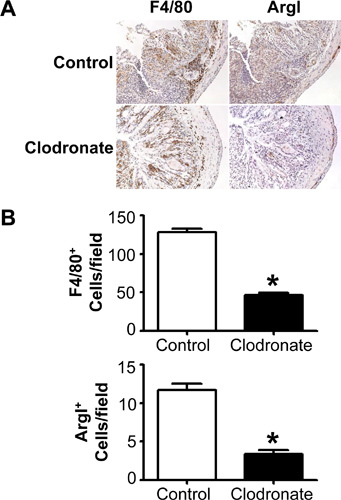 Figure 1. Clodronate-containing liposomes effectively deplete F4/80+ and F480+ArgI+ cells in mouse colons in vivo. An F2 generation of wild type mice on a mixed C57BL/6 ×129Sv background were given 5% DSS for 7 days and injected IP with PBS (Control) or clodronate-containing liposomes (Clodronate) 4 days prior to DSS treatment and on days 0, 2, 4, and 6 during DSS treatment. (A) Colons were removed, fixed, and sections were stained by immunohistochemistry for mature macrophages (F4/80) and the M2 macrophage marker, ArgI. (B) Quantitation of F4/80+ and ArgI+ macrophages. Positively stained cells were counted by two individuals blinded to experimental condition at 40× magnification in 6 fields from 3 sections/mouse and from 6 mice/group. *P<0.01.
Figure 1. Clodronate-containing liposomes effectively deplete F4/80+ and F480+ArgI+ cells in mouse colons in vivo. An F2 generation of wild type mice on a mixed C57BL/6 ×129Sv background were given 5% DSS for 7 days and injected IP with PBS (Control) or clodronate-containing liposomes (Clodronate) 4 days prior to DSS treatment and on days 0, 2, 4, and 6 during DSS treatment. (A) Colons were removed, fixed, and sections were stained by immunohistochemistry for mature macrophages (F4/80) and the M2 macrophage marker, ArgI. (B) Quantitation of F4/80+ and ArgI+ macrophages. Positively stained cells were counted by two individuals blinded to experimental condition at 40× magnification in 6 fields from 3 sections/mouse and from 6 mice/group. *P<0.01.
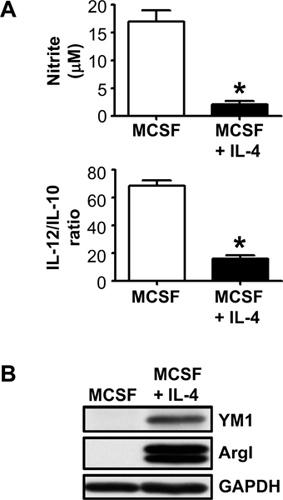 Figure 2. MCSF-derived macrophages express a classically activated phenotype and MCSF-derived macrophages treated with IL-4 express an alternatively activated macrophage phenotype. (A) Consistent with a classically activated phenotype, MCSF-derived macrophages produce higher levels of nitric oxide in response to lipopolysaccharide and can be measured by the Griess assay, which detects its downstream metabolite, nitrite. They also produce a higher IL-12p70/IL-10 ratio, which can be measured by ELISA. *P<0.01 for n=3. (B) Consistent with an alternatively activated phenotype, MCSF-derived macrophages treated with IL-4 constitutively express YM1 and ArgI, which can be detected by Western immunoblotting. Data are representative of 3 independent experiments with similar results.
Figure 2. MCSF-derived macrophages express a classically activated phenotype and MCSF-derived macrophages treated with IL-4 express an alternatively activated macrophage phenotype. (A) Consistent with a classically activated phenotype, MCSF-derived macrophages produce higher levels of nitric oxide in response to lipopolysaccharide and can be measured by the Griess assay, which detects its downstream metabolite, nitrite. They also produce a higher IL-12p70/IL-10 ratio, which can be measured by ELISA. *P<0.01 for n=3. (B) Consistent with an alternatively activated phenotype, MCSF-derived macrophages treated with IL-4 constitutively express YM1 and ArgI, which can be detected by Western immunoblotting. Data are representative of 3 independent experiments with similar results.
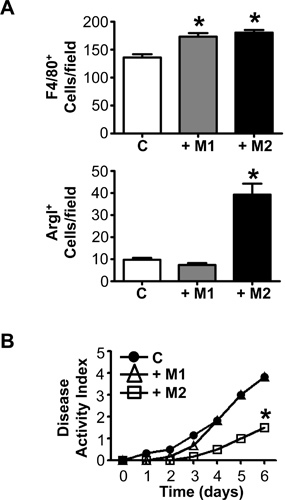 Figure 3. Intravenous injected, ex vivo-derived alternatively activated macrophages traffic to the colon and reduce intestinal inflammation. An F2 generation of wild type mice on a mixed C57BL/6 ×129Sv background were given 5% DSS in their drinking water for six days. M1 and M2 macrophages were injected IV on Days 0 and 4 during DSS treatment. (A) Colons were removed, fixed, and sections were stained by immunohistochemistry for mature macrophages (F4/80) and an M2 macrophage marker (ArgI). Quantitation of macrophages for control PBS injected mice (C), mice injected with M1 macrophages (+M1) and mice injected with M2 macrophages (+M2) were performed by counting positively stained cells at 40× magnification in 6 fields from 3 sections/mouse and from 6 mice/group. Counting was performed by two individuals blinded to experimental condition. (B) Disease activity was monitored and scored daily during treatment and is an additive score based on weight loss, rectal bleeding, and decreased stool consistency. *P<0.05 for n= 6 mice in 2 independent experiments.
Figure 3. Intravenous injected, ex vivo-derived alternatively activated macrophages traffic to the colon and reduce intestinal inflammation. An F2 generation of wild type mice on a mixed C57BL/6 ×129Sv background were given 5% DSS in their drinking water for six days. M1 and M2 macrophages were injected IV on Days 0 and 4 during DSS treatment. (A) Colons were removed, fixed, and sections were stained by immunohistochemistry for mature macrophages (F4/80) and an M2 macrophage marker (ArgI). Quantitation of macrophages for control PBS injected mice (C), mice injected with M1 macrophages (+M1) and mice injected with M2 macrophages (+M2) were performed by counting positively stained cells at 40× magnification in 6 fields from 3 sections/mouse and from 6 mice/group. Counting was performed by two individuals blinded to experimental condition. (B) Disease activity was monitored and scored daily during treatment and is an additive score based on weight loss, rectal bleeding, and decreased stool consistency. *P<0.05 for n= 6 mice in 2 independent experiments.
Discussion
Macrophages are phagocytic cells that play an important role in the immune system. They are responsible for initiating the innate immune response and directing the acquired immune response. Typically, classically activated macrophages are activated by IFNγ or LPS and are responsible for eliminating pathogens and mounting an inflammatory response1. Conversely, alternatively activated macrophages are activated by IL-4 or IL-13 and play a role in debris scavenging and tissue remodeling during the resolution of inflammation1. Specialized macrophages in the gut retain their bactericidal activity but do not mount a pro-inflammatory response2. This tolerance to beneficial flora and food antigen allows for the clearance of material that breaches the epithelial barrier without initiating an inappropriate and potentially pathological immune response3. Aberrant colonic macrophage function can contribute to pathological conditions including inflammatory bowel diseases, irritable bowel syndrome, and colorectal cancer3-5. Macrophage phenotype is dependent on the local microenvironment so defining their role in health or disease can be challenging. The protocol described here allows for macrophage depletion from the complex in vivo environment. In addition, to examine the role of macrophage phenotype in situ, phenotypically defined polarized macrophages can be derived ex vivo from bone marrow aspirates and adoptively transferred to mice, with or without prior depletion of macrophages.
Clodronate-containing liposomes promote macrophage "suicide" and have been used to deplete macrophages in a variety of tissues6. Macrophages phagocytose clodronate-containing liposomes, which are subsequently degraded by lysosomal phospholipases releasing the clodronate into the cell and inducing apoptosis12. Clodronate (0.25 g/ml aqueous solution) is encapsulated in liposomes and is stable at 4 °C for one month12. Liposomes must be evenly distributed in solution before loading into the syringe and before injection into the mouse, regardless of the route of administration or target tissue. PBS injections and injection of PBS-containing liposomes are required controls when depleting macrophages in vivo with clodronate-containing liposomes7. PBS-containing liposomes would seem to be a better control than PBS injections alone. However, it is important to note that while some reports have successfully used PBS-containing liposomes as a control; we, and others, have reported that PBS-containing liposomes can deplete macrophages in some experimental conditions8, 9. In addition, liposomes alone have been reported to inhibit phagocytosis temporarily10. Macrophage depletion by PBS-containing liposomes may be dependent on phagocytic ability and may differ according to mouse strain, genetic background, tissue, and/or experimental condition. The use of PBS-containing liposomes as a control and their effect on macrophage depletion in a specific application needs to be determined experimentally. Therefore, we recommend that both PBS and PBS-containing liposomes be used as injection controls during initial experiments.
A critical consideration in designing macrophage depletion experiments is the route of administration of clodronate-containing liposomes. We have successfully targeted colonic macrophages in mice using intraperitoneal injection8. Others have used intrarectal administration or intravenous injections9, 11. Intrarectal and intraperitoneal administration have both been reported to deplete approximately 50% of colonic macrophages whereas intravenous injection has been reported to deplete up to 90% of macrophages from the lamina propria8, 9, 11. We chose to perform intraperitoneal injections to deliver clodronate-containing liposomes due to the simplicity of the technique and because studies similar to ours have successfully used this route of administration7. However, if higher levels of depletion in the colon are required for an experiment, intravenous injection may improve depletion.
Route of administration is an important consideration for macrophage depletion in different tissues. Intraperitoneal injections have been used to deplete peritoneal, omentum, parathymic lymph node, liver, and splenic macrophages12. Intravenous injections have been used to deplete macrophages in the liver, spleen, and bone marrow12. Intraventricular administration of clodronate-containing liposomes has been used to deplete perivascular and meningeal macrophages from the cerebellum, cerebrum, and spinal cord12. Intratracheal and intranasal administration of clodronate-containing liposomes have been used to deplete alveolar macrophages. Finally, local administration has been successfully used to deplete testicular and vitreal macrophages12, 13. The efficacy of macrophage depletion in a target tissue must be determined experimentally and validated by staining tissues by immunohistochemistry (Figure 1A) or by flow cytometry. In some cases it may be necessary to increase the macrophage depletion rate. The variables that can be optimized to increase depletion include changing the route of clodronate-containing liposome administration, the amount of clodronate-containing liposomes per injection, and/or the frequency of injection.
An important consideration in interpretation of data when depleting macrophages with clodronate-containing liposomes is that all professional phagocytes are depleted, including dendritic cells. To ensure that a biological effect is macrophage-dependent, complementary reconstitution experiments are required. Deriving macrophages from bone marrow aspirates is a useful tool for reconstitution experiments as well as research investigating macrophage function. We have successfully used this protocol for in vitro studies exploring the role of the PI3K signaling pathway in the alternative activation of macrophages14. There are two critical steps in the derivation process. First, adherence depletion step is crucial to remove adherent cells from bone marrow aspirates, either contaminating mesenchymal cells or mature hematopoietic cells. Second, macrophage phenotype should be confirmed prior to their use in experimental procedures. A modification to this protocol is that different genetic models can be used for macrophage derivation and reconstitution, permitting investigation of the role of specific genes in macrophage phenotype and function in vitro and in vivo. For reconstitution experiments, intravenous injection is the most common mode of macrophage delivery but both cell quantity and injection frequency can be optimized for a specific study. Retro-orbital injections may be used for reconstitution and should provide similar results. Of note, macrophages are recruited to sites of inflammation and their phenotype may be changed in response to signals from their microenvironment. Finally, adoptive transfer may be sufficient to deliver macrophages to a target tissue but prior depletion of resident tissue macrophages may be necessary to permit establishment of adoptively transferred macrophages.
The protocol presented here has a number of advantages over existing methods. An alternative method to deplete macrophages is by using the conditional ablation transgenic mouse, CD11b-DTR15. In this mouse, the human diphtheria toxin (DT) receptor is expressed under the control of the macrophage-specific CD11b promoter. This confers toxin sensitivity and DT injection causes macrophage depletion15. Though the percentage macrophage depletion is greater in this model, clodronate-containing liposomes offers two important advantages. Clodronate-containing liposome injection can be used to deplete macrophages in any mouse strain. Macrophages can be depleted from genetically modified mice without the time and cost required for backcrossing that is required to use the CD11b-DTR mouse method. For the same reason, macrophages can be depleted from mice on any background, which is an important consideration if the model being investigated requires a unique or mixed background. Similarly, the adoptive transfer experiments using ex vivo derived macrophages from a mouse line avoids any problems with alloreactivity.
The protocols described here can be applied to a variety of research questions. We have used these techniques to study the role of macrophages in inflammation in the colon. Clodronate-containing liposome-mediated macrophage depletion and adoptive transfer of macrophages can be used to target a large number of tissues. Macrophage adoptive transfer can be used to reconstitute depleted macrophages or direct macrophages to sites of inflammation in genetic or inducible models of inflammation. This protocol also provides a platform for examining macrophage targeted therapeutics in disease models where macrophages or their polarization are associated with pathology.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by a 'Grant in Aid of Research' from the Crohn's and Colitis Foundation of Canada to LMS, who is supported by a Canadian Association of Gastroenterology/Canadian Association of Health Research/Crohn's and Colitis Foundation of Canada New Investigator Award.
References
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Lopatin U, Chieppa M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: implications for immune tolerance. Curr. Opin. Immunol. 2008;20:669–675. doi: 10.1016/j.coi.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Heinsbroek SE, Gordon S. The role of macrophages in inflammatory bowel diseases. Expert Rev. Mol. Med. 2009;11:e14. doi: 10.1017/S1462399409001069. [DOI] [PubMed] [Google Scholar]
- Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut. 2001;49:743–745. doi: 10.1136/gut.49.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S. Colon cancer-derived factors activate NF-kappaB in myeloid cells via TLR2 to link inflammation and tumorigenesis. Mol. Med. Report. 2011;4:1083–1088. doi: 10.3892/mmr.2011.545. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: perspectives in research and therapeutics. J. Liposome Res. 2002;12:81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- Hunter MM. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Weisser SB. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J. Leukoc. Biol. 2011;90:483–492. doi: 10.1189/jlb.0311124. [DOI] [PubMed] [Google Scholar]
- Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Smith P. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 2007;178:4557–4566. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- Kataoka K. The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest. Ophthalmol. Vis. Sci. 2011;52:1431–1438. doi: 10.1167/iovs.10-5798. [DOI] [PubMed] [Google Scholar]
- Weisser SB. Alternative activation of macrophages by IL-4 requires SHIP degradation. Eur. J. Immunol. 2011;41:1742–1753. doi: 10.1002/eji.201041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhier JF. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]


