Abstract
There is a considerable discrepancy between oxygen supply and demand in the liver because hepatic oxygen consumption is relatively high but about 70% of the hepatic blood supply is poorly oxygenated portal vein blood derived from the gastrointestinal tract and spleen. Oxygen is delivered to hepatocytes by blood flowing from a terminal branch of the portal vein to a central venule via sinusoids, and this makes an oxygen gradient in hepatic lobules. The oxygen gradient is an important physical parameter that involves the expression of enzymes upstream and downstream in hepatic microcirculation, but the lack of techniques for measuring oxygen consumption in the hepatic microcirculation has delayed the elucidation of mechanisms relating to oxygen metabolism in liver. We therefore used FITC-labeled erythrocytes to visualize the hepatic microcirculation and used laser-assisted phosphorimetry to measure the partial pressure of oxygen in the microvessels there. Noncontact and continuous optical measurement can quantify blood flow velocities, vessel diameters, and oxygen gradients related to oxygen consumption in the liver. In an acute hepatitis model we made by administering acetaminophen to mice we observed increased oxygen pressure in both portal and central venules but a decreased oxygen gradient in the sinusoids, indicating that hepatocyte necrosis in the pericentral zone could shift the oxygen pressure up and affect enzyme expression in the periportal zone. In conclusion, our optical methods for measuring hepatic hemodynamics and oxygen consumption can reveal mechanisms related to hepatic disease.
Keywords: Medicine, Issue 66, Physics, Biochemistry, Immunology, Physiology, microcirculation, liver, blood flow, oxygen consumption, phosphorescence, hepatitis
Protocol
1. Labeling Erythrocytes with Fluorescein Isothiocyanate Isomer I (FITC)
All the experimental protocols we used were approved by the Animal Care Committee of Keio University School of Medicine.
Anesthetize a donor mice, and after making a celiotomy incision withdraw whole blood from the inferior vena cava with a 1-ml syringe containing small amount of heparin. The mouse was immediately euthanized by sodium pentobarbital injection (100 mg/kg i.p.).
Wash the whole blood twice with PBS (pH 7.4) by centrifugation for 5 min at 400 g in a swinging-bucket rotor.
Dissolve 10 mg of FITC in 5 ml of 100 mM Na2HPO4 and filter it with a membrane having 0.22-μm pores.
In a test tube mix 1 ml of the FITC solution with 0.15 ml of 3 mM glucose, 0.25 ml of 180 mM NaH2PO4, and 1.5 ml of 100 mM Na2HPO4. Add 0.2 ml of the RBC pellet, and tap the tube for mixing.
Keep the tube for 2 hours at 4 °C and then wash the stained RBCs in PBS twice.
Put a small amount of the RBC suspension on a slide grass and see if the RBCs look healthy (i.e., that their shape and size are normal) and that the fluorescence intensity is sufficient.
Suspend the 0.1 ml of RBCs in 0.9 ml of PBS at pH 7.4, and keep the suspension at 4 °C until injection.
2. Preparation of Oxygen-sensitive Dye
Dissolve 500 mg of BSA in 10 ml of PBS at pH 7.4.
Add 30 mg of Pd(II)-meso-tetra(4-carboxyphenyl)porphine (Pd-TCPP) to the BSA solution and stir overnight.
(Optional) Extract BSA-bound Pd-TCPP by using gravity-flow chromatography or a spin column to separate it from free Pd-TCPP.
Centrifuge the solution to remove undissolved Pd-TCPP, and filter the supernatant with a membrane filter having 0.22-μm pores.
Store 1-ml aliquots in tubes at -20 °C. Avoid repeated freeze-thaw cycles.
3. Animal Preparation
For microscopic observation of the microcirculation prepare a plastic plate with a hole 20 mm in diameter, and tape a 30-mm-square square cover glass over the hole.
Anesthetize a mouse, remove the fur, and prep the skin. Place a catheter in the tail vain for drug injection by using a 30 G needle connected to a 10-cm polyethylene (PE 10) catheter filled with heparinized PBS.
After median incision, extend a main lobule of the liver on the plastic plate, and place the mouse in sternal recumbency.
Prepare small slips with kitchen wrap (3 mm x 8 mm) and tile them around the edge of the hepatic lobe to inhibit moving of the liver with respiration and keeping it from drying.
Observe the hepatic microcirculation under a microscope (with transmitted light), and confirm that the blood flow has no stasis in the field of view for at least 15 min.
Slowly inject 0.2 ml of fluorescently labeled RBCs for blood flow observation or 0.2 ml of Pd-TCPP solution for pO2 measurement. This amount of FITC-labeled RBCs will account for 1/50 of all the RBCs in circulation in the visualized region.
4. Blood Flow Visualization
Excite the FITC by irradiating it with mercury lamp light that has passed through a bandpass filter (450–490 nm)1.
Record the fluorescent image with a CCD camera.
5. pO2 Measurement
Pd-TCPP phosphorescence is relatively weak and should be detected with a high–sensitivity detector. All experiments need to be performed in a dark room.
The absorption peaks of Pd-TCPP are at 410 nm and 532 nm, so the second-harmonic wavelength 532 nm is recommended for excitation. This wavelength can be generated by a Nd:YAG pulse laser2.
Feed the beam from the Nd:YAG laser into the appropriate port on the inverted microscope, and adjust the beam spot to a central position in the focal plane. The spatial resolution depends on the laser spot size, which is changed by passing the beam through a pinhole.
To detect the phosphorescence, attach a long-pass filter (>620 nm) and a photomultiplier tube (PMT) to the other port of the microscope. The PMT should be sensitive to red wavelengths, especially around 700 nm.
Sample the phosphorescence signal (500 points sampled at 200 kHz) and calculate the time constant of the decay by fitting an exponential function to the decay.
For calibration prepare two set of samples of Pd-TCPP solution with pO2 150 mmHg and 0 mmHg. Add dithionite sodium 1% in volume to sample to produce absence of oxygen. Keep the pH of the samples at 7.4 and the temperature at 37 °C during calibration.
Fix the Stern-Volmer constant kq and oxygen-free luminescence decay time τ0 in the equation (1)3. In our case, with Pd-TCPP solutions at 37 °C and a pH of 7.4, kq is 374 mmHg-1 s-1 and τ0 is 0.74 ms. These measurement parameters depend on the equipment (i.e. laser, detector, other optical devices) and the signal processing method, so calibration is needed in each measurement system4. τ0/τ = 1 + kq • τ0 • pO2 (1)
Set the mouse on the plate on the microscope stage, observe the hepatic microcirculation, and adjust the position of the target microvessel to the point of the laser spot.
6. Hepatitis Model
Fast mice with free access to water overnight.
Prepare a 20 mg per ml solution of acetaminophen (APAP) in DMSO.
Inject it intraperitonealy at 1 ml/100 g bw (i.e., 200 mg/kg bw). After the injection, the mouse can be given free access to food and water 5.
24 hours after injection, anesthetize the mouse and expose the liver by making a median incision. If hepatitis is achieved, necrosis in the pericentral region will be easily visible to the naked eye.
7. Representative Results
Example images of hepatic microcirculation are shown in Figure 1, where (a) is a transmitted–light image and (b) is a fluorescence image. Individual FITC-labeled erythrocytes are observed in the video movie, and portal venules, sinusoids, and central venules are recognized by blood flow directions.
As shown in Figure 2, the laser spot irradiating a central venule through a x100 objective lens was approximately 10 μm in diameter. Intravascular pO2 in the hepatic microcirculation was measured in male C57BL/6 mice, and Figure 3(a) shows the relations between pO2 and vessel diameter in portal and central venules. The pO2 gradient from portal to central venules indicates that RBCs release oxygen efficiently while passing through sinusoids. As shown in Figure 3(b), the average pO2 was 59.8 mmHg in portal vessels, 48.2 mmHg in sinusoids, and 38.9 mmHg in central venules.
When we produced acute hepatitis by injecting APAP intraperitonealy, intravascular pO2 was significantly elevated in each part of the hepatic microcirculation: p<0.05 in portal veins and p<0.01 in sinusoids and central veins (Figure 3(c)).
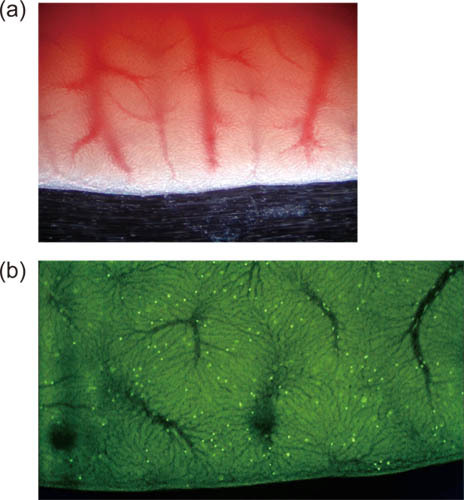 Figure 1. Low-magnification images showing hepatic microcirculation with (a) transmitted light and (b) fluorescence.
Figure 1. Low-magnification images showing hepatic microcirculation with (a) transmitted light and (b) fluorescence.
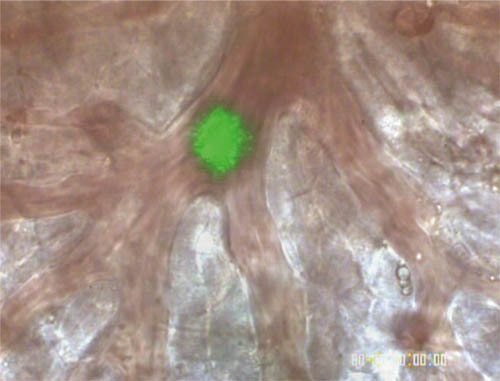 Figure 2. Image of laser spot irradiating a targeted central venule for pO2 measurement.
Figure 2. Image of laser spot irradiating a targeted central venule for pO2 measurement.
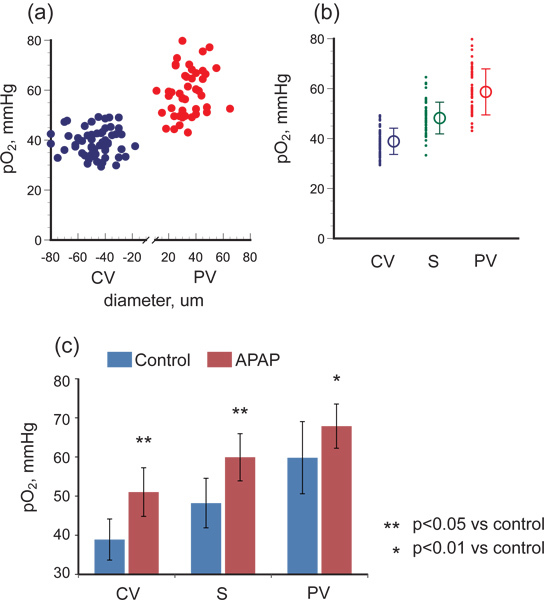 Figure 3. pO2 gradient in hepatic microcirculation of healthy control mice and of mice with acetaminophen-induced hepatic injury. (a) shows pO2 versus vessel diameter for portal venules (PV) and central venules (CV), and (b) shows the oxygen gradient from PV to CV via sinusoids (S). The pO2 difference between PV and CV reflects the oxygen consumption of hepatocytes or other parenchymal cells. (c) shows that in hepatitis conditions induced by APAP administration, pO2 was significantly elevated in PV, S, and CV and the pO2 gradient decreased, indicating that hepatic oxygen consumption was compromised.
Figure 3. pO2 gradient in hepatic microcirculation of healthy control mice and of mice with acetaminophen-induced hepatic injury. (a) shows pO2 versus vessel diameter for portal venules (PV) and central venules (CV), and (b) shows the oxygen gradient from PV to CV via sinusoids (S). The pO2 difference between PV and CV reflects the oxygen consumption of hepatocytes or other parenchymal cells. (c) shows that in hepatitis conditions induced by APAP administration, pO2 was significantly elevated in PV, S, and CV and the pO2 gradient decreased, indicating that hepatic oxygen consumption was compromised.
Discussion
Delivering oxygen to tissues is an essential role of the microcirculation, and a lot of papers describe diseases related to microvessel anatomy and rheology6. Liver blood flow is a combination of arterial and venous blood. About 30% of the blood flow to the liver is well-oxygenated blood provided by the hepatic artery and the other 70%, provided by the portal vein, is the poorly oxygenated blood venous outflow from the spleen and gastrointestinal tract7. Liver diseases such as fatty liver, shock, or tumor change the blood flow in the hepatic microcirculation. To determine tissue oxygenation, one needs to measure both the blood flow and oxygen concentration in the microcirculation. The lack of commercially available instruments for making these measurements in the hepatic microcirculation has made it hard to obtain physiological data in this field.
An important point in animal preparation steps is the fixation of the liver on a plastic plate. Loose fixation results in liver movement synchronized with respiration, which interferes with microscopic observation and makes analysis difficult. Tight fixation, however, causes blood flow stasis. Blood flow conditions should be observed though a low-power microscope during the operative procedure. High-power lasers easily injure microvessels, so the frequency of the irradiation should be minimized. 1 Hz is usually enough for temporal measurement8. However, a single measurement of pO2 takes less than 1 ms, which means that the repetition frequency can be increased to 1 kHz for rapid pO2 change, as necessary. Furthermore, the photochemical reaction of Pd-TCPP generates singlet oxygen that damages microvessels and induces platelet aggregation9. The combination of fluorescence imaging and pO2 measurement can measure blood flow dynamics and oxygen concentration all together. The mercury lamp irradiation exciting FITC also excites Pd-TCPP, however, thereby generating much singlet oxygen and leading to blood flow stasis. When both blood flow and oxygen tension are measured in the same animal, the FITC imaging should be done before Pd-TCPP is administered. Additionally, systemic oxygen level is indicative of the oxygen consumption in the tissue. It is preferable to perform respiratory management during experiments or analyze blood gas levels after experiments.
We used APAP to induce acute hepatitis, in part because a high dose of APAP is known to lead to cell necrosis in the pericentral region10. Partial oxygen pressure measurement showed the 20.9-mmHg pO2 difference between portal venules and central venules in control mice decreased to 16.9 mmHg in the APAP-treated mice and that the pO2 was higher throughout the hepatic microcirculation. The oxygen gradient in the hepatic microcirculation is mainly the result of oxygen supplied from portal venules being consumed in hepatocytes. The higher dose of APAP induces necrosis in the pericentral region11, 12, and which could decrease oxygen consumption downstream as shown in Figure 3c. To determine detailed mechanisms underlying liver hepatitis, especially relationship between tissue necrosis and oxygen level, quantification of the extent of necrosis, and serum levels of bilirubin, ALT, AST, and inflammatory cytokines need to be measured. A lot of liver diseases are related to hepatic microcirculation, in other words, to the blood flow distribution, oxygen delivery, and oxygen consumption in liver tissue. In cases of chronic alcohol intake, about 10–15 % of alcohol is metabolized by the microsomal ethanol-oxidizing system, and the alcohol-induced molecule P450IIE1 generates NAPQI13, a toxic metabolite that leads to pericentral hepatocyte necrosis14.
In conclusion, our optical measurement method can be used to investigate physiological and pathological mechanisms by analyzing microvascular structure, diameter, blood flow velocity, and oxygen tension.
Disclosures
We have nothing to disclose.
Acknowledgments
Authors thank Ms. Risa Otsuka for technical assistance and helping experiments. This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Young Scientists (B), 2010, 22700476, and Suzuken Memorial Foundation 2010 for K.T. And this study was supported by Research and Development of the Next-Generation Integrated Simulation of Living Matter, a part of the Development and Use of the Next-Generation Supercomputer Project of MEXT, and in part by JST ERATO Suematsu Gas Biology Project.
References
- Ishikawa M, Sekizuka E, Shimizu K, Yamaguchi N, Kawase T. Measurement of RBC velocities in the rat pial arteries with an image-intensified high-speed video camera system. Microvasc. Res. 1998;56:166–172. doi: 10.1006/mvre.1998.2100. [DOI] [PubMed] [Google Scholar]
- Tsukada K, Sekizuka E, Oshio C, Tsujioka K, Minamitani H. Red blood cell velocity and oxygen tension measurement in cerebral microvessels by double-wavelength photoexcitation. J. Appl. Physiol. 2004;96:1561–1568. doi: 10.1152/japplphysiol.00764.2003. [DOI] [PubMed] [Google Scholar]
- Stern O, Volmer M. Über die Abklingzeit der Fluoreszenz. Physik. Zeitschr. 1919;20:183–188. [Google Scholar]
- Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science. 1988;241:1649–1651. doi: 10.1126/science.241.4873.1649. [DOI] [PubMed] [Google Scholar]
- Soga T. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006;281:16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- Goda N. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J. Clin. Invest. 1998;101:604–612. doi: 10.1172/JCI1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote J. Physiologie der Menschen. 20th edn. New York: Springer-Verlag; 1980. p. 560. [Google Scholar]
- Suganuma K. Erythrocytes with T-state-stabilized hemoglobin as a therapeutic tool for postischemic liver dysfunction. Antioxid Redox Signal. 2006;8:1847–1855. doi: 10.1089/ars.2006.8.1847. [DOI] [PubMed] [Google Scholar]
- Vanderkooi JM, Maniara G, Green TJ, Wilson DF. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J. Biol. Chem. 1987;262:5476–5482. [PubMed] [Google Scholar]
- Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010;169:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab. Rev. 2004;36:805–822. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Medical disorders of alcoholism. N. Engl. J. Med. 1995;333:1058–1065. doi: 10.1056/NEJM199510193331607. [DOI] [PubMed] [Google Scholar]
- Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–773. [PubMed] [Google Scholar]


