Abstract
The human organic solute transporter (hOST) is a heterodimer composed of alpha and beta subunits. Physical association of hOSTα and β subunits is essential for their polarized basolateral plasma membrane localization and function in the export of bile acids and steroids. To understand the role of carboxyl- and amino-tails of OSTβ and mechanisms underlying membrane localization of hOST, the effects of tail deletion of the hOSTβ subunit and biological reagents on membrane distribution and transport function of hOST were investigated in stably transfected MDCK cells. After deletion of 35 amino acids from the amino-tail of hOSTβ, the efflux transport activity and polarized membrane distribution of the truncated hOSTβ was abolished. A co-immunoprecipitation study verified that the amino-tail of hOSTβ is essential for the association with hOSTα subunit. Treatments with acytochalasin D (interrupting ctin-filaments), bafilomycin A1 (inhibiting vacuolar H+-ATPase), brefeldin A (disrupting the Golgi complex), and calphostin C (inhibiting protein kinase C), significantly disrupted the polarized membrane distribution of hOST and markedly reduced transport activity in stably transfected MDCK cells. In summary, the 35 amino acid amino-terminal fragment of hOSTβ contains critical information for interaction with the hOSTα subunit and subsequent trafficking to the plasma membrane. These studies suggest that the membrane sorting process of hOST is mediated by a bafilomycin A1-sensitive vesicular pathway that is associated with the actin-cytoskeleton network. The membrane localization of hOST is also partially mediated through a brefeldin A sensitive mechanism, which controls its transit from the ER to Golgi and is regulated by PKC.
Keywords: Human OST, protein interaction, membrane trafficking, organic anion transporter
Introduction
Bile acids are the end products of cholesterol catabolism and are conserved by an efficient enterohepatic circulation that involves many transport proteins in liver, intestine and kidney. Bile acids play a critical role in a multitude of biological processes including bile secretion and absorption of fat and fat-soluble vitamins. Human OSTα [a 340-amino acid, seven-transmembrane (TM) domain protein] and β (a 128-amino acid, single-TM domain ancillary polypeptide) is a novel heteromeric transporter that was discovered at 2003 [1]. These two proteins are co-expressed to form a heterodimer that transports bile acids and other important steroid-derived drugs via a Na+-independent mechanism at the basolateral membranes of epithelium in the ileum, kidney, and liver [2-4]. hOST (located at basolateral plasma membrane domain) and the ileal apical Na+-dependent bile acid transporter (ASBT) (located at apical plasma membrane domain) are both responsible for reabsorbing the majority of bile acids from the intestinal lumen [2]. Recently, the studies indicate that Ostα-Ostβ is essential for bile acid and sterol disposition, and suggest that the carrier may be involved in human conditions related to imbalances in bile acid or lipid homeostasis [2]. Boyer et al. have indicated that expression of OSTα and β are highly regulated in response to cholestasis and that this response is dependent on the FXR bile acid receptor [5]. Previous studies from our lab and others demonstrated that the physical association of hOSTα and β subunits is essential for their polarized basolateral plasma membrane targeting [6-10]. Co-expression of OSTα and β, but not the individual subunits, stimulated Na+-independent bile acid uptake and the apical-to-basolateral transport of taurocholate [6,9]. Our studies further demonstrated that the extracellular amino-terminal portion of human OSTα plays an important role in the assembly of the heterodimer with hOSTβ, and trafficking to the plasma membrane [6]. However, it is unclear which domain of hOSTβ is required for the formation of a functional heterodimer with hOSTα. Mechanisms involved in the polarized membrane trafficking of hOST proteins, as well as their control mechanisms, are not completely understood. To answer these questions, we investigated the functions of carboxyl- and amino-terminals of the hOSTβ through a combination of cell biological, biochemical, and confocal microscopic methods. Several cytoskeleton, transport vesicle-disrupting reagents and protein kinase inhibitors were used to gain insight into how the cytoskeleton machinery and protein kinases would influence transport function and membrane localization of human OST proteins. These studies may prove useful information for designing novel drugs to reduce bile acid intestinal reabsorption and lower serum cholesterol levels.
Materials and methods
Materials
[3H]taurocholate ([3H]TC), (3.47 Ci/mmol) was purchased from DuPont NEN (Boston, MA). Unlabeled TC was purchased from Sigma Chemical Company (St. Louis, MO). Brefeldin A (BFA) and protein kinase G inhibitor (PKGi) were obtained from Calbiochem (La Jolla, CA). N-[2-(methylamino)ethyl]-5-isoquinoline-sulfonamide (H-89), bafilomycin A1 (BA1), nocodazole, colchicine, cytochalasin D, and calphostin C were acquired from Sigma Chemical Company (St. Louis, MO). Poly-Myc antibody was purchased from Abcam (Cambridge, MA). Cell-culture supplies were obtained from Life Technologies, Inc. (Rockville, MD). Subcloning reagents, restriction enzymes and competent cells were obtained from Stratagene (La Jolla, CA), GIBCO BRL (Gaithersburg, MD), New England BioLabs (Beverly, MA), and Invitrogen (Carlsbad, CA).
Construction of epitope tagged and truncated human OST (hOST)
The human OSTα and β cDNA fragments were amplified from human liver Quick-Clone™ cDNA library [BD Biosciences Clontech] by PCR using specific primers according to manufacturer’s directions as described previously [6]. A combination of restriction enzyme digestion and PCR were used to generate epitope tagged and truncated transporters with human OSTα and β cDNAs as templates. The PCR was performed with oligonucleotide primers generated from DNA sequencing information. Wild type and truncated hOSTα and β cDNAs were subcloned into a mammalian expression vector pcDNA3.1 (Invitrogen) and/or a green fluorescent protein (GFP) fusion protein vector, pEGFPN2 (Clonetech, Palo Alto, CA) at the XhoI/SalI sites. pBudCE4.1 vector (Invitrogene) was designed for simultaneous expression of two genes in mammalian cell lines. The pBudCE4.1 vector contains a human cytomegalovirus (CMV) immediate-early promoter and a human elongation factor 1α-subunit (EF-1α) promoter for independent expression of two recombinant proteins. The full-length wild type human OSTα and β cDNAs, the fragments corresponding to amino acids #1-55 (Carboxyl-end truncated, β-CT) and #36-128 (Amino-end truncated, β-NT) of hOSTβ were amplified by PCR and were inserted in-frame into the PstI/XbaI sites of CMV promoter of pBudCE4.1 vector to generate Myc/His tagged constructs. GFP was fused at Carboxyl-end of wild type and/or truncated hOSTα and β. This GFP fused constructs were inserted in-frame into the NotI/XhoI sites of EF-1α promoter of pBudCE4.1 vector. All of the positive clones containing cDNA inserts were identified by restriction enzyme mapping and sequenced using the ABI automated DNA sequencer model 377 at the DNA Core Facility, Mount Sinai School of Medicine.
Establishment of transfected cell lines
DNA plasmid transfection of MDCK cells were performed as described previously [6]. Briefly, in the stably transfected cell model, the rat ileal sodium-dependent bile acid transporter (rAsbt), wild type or truncated human OSTα and β (both constructed in pBudCE4.1 vector) were stably expressed in MDCK cells as follows. On day 1, 60-mm plates were seeded with 5 x 104 rat Asbt stably transfected MDCK cells. Cells were transfected with wild type or mutated hOSTα and β constructs using the FuGENE 6 transfection reagent (Roche Applied Science). On day 3, cells were split to two 100mm dishes in culture medium containing 0.9mg/ml of G418 (Invitrogen) and 250μg/ml of Zeocin (Invitrogen). After about 15 days for selection, individual colonies were picked, expanded in 35-mm plates, and screened by Na+-dependent bile acid uptake activity for rAsbt, confocal imaging, and immunoblotting for wild type and truncated hOSTα and β protein expression.
Confocal fluorescence microscopy
Confocal microscopy was performed on a confluent monolayer of transfected cells cultured on glass coverslips as described previously [6]. Cells were fixed and permeabilized for 7min in 100% of methanol at -20°C, followed by rehydration in PBS. After being washed with PBS, cells on coverslips were inverted onto a drop of VectaShield mounting medium (Vector Laboratories, Inc., Burlingame, CA). Fluorescence was examined with a Leica TCSSP (UV) 4-channel confocal laser-scanning microscope in the Imaging Core Facility Microscopy Center, Mount Sinai School of Medicine. The semi-quantitative image analysis of protein co-localization was performed as described [6].
Transport assays
Na+-dependent and independent taurocholate (TC) influx and efflux assays were performed in 12 well plates or using a transwell filter culture system as described previously [6]. For Na+-independent bile acid efflux assay in transwell filter system, stably transfected MDCK cells were plated on 24-well plates with 6.4-mm transwell filter inserts (Costar), and cultured in the culture medium containing 0.9mg/ml of G418 (Invitrogen) and 250μg/ml of Zeocin (Invitrogen). Briefly, for the transwell filter system (Costar, Cambridge, MA), transfected and untransfected cells were grown to confluence for 4–5 days on 0.45-μm pore size transwell filter inserts. [3H]TC uptake was performed at 37°C for 10min. Confluent cell monolayers grown on transwell filters were washed twice with warm sodium or choline uptake buffer [116mM NaCl (or choline), 5.3mM KCl, 1.1mM KH2PO4, 0.8mM MgSO4, 1.8mM CaCl2, 11mM D-glucose, and 10mM HEPES; pH 7.4], and each well was incubated from the apical (0.2ml) or basolateral (0.6ml) side with uptake buffer containing 10μM [3H]TC at the final concentration. After a 10-min incubation, uptake assays were terminated by aspirating the medium and the filters were successively dipped into three beakers, each of which contained 100ml of icecold uptake buffer. Filters were excised from cups, and attached cells were solubilized in 0.2ml of 1% SDS and transferred into scintillation vials with 4ml Optifluor (DuPont-New England Nuclear). The protein was determined with the Bio-Rad protein assay kit.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The mRNA level of transfected constructs were quantified by RT-PCR as described previously [6]. Briefly, RNA isolation and cDNA synthesis were carried out by Trizol Method and Superscript Reverse Transcriptase Method (INVITROGEN) as described by the manufacturer. Total RNA was extracted from transfected cells using TRIzol Reagent and precipitated by Iso-propanol. The RNA was quantified by spectrophotometer and equal amount was used for the cDNA preparation from the different transfected cell lines. 2 micro litters of total 20 micro litters volume were used. The PCR carried out according to GoldTaq Green Master Mix from Promega.
Co-Immunoprecipitation (co-IP)
Co-IP was performed as described by Novus Biologicals (www.novusbio.com/protocol) and Abcam. HEK293 cells co-transfected with epitope (GFP or Myc) tagged wild type or truncated hOSTα and hOSTβ proteins were used for co-IP. Cell monolayers in 6-well plates were washed with PBS and lysis with 250 μl/well lysis buffer (M-PER Mammalian Protein Extraction Reagent, Thermo, Protease Inhibitor Cocktail Tablets, Roche, Phosphatase Inhibitor Cocktail Tablets, Roche) for 15min at 4°C. The supernatant was collected following centrifugation. Cell extract (200μl) were treated with protein G-Agarose beads and incubated with 5μg rabbit polyclonal GFP (FL) antibody (Santa-cruz Laboratory) overnight at 4°C, on a rotator. Protein-G Agarose (60μl) was added and incubated overnight at 4°C in a rotating shaker. Immune complexes were recovered by centrifugation, washed three times with PBS and released from the beads by 40μl of 2x reducing sample buffer (Bio-Rad). Immune-complexes (15-20μl) were assayed after separation with 12% SDS-PAGE and Western blot analysis with corresponding antibodies to detect each protein.
Biological reagent treatments
The effects of biological reagents on cell surface expression of hOST were evaluated by taurocholate transport assays and confocal fluorescence microscopy analysis. In this experiment, brefeldin A (BFA) (which inhibits protein secretion by specifically inhibiting the Golgi-associated guanine nucleotide exchange activity of the small GTP-binding protein) was added to a final concentration of 2μM, as described previously for MDCK II cells [11]. Bafilomycin A1 (BA1, a vacuolar H+–ATPases interrupting reagent), colchicine (Col, a microtubule disruption reagent), cytochalasin D (Cyt D, a actin polymerization disruption reagent), nocodazole (Noc, a microtubule disruption reagent) were incubated at a final concentration of 50nM (for BA1), 10μM (for col), 2μM (for cyt D), and 33 μM (for Noc) in culture medium, respectively, for 16 hr at 37°C, as described previously [11,12]. A Protein kinase A (PKA) specific inhibitor, H89, was evaluated at a final concentration of 20μM, as described previously [13]. Calphostin C (Cap C, a PKC highly specific inhibitor) was examined at a final concentration of 1μM as described previously [14,15]. The protein kinase G inhibitor (PKGi) was evaluated at a final concentration of 86μM for 1h at 37°C, as described previously [16].
Statistics analysis
The results were expressed as mean value ±SEM and analyzed by using unpaired Student’s t test for a difference in means between two groups that may have un-equal sizes. The p-values < 0.05 was considered statistically significant.
Results
Roles of the carboxyl- and amino-tail of hOSTβ protein on protein association, transport activity and cellular distribution
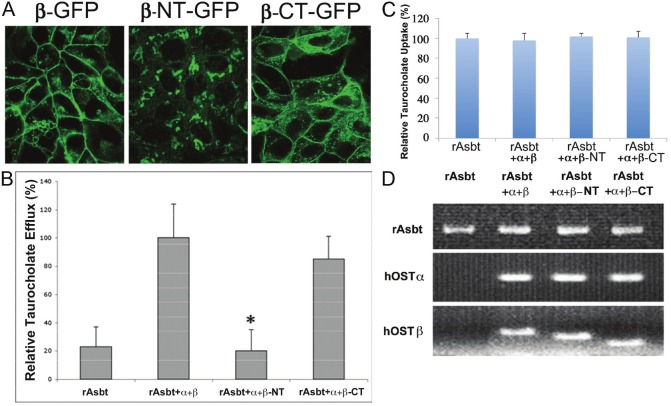
It has been reported that the carboxyl- (C-) and/or amino- (N-) tails of transporter proteins may play an important role in protein-protein interaction, cellular localization and transport functions [6,17,18]. Previous studies also demonstrated that the co-expression and association of hOSTα with β proteins are required for transport function and specific polarized basolateral plasma membrane localization [6-10]. By prediction analysis of protein structure, several potential motifs for membrane targeting and protein interaction (such as di-leu, leucine repeat, and RRK motifs) were identified in the N- and C- tails of hOSTβ protein. To further define the possible protein interaction domain(s) of hOSTβ, the entire 73 amino acids of C-end cytoplasmic tail or the 35 amino acids of N-end extracellular fragment of hOSTβ were deleted. Our previous data demonstrated that fusion of GFP protein at C-end of OSTα and β subunits did not affect OST subunit association, transport function and polarized basolateral membrane targeting [6]. Therefore, -Myc or –GFP tagged hOSTα and hOSTβ and C- or N-end truncated hOSTβ were constructed and were used in this study. To determine the roles of Nand C-end fragments of hOSTβ on membrane localization and efflux transport activity, confocal microscopy and transport assay were performed in stably transfected MDCK cells (see methods). The subcellular distribution of wild type and C- or N-end truncated hOSTβ proteins in stably transfected MDCK cells were demonstrated by confocal microscopy. Figure 1 shows that in cells co-transfected with hOSTα, the GFP-tagged wild type OSTβ (β-GFP) proteins were localized to the basolateral membrane domain of polarized MDCK cells (Figure 1A left panel). In cells co-transfected with hOSTα, the majority of the C-end truncated hOSTβ (β-CTGFP) was also expressed on the basolateral membrane of stably transfected MDCK cells (Figure 1A right panel). However, the semiquantitative image analysis showed that the percentage of intracellular accumulation of the C-end truncated hOSTβ (β-CT-GFP) was increased more than 25% in comparison with the wild type hOSTβ proteins. In contrast, the N-end truncated hOSTβ (β-NT-GFP) showed a predominant intracellular distribution (most likely accumulated on ER and Golgi area) in polarized MDCK cells co-transfected with hOSTα (Figure 1A central panel).
Figure 1.
Confocal microscopy and [3H]taurocholate (TC) efflux studies of human hOSTα and truncated β co-expressed in stably transfected MDCK cells. (A) Confocal fluorescence images: The cells were grown on glass coverslips and fixed with cold 100% methanol. In stably transfected MDCK cells, the hOSTα were co-expressed with a GFP fused wild type (β-GFP), C-terminal truncated (β-CT-GFP) or N-terminal truncated (β-NT-GFP) hOSTβ. TC transport activity of cells transfected with human organic solute transporter (hOST): Na+-independent [3H]TC efflux (B) and Na+-dependent [3H]TC uptake (C) were measured in stably transfected MDCK cells that were transfected with rat Asbt, human OSTα and wild type (β), or C-terminal truncated (β-CT) or N-terminal truncated (β-NT) hOSTβ cDNA. Data represent mean ±S.E. (bars) of triplicate determinations from at least three different cell culture preparations. Asterisks (*, p<0.05) indicate significant differences from wild type hOSTβ co-transfected cells. Messenger RNA levels of rAsbt, wild type and truncated hOSTα and β constructs in stably transfected MDCK cells were obtained using the reverse transcription-polymerase chain reaction (RT-PCR) (D) (see methods), and the reaction products were visualized by staining with ethidium bromide. Figure (C) and (D) indicate that these stably transfected MDCK cells have similar gene expression level for rAsbt, wild type and truncated hOSTα and β, and equally TC uptake activity.
Na+-independent efflux of taurocholate was examined in MDCK cells stably transfected with rat Asbt, human OSTα and β or truncated OSTβ cDNAs. In this model system, the rAsbt acted as an apical bile acid loading pump in the stably transfected MDCK cells. The bile acid efflux activity from the basolateral domain by hOSTα and β or truncated OSTβ proteins was measured by radioactive scintillation counting. We found that the efflux transport activity was decreased about 15% in the cells co-transfected with C-end 73 amino acid tail truncated hOSTβ (β-CT) and hOSTα cDNAs compared with the wild type hOSTβ co-transfected cells (Figure 1B). In contrast, only background efflux transport activity was found in the cells co-transfected with N-terminal truncated hOSTβ (β-NT) and hOSTα (Figure 1B). Na+-dependent taurocholate uptake activity from apical membrane by rAsbt of the stably transfected MDCK cells is shown in Figure 1C. Figure 1D demonstrates the expression levels of rat Asbt (rAsbt), wild type and truncated hOSTβ in stably transfected MDCK cell lines by RT- PCR. Figure 1C and 1D indicate that these stably transfected cells have similar gene expression levels for rAsbt, hOSTα, β and truncated hOSβ and equivalent Na+-dependent taurocholate uptake activity.
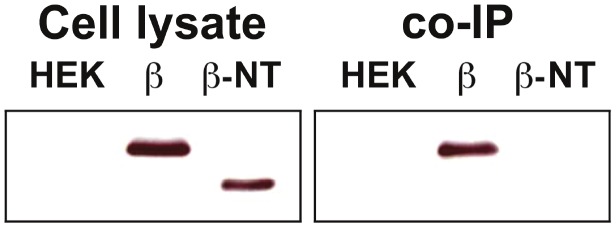
Co-immunoprecipitation (co-IP) was performed to further define the interaction of N-end fragment of hOSTβ with hOSTα protein. The HEK293 cells transiently co-transfected with hOSTα-GFP and -Myc tagged wild type or N-end truncated hOSTβ were tested by co-IP. HEK293 cells, non- transfected and co-transfected with hOSTα-GFP and hOSTβ-Myc were used as negative and positive controls, respectively. The cell extract from the non-transfected and transfected HEK293 cells were immunoprecipitated by a poly-GFP antibody. Co-precipitated proteins were isolated by SDS-PAGE and analyzed by Western blot using the anti-Myc antibody to detect Myc-tagged wild type or N-end truncated hOSTβ protein. Figure 2 showed that co-IP of transfected HEK293 cell lysates with the poly-GFP antibody for hOSTα immunoprecipitated the -Myc fused wild type hOSTβ protein (lane; β), but not the N-end truncated hOSTβ protein (lane; β-NT). This finding is consistent with the confocal image and transport efflux studies. It confirms that after removal of the NH2-terminal tail, the truncated hOSTβ does not interact with hOSTα protein.
Figure 2.
Identification of the hOSTα and β proteins interacting domain. Co-Immunoprecipitation (co-IP) of N-end truncated hOSTβ and wild type hOSTα proteins in transiently co- transfected HEK293 cells. The Myc-tagged wild type (β) or N-terminal truncated hOSTβ (β-NT) were co-transfected with GFP-tagged wild type hOSTα constructs into HEK293 cells, respectively. The total protein extracted from transfected cells was immunoprecipitated by the polyclonal GFP (FL) antibody. The total protein extracted from the transfected HEK293 cells (cell lysate) and co-immunoprecipitated proteins (co-IP) were isolated by SDS-PAGE and analyzed by Western blot using a poly-Myc antibody to detect Myc tagged wild type or N-end truncated hOSTβ protein. The results demonstrated that only the Myc tagged hOSTβ protein (β) was co-immunoprecipitated by hOSTα, but not the amino-terminal truncated hOSTβ (β-NT). [β = wild type hOSTβ; β-NT= 35 amino acid of N-end fragment deleted hOSTβ; ΗΕΚ = Un-transfected HEK293 cells].
These results indicate that the NH2-terminal tail of hOSTβ is essential for association with hOSTα protein, transport function and polarized cellular distribution. Since our previous study demonstrated that the N-end tail of hOSTα is essential for association with hOSTβ subunit [6], these results further suggest the possibility of the N-end tails of hOSTα and hOSTβ interact directly, as an important determinant of cellular distribution and transport function.
Effects of biological reagents on cellular distribution and transport activity of hOST-GFP
The polarized distribution of transport proteins in the apical and basolateral membranes of cells (such as intestine, kidney, and liver) is of critical importance to the absorption and elimination of bile acids and other organic drugs. Previous studies have shown that polarized membrane expression of hepatic transporters is highly regulated by signaling systems including microtubule and actin-mediated pathways and by vesicle-mediated retrieval or insertion of transport proteins into the canalicular (apical) and sinusoid (basolateral) domains [19,20]. To further understand the mechanisms involved in polarized membrane trafficking of hOST proteins into these structurally different domains, we studied MDCK cells stably transfected with rat Asbt, human OSTα and β cDNAs (see above) to detect the effects of various biological reagents on cellular distribution and efflux transport activity. Since the rAsbt acts as an apical bile acid loading pump in the stably transfected MDCK cells and at previous studies have reported that drugs used in this study may effect Asbt uptake function, we used the percentage of TC efflux of hOST to calculate and normalize the effects of these drugs on Asbt uptake [18,21,22].
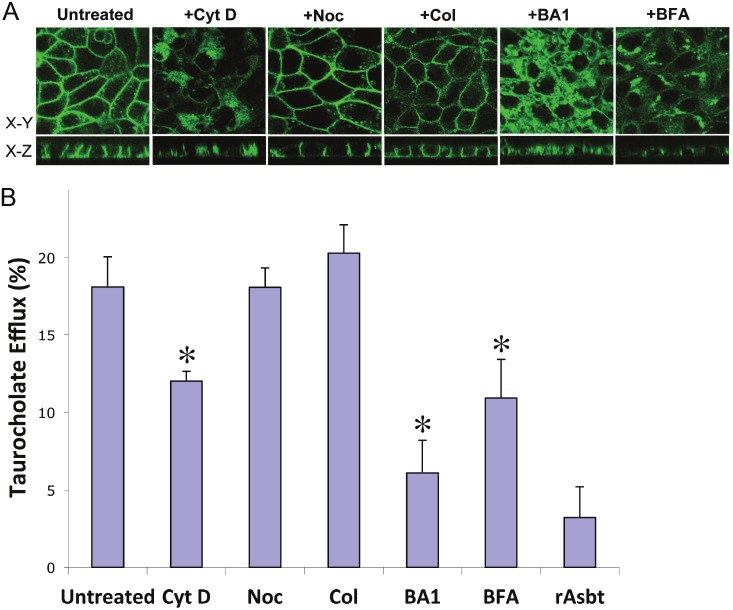
First, we examined whether disruption of cytoskeleton would effect the targeting of hOST proteins to the basolateral membrane. Three biological reagents (nocodazole, colchicines, and cytochalasin D) were used. Nocodazole disrupts microtubules by binding to β-tubulin and preventing formation of one of the two interchain disulfide linkages. Colchicine disrupts microtubules by binding to tubulin and preventing its polymerization. The results of confocal images from both x-y and x-z panels and efflux assay demonstrated that treatments with nocodazole (Noc) and colchicine (Col) did not significantly disrupt hOST protein basolateral membrane localization (Figure 3A) and TC transport activity (Figure 3B). In contrast, 2μM cytochalasin D (Cyt D, a potent inhibitor of actin polymerization) disrupted hOST membrane localization (Figure 3A, x-y and x-z panels) and decreased TC efflux transport activity by about 30% (Figure 3B).
Figure 3.
Effects of biological reagent treatments on cellular distribution and taurocholate (TC) efflux of hOST proteins. The GFP fused hOSTα (α-GFP) constructs were stably co-transfected with hOSTß and rat Asbt in MDCK cells. A: The membrane expression of hOSTα-GFP was detected by confocal fluorescence microscopy and photomicrograph of GFP-fused hOSTα was obtained on a confluent monolayer of the stably transfected MDCK cells cultured on glass coverslips. B: The Na+-independent efflux of taurocholate was examined in the MDCK cells stably transfected with rat Asbt, human OSTα and ß cDNAs. In this model system, the rAsbt acted as an apical bile acid loading pump in the stably transfected MDCK cells. The bile acid efflux activity from the basolateral domain by hOSTα and ß proteins was measured by radioactive scintillation counting. Data are presented as percentage (%) of total TC efflux and represent the mean value ± S.E. of three independent experiments performed in triplicate. Asterisk (*) for each stably transfected MDCK cells treated with drugs indicates significant difference (p<0.05) from drug untreated cells by unpaired t -test. The stably transfected MDCK cells were treated with 33μM nocodazole (Noc), 10μM colchicine (Col), 2μM cytochalasin D (Cyt D), 50nM bafilomycin A1(BA1), and 2μM brefeldin A (BFA), respectively, at 37°C for 16 hr. (Untreated = drug untreated stably transfected MDCK cells; rAsbt = MDCK cells transfected with rAsbt only).
To determine whether Golgi complex and the vacuolar H+-ATPase (a vacuolar proton pump) related vesicular pathway involved in the polarized membrane localization of hOST, the effects of brefeldin A (BFA, a secretory pathway inhibitor which disrupts the Golgi and blocks movement of membrane proteins from an intracellular pool to the cell surface) and bafilomycin A1 (BA1, a specific inhibitor of vacuolar H+-ATPase) were evaluated. In Figure 3A, the x-y and x-z confocal images showed a marked decrease of plasma membrane expression and significant increase of intracellular accumulation of hOSTα-GFP expressed in polarized (MDCK) cells treated with 50nM brefeldin A for 16h at 37°C. This interruption of membrane sorting of hOSTα-GFP was associated with a significant reduction of TC efflux activity by 40% in the brefeldin A (BFA) treated cells when compared with untreated cells (Figure 3B). Similarly, the confocal images demonstrated a large increase in intracellular accumulation of hOST-GFP in polarized (MDCK) cells after culture with 2μM bafilomycin A1 for 16h at 37°C (Figure 3A, x-y and x-z panels). The TC efflux activity was decreased more than 70% in transfected MDCK cells after treatment of 2 μM bafilomycin A1 (BA1) compared with untreated cells (Figure 3B). These results suggest that membrane sorting of hOST-GFP is mediated by a pathway that involve actin, the Golgi complex and bafilomycin A1 sensitive vesicles.
Effects of protein kinase inhibition on cellular localization and transport activity of hOST-GFP
Previous studies demonstrated that protein kinase (PK) phosphorylation might also be a factor regulating membrane localization of bile acid transporters, such as ASBT and human organic anion-transporting polypeptide C (hOATPC) [21,23]. Protein sequence analysis suggests that there are potential protein kinase (e.g. PKC) phosphorylation motifs in the intracellular loop and cytoplasmic tail of human OSTα and β proteins. To determine whether phosphorylation influences membrane trafficking of hOST, the effects of the PKA (H89), PKG (PKGi), and PKC (Calphostin C) inhibitors on membrane sorting of hOSTα-GFP were examined. Confocal imaging (Figure 4A, x-y and x-z panels) demonstrated no significant changes in plasma membrane expression and intracellular distribution of hOSTα-GFP co-expressed with hOSTβ in polarized (MDCK) cells after treated with 10μM H89 or 86 μM PKGi for 1h at 37°C. In contrast, confocal microscopy and TC efflux transport activity assay showed significant changes in cellular distribution and transport activity of hOSTβ cotransfected MDCK cells after treatment with 1μM protein kinase C inhibitor, calphostin C (Cal C) for 1h at 37°C (Figure 4). Confocal image analysis demonstrated a significantly increased intracellular accumulation of hOSTα-GFP in Cal C treated cells (Figure 4A, x-y and x-z panels). Consistent with the results of confocal microscopy, TC efflux transport activity was reduced by ~30% after treatment with 1μM Cal C for 1 hour at 37°C (Figure 4B). These results suggest that membrane targeting of hOST-GFP is regulated by PKC, but not PKA or PKG.
Figure 4.
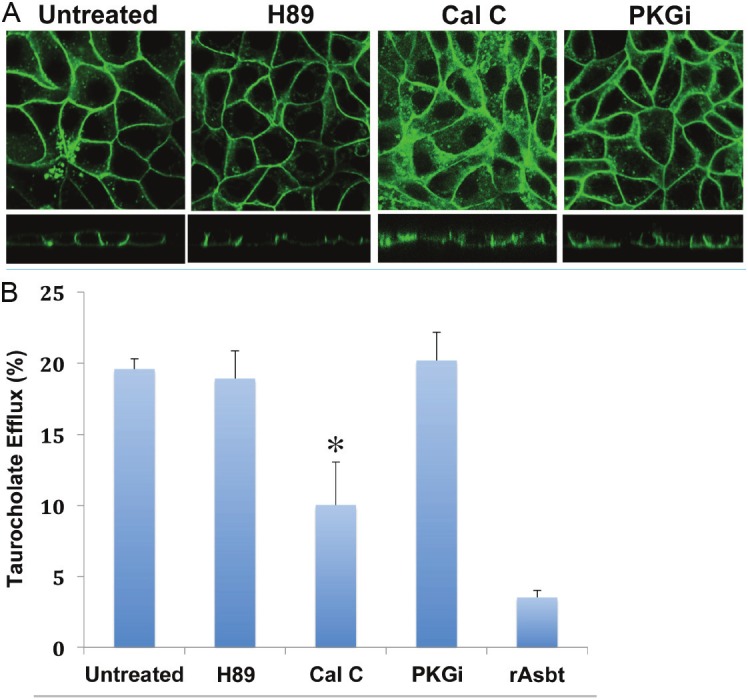
Effects of protein kinase inhibitor treatment on membrane localization and taurocholate (TC) transport activity of hOST protein in stably transfected MDCK cells.The GFP fused hOSTα (α-GFP) constructs were stably co-transfected with hOSTβ and rat Asbt in MDCK cells. The stably transfected MDCK cells were pretreated with (+) or without H89 (a PKA inhibitor, 10μM), Calphostin C (Cal C, a PKC specific inhibitor, 1μM), and Protein Kinase G inhibitor (PKGi, 86μM), respectively, for 1 hr., at 37°C. A: confocal images, B: The Na+-independent bile acid transport activity by hOSTα and ß protein was measured by radioactive scintillation counting. Data are presented as percentage (%) of total TC efflux, and the mean value ± S.E. of two independent experiments performed in triplicate. Asterisk (*) for each stably transfected MDCK cells treated with protein kinase inhibitors indicates significant difference (p<0.05) from untreated cells by unpaired t -test. (Untreated = drug untreated stably transfected MDCK cells; rAsbt = MDCK cells transfected with rAsbt only).
Discussion
Protein-protein interaction is an important mechanism to regulate membrane protein biological function and cellular distribution [24]. For some proteins, these interactions enhance both activity and number of proteins on the cell surface. The carboxyl-terminal and amino-terminal tails of membrane proteins are important for functional activity through specific motifs for interaction with other proteins that are required for proper sorting of the plasma membrane. Subramanian et al [17] showed that the COOH tail of human sodium-dependent multivitamin transporter (hSMVT) contains several determinants that are important for polarized targeting and biotin transport. Some transporter proteins must be physically associated to form a functional homo- or hetero-dimer for transport function and intracellular trafficking. Previous studies from our laboratory and others demonstrated that protein interaction (dimerization) of hOSTα and β subunits is required for transport function and polarized surface localization [6-10]. Individually, hOSTα or hOSTβ subunits lack transport activity and lose the ability for polarized targeting to basolateral plasma membrane.
The results from this study demonstrated that deletion of the C-terminal 73 amino acid tail of hOSTβ slightly increased intracellular accumulation and modestly reduced efflux transport activity. On the other hand, when the N-end of 35 amino acid tail of hOSTβ was removed, the efflux transport activity and polarized membrane distribution of the N-terminal truncated hOSTβ proteins were abolished. Co-IP studies further verified that the N-end tail of hOSTβ may be directly involved in association with hOSTα subunit. These results suggest that the N-end 35 amino acid tail of hOSTβ is essential for assembly of the functional heterodimer with the hOSTα protein, which is required for transport function and polarized cellular distribution.
The cytoskeletal network including microtubules and actin plays an important role in intracellular trafficking of proteins and transport function [25-29]. Dranoff et al [30] have shown that delivery of Na+-taurocholate cotransport polypeptide (Ntcp) to the basolateral region of the plasma membrane is via microtubule network and insertion of Ntcp into the plasma membrane in a microfilament- and cAMP-sensitive fashion. Our previous studies demonstrated that trafficking of ASBT to plasma membrane is by means of a vesicle-associated pathway that is partially interrupted by bafilomycin A1 [18]. This reagent inhibits the vacuolar H+-ATPase (V-ATPase) activity and prevents endosome acidification. The B subunits of V-ATPases have been shown to bind actin filaments, and actin-binding activity by the B subunit is required for targeting of V-ATPases to the plasma membrane [31]. It is generally accepted that integral plasma membrane proteins are typically transported in a secretory pathway from the endoplasmic reticulum (ER) and Golgi complex. However, Schotman et al [32] demonstrated that the newly synthesized integrin (basolateral) plasma membrane proteins are deposited via a mechanism that appears to bypass the Golgi. Batistic et al [33] also indicated that for CBL/CIPK Ca2+ signaling complexes, the S-acylation is crucial for endoplasmic reticulum-to-plasma membrane trafficking via a novel cellular targeting pathway that is insensitive to brefeldin A.
To investigate the subcellular location and the mechanism of hOST membrane trafficking, we expressed human OSTα and β in stably transfected MDCK cells and examined the effects of nocodazole, colchicine, cytochalasin D, bafilomycin A1, and brefeldin A on transport activity and sorting to the plasma membrane. Disruption of the actin cytoskeleton with cytochalasin D, but not microtubules with nocodazole and colchicine, led to the accumulation of hOST intracellularly, and reduced the level of basolateral membrane expression and transport activity. These findings strongly suggest that hOST is targeted to the cell membrane in an actin-dependent manner. Inhibiting vacuolar H+-ATPase by bafilomycin A1 also disrupted hOST intracellular trafficking and reduced transport activity, indicating that hOST is delivered to the plasma membrane via a bafilomycin A1-sensitive vesicular machinery. The involvement of vacuolar H+-ATPase with the B subunit that is connected with actin, further supports the involvement of actin components in hOST trafficking. These results suggest that a key role for the actin-network in trafficking of hOST, whose movement could occur via a bafilomycin A1-sensitive vesicular pathway. Our results show that brefeldin A treatment, which effectively disperses the ER and Golgi membranes, leads to partial retention of hOST within ER and Golgi regions and reduced transport activity. Interestingly, the confocal image of N-end truncated hOSTβ (β-NT-GFP) showed a similar predominant intracellular distribution, as observed with Brefeldin A treatment. This suggests that the N-end fragment of hOSTβ may contain information involving the protein complex secretory pathway from ER and Golgi complex.
Protein kinases have also been implicated in regulating intracellular trafficking of proteins and transporter function. We and others have shown that Asbt follows a transport vesicle-mediated, apical sorting pathway that is brefeldin A-sensitive and occurs via PKC- and PKA-regulating mechanisms [18,21,22]. In this study, we demonstrated that a broad-spectrum PKC inhibitor, Calphostin C, but not PKA and PKG inhibitors, partially prevented hOST polarized basolateral membrane trafficking, increased intracellular accumulation of hOST and significantly reduced hOST efflux transport activity. These results suggest that a PKC-sensitive machinery modulates membrane targeting of hOST-GFP. Protein kinase C is an important family of serine/threorine protein kinases that has various isoforms (such as isotypes, alpha, beta 1, delta, gamma, lambda, epsilon, eta) with different subcellular distributions [14,15,34]. More studies are required to identify which PKC isoforms play a role in hOST subcellular trafficking.
In conclusion, our studies indicate that the 35 amino acid amino-terminal fragment of hOSTβ contains critical information for association with hOSTα and functional polarized membrane localization. The membrane sorting process of hOST is mediated by a bafilomycin A1-sensitive vesicular pathway that is associated with the actin-cytoskeleton network. The membrane localization of hOST is also partially mediated through a brefeldin A sensitive mechanism, which controls its transit from the ER to Golgi and is regulated by PKC.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants 5R37HD020632-21 and DK084434 (to F. J. S.). Confocal laser scanning microscopy was performed at the MSSMCLSM core facility, supported with funding from NIH-NCI shared resources grant (5R24 CA095823-04), NSF Major Research Instrumentation grant (DBI-9724504) and NIH shared instrumentation grant (1 S10 RR0 9145-01).
Abbreviations
- BA1
bafilomycin A1
- BFA
brefeldin A
- Cal C
calphostin C
- Col
Colchicines
- Cyt D
Cytochalasin D
- GFP
green fluorescent protein
- H89
N-[2-(methylamino)ethyl]-5-isoquinolinesulfonamide
- hOST
human organic solute transporter
- hOST-GFP
GFP-fused human OST
- Noc
Nocodazole
- PKGi
protein kinase G inhibitor
- TC
Taurocholate
References
- 1.Seward DJ, Koh AS, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, OSTalpha-OSTbeta. J Biol Chem. 2003;278:27473–82. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- 2.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–9. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 3.Ballatori N, Li N, Fang F, Boyer JL, Christian WV, Hammond CL. OST alpha-OST beta: a key membrane transporter of bile acids and conjugated steroids. Front Biosci. 2009;14:2829–44. doi: 10.2741/3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol. 2008;295:G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–30. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sun AQ, Balasubramaniyan N, Xu K, Liu CJ, Ponamgi VM, Liu H, Suchy FJ. Protein-protein interactions and membrane localization of the human organic solute transporter. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1586–93. doi: 10.1152/ajpgi.00457.2006. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking and membrane topology of the two proteins, Ost alpha and Ost beta, that constitute the organic solute and steroid transporter. Biochem J. 2007;407:363–72. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballatori N. Biology of a novel organic solute and steroid transporter, OSTalpha-OSTbeta. Exp Biol Med (Maywood) 2005;230:689–98. doi: 10.1177/153537020523001001. [DOI] [PubMed] [Google Scholar]
- 9.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alphabeta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–8. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson PA, Hubbert ML, Rao A. Getting the mOST from OST: Role of organic solute transporter, OSTalpha-OSTbeta, in bile acid and steroid metabolism. Biochim Biophys Acta. 2010;180:994–1004. doi: 10.1016/j.bbalip.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders C, Limbird LE. Disruption of microtubules reveals two independent apical targeting mechanisms for G-protein-coupled receptors in polarized renal epithelial cells. J Biol Chem. 1997;272:19035–45. doi: 10.1074/jbc.272.30.19035. [DOI] [PubMed] [Google Scholar]
- 12.Sun AQ, Balasubramaniyan N, Liu CJ, Shahid M, Suchy FJ. Association of the 16 kDa subunit C of vacuolar proton pump with ileal Na+-dependent bile acid transporter: protein-protein interaction and intracellular trafficking. J Biol Chem. 2004;279:16295–16300. doi: 10.1074/jbc.M312838200. [DOI] [PubMed] [Google Scholar]
- 13.Potter EA, Stewart G, Smith CP. Urea flux across MDCK-mUT-A2 monolayers is acutely sensitive to AVP, cAMP, and [Ca2+] i. Am J Physiol Renal Physiol. 2006;291:F122–8. doi: 10.1152/ajprenal.00423.2005. [DOI] [PubMed] [Google Scholar]
- 14.Adams JC, Clelland JD, Collett GD, Matsumura F, Yamashiro S, Zhang L. Cell-matrix adhesions differentially regulate fascin phosphorylation. Mol Biol Cell. 1999;10:4177–90. doi: 10.1091/mbc.10.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew CS, Zhou CJ, Parente JA Jr. Ca2+-independent protein kinase C isoforms may modulate parietal cell HCl secretion. Am J Physiol. 1997;272:G246–56. doi: 10.1152/ajpgi.1997.272.2.G246. [DOI] [PubMed] [Google Scholar]
- 16.Glass DB. Differential responses of cyclic GMPdependent and cyclic AMP-dependent protein kinases to synthetic peptide inhibitors. Biochem J. 1983;213:159–64. doi: 10.1042/bj2130159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodiumdependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol. 2009;296:C663–71. doi: 10.1152/ajpcell.00396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun AQ, Salkar R, Sachchidanand , Xu S, Zeng L, Zhou MM. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem. 2003;278:4000–9. doi: 10.1074/jbc.M207163200. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Suzuki H, Horie T, Sugiyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22:1559–77. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- 20.Roma MG, Milkiewicz P, Elias E, Coleman R. Control by signaling modulators of the sorting of canalicular transporters in rat hepatocyte couplets: role of the cytoskeleton. Hepatology. 2000;32:1342–56. doi: 10.1053/jhep.2000.20519. [DOI] [PubMed] [Google Scholar]
- 21.Sarwar Z, Annaba F, Dwivedi A, Saksena S, Gill RK, Alrefai WA. Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G532–8. doi: 10.1152/ajpgi.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlattjan JH, Benger S, Herrler A, von Rango U, Greven J. Regulation of taurocholate transport in freshly isolated proximal tubular cells of the rat kidney by protein kinases. Nephron Physiol. 2005;99:p35–42. doi: 10.1159/000082870. [DOI] [PubMed] [Google Scholar]
- 23.Sun AQ, Ponamgi VM, Boyer JL, Suchy FJ. Membrane trafficking of the human organic anion transporting polypeptide C (hOATPC) Pharm Res. 2008;25:463–74. doi: 10.1007/s11095-007-9399-9. [DOI] [PubMed] [Google Scholar]
- 24.Brzóska E, Grabowska I, Wróbel E, Moraczewski J. Syndecan-4 distribution during the differentiation of satellite cells isolated from soleus muscle treated by phorbol ester and calphostin C. Cell Mol Biol Lett. 2003;8:269–78. [PubMed] [Google Scholar]
- 25.Subramanian VS, Marchant JS, Ye D, Ma TY, Said HM. Tight junction targeting and intracellular trafficking of occludin in polarized epithelial cells. Am J Physiol Cell Physiol. 2007;293:C1717–26. doi: 10.1152/ajpcell.00309.2007. [DOI] [PubMed] [Google Scholar]
- 26.Hennig B, Schultheiss G, Kunzelmann K, Diener M. Ca2+-induced Cl- efflux at rat distal colonic epithelium. J Membr Biol. 2008;221:61–72. doi: 10.1007/s00232-007-9078-0. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Imamura T, Babendure JL, Lu JC, Olefsky JM. Disruption of microtubules ablates the specificity of insulin signaling to GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 2005;280:42300–6. doi: 10.1074/jbc.M510920200. [DOI] [PubMed] [Google Scholar]
- 28.Wersinger C, Sidhu A. Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry. 2005;44:13612–24. doi: 10.1021/bi050402p. [DOI] [PubMed] [Google Scholar]
- 29.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol. 2009:133–57. doi: 10.1007/978-3-540-79885-9_6. [DOI] [PubMed] [Google Scholar]
- 30.Dranoff JA, McClure M, Burgstahler AD, Denson LA, Crawford AR, Crawford JM, Karpen SJ, Nathanson MH. Short-term regulation of bile acid uptake by microfilament-dependent translocation of rat ntcp to the plasma membrane. Hepatology. 1999;30:223–9. doi: 10.1002/hep.510300136. [DOI] [PubMed] [Google Scholar]
- 31.Zuo J, Vergara S, Kohno S, Holliday LS. Biochemical and functional characterization of the actin binding activity of the B subunit of yeast vacuolar H+-ATPase. J Exp Biol. 2008;211:1102–8. doi: 10.1242/jeb.013672. [DOI] [PubMed] [Google Scholar]
- 32.Schotman H, Karhinen L, Rabouille C. dGRASP-mediated noncanonical integrin secretion is required for Drosophila epithelial remodeling. Dev Cell. 2008;14:171–82. doi: 10.1016/j.devcel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Batistic O, Sorek N, Schültke S, Yalovsky S, Kudla J. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell. 2008;20:1346–62. doi: 10.1105/tpc.108.058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faghiri Z, Bazan NG. Selective relocalization and proteasomal downregulation of PKCalpha induced by platelet-activating factor in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2006;47:397–404. doi: 10.1167/iovs.05-0290. [DOI] [PubMed] [Google Scholar]