Abstract
The planarian, a freshwater flatworm, has proven to be a powerful system for dissecting metazoan regeneration and stem cell biology1,2. Planarian regeneration of any missing or damaged tissues is made possible by adult stem cells termed neoblasts3. Although these stem cells have been definitively shown to be pluripotent and singularly capable of reconstituting an entire animal4, the heterogeneity within the stem cell population and the dynamics of their cellular behaviors remain largely unresolved. Due to the large number and wide distribution of stem cells throughout the planarian body plan, advanced methods for manipulating subpopulations of stem cells for molecular and functional study in vivo are needed.
Tissue transplantation and partial irradiation are two methods by which a subpopulation of planarian stem cells can be isolated for further study. Each technique has distinct advantages. Tissue transplantation allows for the introduction of stem cells, into a naïve host, that are either inherently genetically distinct or have been previously treated pharmacologically. Alternatively, partial irradiation allows for the isolation of stem cells within a host, juxtaposed to tissue devoid of stem cells, without the introduction of a wound or any breech in tissue integrity. Using these two methods, one can investigate the cell autonomous and non-autonomous factors that control stem cell functions, such as proliferation, differentiation, and migration.
Both tissue transplantation5,6 and partial irradiation7 have been used historically in defining many of the questions about planarian regeneration that remain under study today. However, these techniques have remained underused due to the laborious and inconsistent nature of previous methods. The protocols presented here represent a large step forward in decreasing the time and effort necessary to reproducibly generate large numbers of grafted or partially irradiated animals with efficacies approaching 100 percent. We cover the culture of large animals, immobilization, preparation for partial irradiation, tissue transplantation, and the optimization of animal recovery. Furthermore, the work described here demonstrates the first application of the partial irradiation method for use with the most widely studied planarian, Schmidtea mediterranea. Additionally, efficient tissue grafting in planaria opens the door for the functional testing of subpopulations of naïve or treated stem cells in repopulation assays, which has long been the gold-standard method of assaying adult stem cell potential in mammals8. Broad adoption of these techniques will no doubt lead to a better understanding of the cellular behaviors of adult stem cells during tissue homeostasis and regeneration.
Keywords: Developmental Biology, Issue 66, Neuroscience, Molecular Biology, Medicine, transplantation, partial irradiation, rescue, immobilization, planaria, flatworm, stem cell, regeneration
Protocol
Note: this protocol suggests the use of potentially hazardous materials (lead and chloretone). Acquire, read, and follow MSDS for all potentially hazardous materials.
1. Animal Culture, Selection, and Preparation
For animal culture and handling use planarian water (1X Montjuïc salts9) and plastic transfer pipettes.
Sexual biotype Schmidtea mediterranea can be used when raised in the laboratory under normal culture conditions10. To produce asexual specimens of requisite size, S. mediterranea raised at room temperature under normal conditions9 should be fed at double to triple normal frequency (2-3 times per week) for one to two months prior to use. Alternatively, asexual animals fed at normal frequency may be kept at 10 degrees Celsius indefinitely in order to increase their average size.
Select animals that are between 1 to 2 cm in length and wider than 2 mm then starve animals 3-7 days prior to use.
If performing any pharmacological or radiological treatments on intended hosts, donors, or partially irradiated animals, perform treatment(s) at this point.
If pharmacological treatments were performed that required feeding the animals, starve animals an additional 3 to 7 days prior to use.
2. Preparation of Solutions and Materials
Prepare chloretone solution, a mild local anesthetic, by dissolving 0.1-0.2% w/v chloretone in planarian water and chilling the solution on ice.
If performing partial irradiation only, proceed to step 2.7. For tissue transplantation continue on to step 2.3.
Using a Bunsen burner, bend 0.75 mm interior diameter, used for cutting the graft tissue, and 0.7 mm exterior diameter, used for creating a hole in the host which will receive the graft, capillary tubes to a 90° angle at 1-2 cm from the end of each tube. To save materials, bend both ends of each capillary tube and break them in half to produce two tools. Take care not to flame the very ends of the tubes.
- Cut the following papers to the indicated sizes:
- Black filter paper (cut into rectangles approx. 2.5 cm x 1.5 cm)
- Whatman #3 filter paper (cut into rectangles approx. 2 cm x 0.5 cm)
- Kimwipe (folded and cut into wads approx. 3 cm x 0.5 cm x 4 ply)
- Cigarette rolling paper (remove gum strip and cut into rectangles approx. 3 cm x 2 cm)
Prepare modified Holtfreter's solution (3.5 g/L NaCl, 0.2 g/L NaHCO3, 0.05 g/L KCl, 0.2 g/L MgSO4, 0.1 g/L CaCl2, pH 7.0-7.5) and casein saturated Holtfreter's solution and chill both to 4 °C.
Attach a folded Kimwipe to a square of Parafilm and place on Peltier cooler plate or other cooling device situated under a dissecting microscope. Saturate the Kimwipe with chilled Holtfreter's solution and place two black filter paper rectangles on the Kimwipe.
Line Petri dishes with Whatman #2 filter paper. Moisten the filter paper with Holtfreter's solution and chill the dishes on ice. For partial irradiation a larger dish and filter paper liner may be used.
3. Anesthetization and Immobilization
Fill a Petri dish with chilled chloretone solution and pipette worms into the dish. For transplantation only anesthetize one host and one donor at a time. For partial irradiation many (n > 10) animals can be anesthetized at once.
Allow worms to soak in the chloretone solution until they become motionless (5-10 min).
Rinse worms by pipetting them into a dish filled with chilled Holtfreter's solution.
Immobilize animals by pipetting them onto black filter paper saturated with chilled Holtfreter's solution and orient them ventral side down with forceps. If animals are able to locomote, soak up excess Holtfreter's solution, slightly decrease the temperature of your Peltier or cold plate, or increase the length of chloretone treatment.
4. Partial Irradiation
Note: Follow these steps to prepare animals for partial irradiation. If performing transplantation instead, proceed to section 5.
Place a chilled Petri dish from step 2.7 on ice in an ice bucket that will fit inside a top source X-ray irradiator.
Arrange anesthetized animals in a Petri dish by moving the black filter paper on which they are immobilized. Using forceps to move anesthetized worms directly may injure them.
Transport arranged animals to a top-source X-ray irradiator and situate the ice bucket such that the distance from the cathode tube to the animals is minimized, thus maximizing the effective dose rate.
Position lead shield(s) (Figure 1) between the animals and the cathode tube as desired. Shields should be 4.5 to 6 mm thick to allow for 97 to 99% attenuation of a 325kV X-ray beam11.
Deliver X-ray dose. If complete stem cell ablation from non-shielded regions is desired, deliver 30 Gy or greater using an X-ray irradiator. For reference, 30 Gy is equivalent to 3.6 minutes at 320 kilovolts and 10 milliamps in a Precision X-Ray Inc. XRAD320 with a field-to-source distance of 30 centimeters.
Immediately after dosing is complete, handling worms by the black filter paper, transfer animals into chilled planarian water. Allow the planarian water to warm to room temperature and the animals to dislodge themselves from the black filter paper. The partial irradiation procedure is now complete.
5. Tissue Transplantation
Using a transfer pipette and forceps, arrange anesthetized host and donor worms on separate rectangles of black filter paper on the Kimwipe which has been cooled on the Peltier or cooler plate under the dissecting microscope.
Using a 0.75 mm inner diameter capillary tube cut out the graft plug from the donor and, using forceps, place it on an out of the way portion of the host. If graft material gets stuck in the capillary tube, dislodge with forceps.
Using a 0.7 mm outer diameter capillary tube remove a plug from the host and using forceps position the graft into the hole that is left behind.
Transfer the transplanted host on its black filter paper rectangle into the Petri dish prepared in step 2.7.
Wet a piece of rolling paper with casein saturated Holtfreter's solution and place it on top of the transplanted host as diagramed in Figure 2A.
Soak four pieces of filter paper in casein saturated Holtfreter's solution and encase the transplanted host as diagramed in Figure 2B.
Soak four wads of cut Kimwipe in casein saturated Holtfreter's solution and lay them over the filter paper from step 5.6 (Figure 2B). Replace the lid and put the Petri dish on ice.
Transfer the donor worm into planarian water to recover, heal, and regenerate.
When all transplants are completed, place the transplanted worms into a 10 °C incubator overnight.
The following morning, taking care not to disturb the graft, uncover the worm and transfer it (on its black filter paper) to a Petri dish filled with planaria water.
Either allow the worm to dislodge itself from the filter paper or gently remove it with forceps.
Change the planarian water once every 2-3 days.
6. Representative Results
Immediately following partial irradiation planaria will appear normal and unaffected. Depending on the delivered dose and the geometry of the shield used, irradiated tissue may regress and even disintegrate7. Shielded tissue should remain intact. Following tissue regression and a loss of tissue integrity, a blastema will form and missing structures will be regenerated (Figure 3A). If an amputation is made in the partially irradiated region, the irradiated tissue will be rescued (i.e. prevented from regressing or disintegrating) (Figure 3B). In both the uninjured and the amputated case regeneration will be delayed as compared to an amputated non-irradiated planaria (Figure 3C). If an X-ray dose of 30 Gy was delivered and the partially irradiated animal was uninjured, successful stem cell ablation in a pattern corresponding with the lead shield used can be confirmed 2 to 3 days following partial irradiation by in situ hybridization for the stem cell marker Smed-piwi-112 (a.k.a. smedwi-1) (Figure 4).
The morning following transplantation, a successful graft of the transplanted tissue should be obvious within the host tissue, having adhered to both the dorsal and ventral surfaces of the host (Figure 5A). Occasionally, the graft will adhere to only the ventral or dorsal surface. If the transplantation was completely unsuccessful, no sign of the graft will be visible from either the dorsal or ventral surface of the host (Figure 5B). Shortly following a successful graft of non-irradiated tissue into a host that had been ablated of stem cells by lethal irradiation13,14 , in situ hybridization for Smed-piwi-1 will reveal that stem cells are present primarily within the graft (Figure 5C). Additionally, successful grafts of non-irradiated tissue into lethally irradiated hosts will result in rescue of host tissue and long-term survival of the host15.
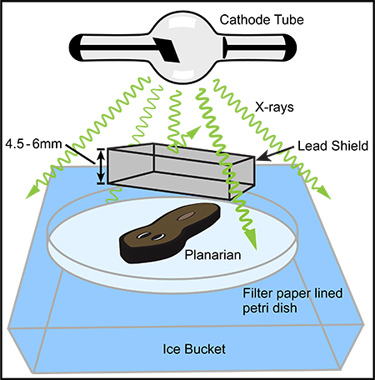 Figure 1. General arrangement of basic components for partial irradiation. In an X-ray irradiator with a top positioned X-ray source (cathode tube) the anesthetized planarian is positioned directly below the X-ray source within the irradiation field. In order to maximize the X-ray dose rate, the distance between the planarian and the X-ray source should be minimized. A lead shield should be positioned between the cathode tube and the anesthetized worm, as close to the worm as practical. The lead shield should be designed, manufactured, and positioned so that it shields the desired tissue but completely exposes the rest of the worm. Many commercial manufactures will produce custom lead shields from your simple design diagram; we have successfully used Alpha Systems Corp. (Bluffdale, UT). The lead should be sufficiently thick to allow for the desired amount of shielding. For example, if near complete shielding of a 320 kV X-ray beam is desired, the lead should be 4.5 to 6 mm thick. The planarian and shield are positioned on a Holtfreter's soaked filter paper lined Petri dish which rests in an ice bucket. Given a sufficiently large X-ray irradiation field and a number of identical lead shields, many specimens may be partially irradiated at one time (not pictured).
Figure 1. General arrangement of basic components for partial irradiation. In an X-ray irradiator with a top positioned X-ray source (cathode tube) the anesthetized planarian is positioned directly below the X-ray source within the irradiation field. In order to maximize the X-ray dose rate, the distance between the planarian and the X-ray source should be minimized. A lead shield should be positioned between the cathode tube and the anesthetized worm, as close to the worm as practical. The lead shield should be designed, manufactured, and positioned so that it shields the desired tissue but completely exposes the rest of the worm. Many commercial manufactures will produce custom lead shields from your simple design diagram; we have successfully used Alpha Systems Corp. (Bluffdale, UT). The lead should be sufficiently thick to allow for the desired amount of shielding. For example, if near complete shielding of a 320 kV X-ray beam is desired, the lead should be 4.5 to 6 mm thick. The planarian and shield are positioned on a Holtfreter's soaked filter paper lined Petri dish which rests in an ice bucket. Given a sufficiently large X-ray irradiation field and a number of identical lead shields, many specimens may be partially irradiated at one time (not pictured).
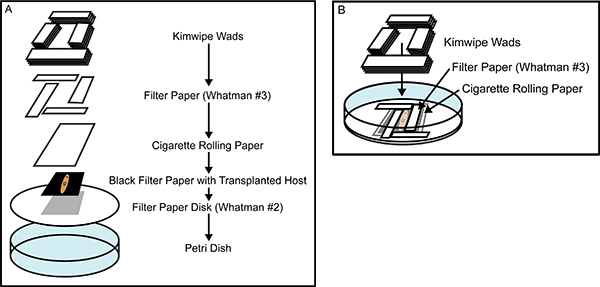 Figure 2. Building the recovery chamber. (A) An exploded view of the tissue transplantation recovery chamber, showing all of the components that are layered on top of one another after being soaked in casein saturated Holtfreter's Solution. (B) A nearly completed recovery chamber, illustrating the interlocking placement of the Whatman #3 filter paper rectangles which snugly encase the anesthetized planarian, preventing movement during healing. The careful construction of the recovery chamber prevents animal movement and desiccation, promoting rapid healing and greater efficacy of tissue transplantation.
Figure 2. Building the recovery chamber. (A) An exploded view of the tissue transplantation recovery chamber, showing all of the components that are layered on top of one another after being soaked in casein saturated Holtfreter's Solution. (B) A nearly completed recovery chamber, illustrating the interlocking placement of the Whatman #3 filter paper rectangles which snugly encase the anesthetized planarian, preventing movement during healing. The careful construction of the recovery chamber prevents animal movement and desiccation, promoting rapid healing and greater efficacy of tissue transplantation.
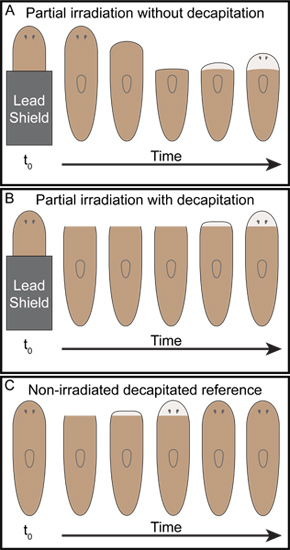 Figure 3. Representative outcomes of simple posterior partial irradiation. As Dubois described7, (A) when the posterior half of planarians were shielded with lead and then exposed to x-ray irradiation the anterior tissue was observed to regress back to the boundary between the irradiated and shielded tissue at which point unpigmented blastemas formed and the animals began to regenerate. (B) On the other hand, when animals were decapitated following the same partial irradiation performed in (A), tissue regression was not observed and the remaining irradiated anterior tissue was rescued. The decapitated partially irradiated animals regenerated heads (B), however, regeneration was significantly delayed as compared to unirradiated decapitated controls (C).
Figure 3. Representative outcomes of simple posterior partial irradiation. As Dubois described7, (A) when the posterior half of planarians were shielded with lead and then exposed to x-ray irradiation the anterior tissue was observed to regress back to the boundary between the irradiated and shielded tissue at which point unpigmented blastemas formed and the animals began to regenerate. (B) On the other hand, when animals were decapitated following the same partial irradiation performed in (A), tissue regression was not observed and the remaining irradiated anterior tissue was rescued. The decapitated partially irradiated animals regenerated heads (B), however, regeneration was significantly delayed as compared to unirradiated decapitated controls (C).
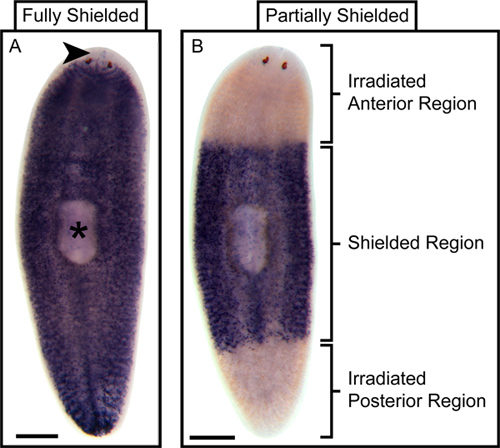 Figure 4. Representative outcome of partial stem cell ablation following partial irradiation. Whole-mount In situ hybridization (WISH) for the stem cell marker Smed-piwi-1 reveals that wild type planaria have stem cells distributed throughout their bodies with the exception of the tissue anterior to the photoreceptors (arrowhead) and the pharynx proper (asterisk)14. (A) WISH for Smed-piwi-1 in irradiated but fully shielded control planaria fixed three days following irradiation shows a stem cell distribution that is indistinguishable from that of wild type planaria. (B) On the other hand, WISH for Smed-piwi-1 in animals that were only partially shielded, leaving the anterior and posterior exposed, but were also fixed three days following irradiation shows that stem cells are ablated from the non-shielded regions. Scale bars are 500 microns.
Figure 4. Representative outcome of partial stem cell ablation following partial irradiation. Whole-mount In situ hybridization (WISH) for the stem cell marker Smed-piwi-1 reveals that wild type planaria have stem cells distributed throughout their bodies with the exception of the tissue anterior to the photoreceptors (arrowhead) and the pharynx proper (asterisk)14. (A) WISH for Smed-piwi-1 in irradiated but fully shielded control planaria fixed three days following irradiation shows a stem cell distribution that is indistinguishable from that of wild type planaria. (B) On the other hand, WISH for Smed-piwi-1 in animals that were only partially shielded, leaving the anterior and posterior exposed, but were also fixed three days following irradiation shows that stem cells are ablated from the non-shielded regions. Scale bars are 500 microns.
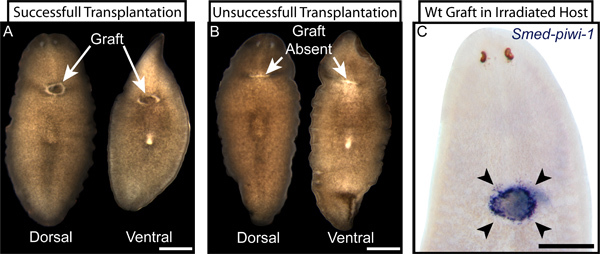 Figure 5. Examples of successful and unsuccessful tissue transplantation. (A) Live images of dorsal and ventral views of a successfully transplanted planarian three days following transplantation. The graft (indicated) is clearly visible on both dorsal and ventral surfaces and is surrounded with characteristic unpigmented tissue at the graft-host interface. (B) Correspondingly, unsuccessful transplantations display no visible graft tissue at the transplantation site (indicated) and instead show a healed, unpigmented, lateral wound from the failed transplantation. A graft that adheres to only the dorsal or the ventral surface can resemble a successful transplantation (A) when viewed from one side and an unsuccessful transplantation (B) when viewed from the other. (C) When wild type (wt) tissue is grafted into an irradiated host that has been ablated of resident stem cells and the transplanted stem cells are later revealed by WISH for Smed-piwi-1 two days following transplantation, the success of the transplantation is clearly displayed by the specific presence of stem cells only in or around the graft location (arrowheads). Scale bars are 500 microns.
Figure 5. Examples of successful and unsuccessful tissue transplantation. (A) Live images of dorsal and ventral views of a successfully transplanted planarian three days following transplantation. The graft (indicated) is clearly visible on both dorsal and ventral surfaces and is surrounded with characteristic unpigmented tissue at the graft-host interface. (B) Correspondingly, unsuccessful transplantations display no visible graft tissue at the transplantation site (indicated) and instead show a healed, unpigmented, lateral wound from the failed transplantation. A graft that adheres to only the dorsal or the ventral surface can resemble a successful transplantation (A) when viewed from one side and an unsuccessful transplantation (B) when viewed from the other. (C) When wild type (wt) tissue is grafted into an irradiated host that has been ablated of resident stem cells and the transplanted stem cells are later revealed by WISH for Smed-piwi-1 two days following transplantation, the success of the transplantation is clearly displayed by the specific presence of stem cells only in or around the graft location (arrowheads). Scale bars are 500 microns.
Discussion
Importance of immobilization
Immobilization is by far the most critical step for the successful completion of any of these processes. If planaria are improperly immobilized prior to partial irradiation, they may move beneath the lead shield, producing inconsistent and confounding results. Additionally, if animals are insufficiently immobilized following transplantation, the host worm will likely move away from the graft tissue, resulting in a failure of the graft to heal properly to the host. Proper immobilization is achieved by adjusting the concentration or length of chloretone treatment and the temperature and moisture of the filter paper on which the planaria rest. Chloretone treatments at 0.2 percent of up to one half hour do not appear to be toxic, however, treatments of that length may induce ejection of the pharynx. Planaria remain still and amenable to partial irradiation during this length of time. Our experience has shown that moisture, rather than chloretone treatment, is the most crucial factor - too wet and the animals move easily; too dry and the animals will desiccate. In all cases filter paper should be completely saturated but not to the point of creating standing water within the container or on the surface. Excess liquid can be shaken from the Petri dish. The amount of liquid used to saturate the filter paper will no doubt need to be adjusted according to the specific environmental humidity.
Speed of transplantation
Speed is essential in increasing the efficacy of both partial irradiation and transplantation. The longer partially irradiated animals are immobilized the greater the chances of an injury or a breech in tissue integrity which may skew the results of a particular assay. The speed at which transplantations are preformed roughly correlates with the successful graft rate. If the hole in the host that is to receive the graft is left empty for too long, the wounded ventral and dorsal surfaces will begin to heal to each other rather than to the graft tissue, thus resulting in an unsuccessful transplantation. In our hands, the graft plug only occasionally becomes lodged in the capillary tube. On those occasions, the plug can normally be dislodged quickly with fine forceps or by forcing air through the capillary tube by mouth. Once one becomes practiced with the transplantation procedure, the time needed to produce many grafted animals can be greatly reduced by anesthetizing the next donor-host pair while grafting the previous pair. By performing anesthetization and transplantation concurrently, the actual time necessary to produce each grafted animal can drop to as little as five minutes.
The versatility of partial irradiation and transplantation
Once the conditions for anesthetization and partial irradiation are optimized according to the specific experimental environment and the available X-ray irradiator, the number of different experiments that can be performed using the partial irradiation technique becomes large. Advanced lead machining techniques allow for the production of not only intricate irradiation shields that can target specific organs and tissues, but also the manufacture of large numbers of identical shields, permitting the rapid creation of many biological replicates. Future investigations will, no doubt, take advantage of this method to test the in vivo cellular responses to localized stem cell ablation and the functional differences between stem cell populations present in different parts of the planarian. The versatility of partial irradiation, as it pertains to the scientific question of interest, is limited only by current lead machining techniques and one's imagination. Therefore, the true power of the partial irradiation technique may lie in the ability to precisely shield and ablate nearly any differently positioned subpopulations of stem cells.
Whereas transplantation also allows for the isolation of a subpopulation of stem cells, the more significant advantage of this technique is the ability to differentially treat the host or donor prior to transplantation. Treatment of host or donor with a drug or RNA interference, will allow for the investigation of how untreated transplanted cells behave in a treated environment or how treated cells behave in an untreated environment. These types of experiments will certainly help to tease apart the autonomous and non-autonomous molecular contributions to stem cell function.
Additionally, as classical grafting experiments in planaria have already shown5,6, tissue transplantation is an ideal technique for exploring the inductive potential of different tissues during regeneration. As planarian research enters the molecular age, we have begun to uncover very specific localized expression of important signaling molecules which directly control regeneration16. Transplanting tissues that contain these signaling molecules to ectopic sites during regeneration may help us to better understand their role in directing the morphogenesis of regenerating structures.
Advantages of partial irradiation and tissue transplantation over other techniques
Besides the inherent specific strengths that partial irradiation and tissue transplantation have when compared to one another, they also provide particular advantages over other techniques used in the planarian regeneration field. For instance, low dose irradiation has been utilized as a way to isolate a small number of stem cells within an otherwise stem cell devoid host4,17 , however, because we do not know how sublethal irradiation affects planarian stem cells, we cannot be sure that the cellular behaviors observed in these experiments are characteristically normal. This caveat of low dose irradiation is less of a concern in partial irradiation because radiation exposure can be easily attenuated more than 99 percent with lead of the proper thickness. Furthermore, transplantation completely avoids this caveat because a small population of stem cells can be isolated within a stem cell devoid host without ever exposing the transplanted cells to radiation. Furthermore, the exact position of the healthy stem cells can be known and controlled in both partial irradiation and transplantation, whereas the cells surviving low dose irradiation are positioned randomly throughout the animal. Finally, whereas single cell transplantation has elegantly demonstrated the high potential of a single planarian stem cell4, tissue transplantation may prove an equally powerful technique because it is faster, less tedious, and the function and behavior of a number of cells can be observed simultaneously in each host. The analysis of a small population of cells versus a single cell not only allows for more efficient gathering of data but it may also reveal cell-cell interactions that would be missed in a single cell transplantation assay.
Conclusion
Partial irradiation and tissue transplantation, although old techniques, will play an important role in dissecting the functions of planarian stem cells as well as the general molecular mechanisms underlying regeneration. Modernization of these classical techniques has brought higher throughput and greater consistency while allowing for the integration of modern functional assays and molecular techniques. These classical techniques that were once used to outline major questions of planarian regeneration can now be used to discover the answers to those questions and therefore further our understanding of stem cell biology.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Chiyoko Kobayashi and Kiyokazu Agata for helpful advice on planarian transplantation as well as past and present members of the Sánchez lab for invaluable discussions during the development of these techniques. This work was supported by NIH Training Grant (5T32 HD0791) to OCG and NIH R37GM057260 to ASA. ASA is a Howard Hughes Medical Institute Investigator.
References
- Morgan T. Experimental studies of the regeneration of Planaria maculata. Arch. Entw. Mech. Org. 7:364–397. [Google Scholar]
- Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Randolph H. The regeneration of the tail in lumbriculus. J. Morphol. 7:317–344. [Google Scholar]
- Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science. 2011;332:811–816. doi: 10.1126/science.1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F. Studies on transplantation in planaria. Biological Bulletin. 1929;57:188–197. [Google Scholar]
- Morgan L. Regeneration of grafted pieces of planarians. J. Exp. Zoöl. 1906;3:269–294. [Google Scholar]
- Dubois F. Contribution á l 'ètude de la migration des cellules de règènèration chez les Planaires dulcicoles. Bull. Biol. Fr. Belg. 1949;83:213–283. [Google Scholar]
- Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell. 2007;1:263–270. doi: 10.1016/j.stem.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Cebria F, Newmark PA. Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development. 2005. pp. 132–3691. [DOI] [PubMed]
- Newmark PA, Sánchez Alvarado A. Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev. Biol. 2000;220:142–153. doi: 10.1006/dbio.2000.9645. [DOI] [PubMed] [Google Scholar]
- Miller W, Kennedy RJ. X-ray attenuation in lead, aluminum, and concrete in the range 275 to 525 kilovolts. Radiology. 1955;65:920–925. doi: 10.1148/65.6.920. [DOI] [PubMed] [Google Scholar]
- Pearson BJ. Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev. Dyn. 2009;238:443–450. doi: 10.1002/dvdy.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Asami M, Higuchi S, Shibata N, Agata K. Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting. Dev. Growth Differ. 2006;48:371–380. doi: 10.1111/j.1440-169X.2006.00876.x. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Oviedo NJ, Jennings JR, Jenkin JC, Sánchez Alvarado A. SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science. 2005;310:1327–1330. doi: 10.1126/science.1116110. [DOI] [PubMed] [Google Scholar]
- Stéphan-Dubois F. Les cellules de régénération chez la planaire Dendrocoleum lacteum. Bulletin de la Société Zooologique de France. 1961;86:172–185. [Google Scholar]
- Gurley KA. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev. Biol. 2010;347:24–39. doi: 10.1016/j.ydbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti A. Adult stem cell plasticity: neoblast repopulation in non-lethally irradiated planarians. Dev. Biol. 2009;328:305–314. doi: 10.1016/j.ydbio.2009.01.029. [DOI] [PubMed] [Google Scholar]


