Abstract
Most cells activate intracellular signalling to recover from heat damage. An increase of temperature, known as HS (heat shock), induces two major signalling events: the transcriptional induction of HSPs (heat-shock proteins) and the activation of the MAPK (mitogen-activated protein kinase) cascade. We performed the present study to examine the effects of HS, induced by different experimental conditions, on various kinases [ERK (extracellular-signal-regulated kinase), JNK (c-Jun N-terminal kinase), p38, Akt, AMPK (AMP-activated protein kinase) and PKC (protein kinase C)]. We investigated by Western blot analysis the phosphorylation of MAPK as a measure of cellular responsiveness to heat shift (37°C) and mild HS (40°C) in different cell lines. The results of the study indicate that every cell line responded to heat shift, and to a greater extent to HS, increasing ERK and JNK phosphorylation, whereas variable effects on activation or inhibition of PKC, AMPK, Akt and p38 were observed. Besides the implications of intracellular signalling activated by heat variations, these data may be of technical relevance, indicating possible sources of error due to different experimental temperature conditions.
Keywords: cell culture, heat damage, signal transduction
Abbreviations: AMPK, AMP-activated protein kinase; CGC, cerebellar granule cell; CHO, Chinese-hamster ovary; ERK, extracellular-signal-regulated kinase; HS, heat shock; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; PKC, protein kinase C
1. Introduction
HS (heat shock) response is one of the primordial intracellular defence mechanisms against stressful conditions. The cellular stress response can be viewed as an adaptative or ‘survival instinct’ response for the defence and maintenance of its structural and functional integrity (Widmann et al., 1999). HS induces two major signalling events: the transcriptional induction of HSPs (heat-shock proteins) (Young et al., 2003) and the activation of the MAPK (mitogen-activated protein kinase) cascade (Lee et al., 2005), which is one of the most ancient and evolutionarily conserved signalling pathways from unicellular organisms such as brewers’ yeast to complex organisms such as humans (Widmann et al., 1999). Three major groups of MAPKs in mammalian cells are regulated by stress: ERK (extracellular-signal-regulated kinase), p38 MAPK and JNK (c-Jun N-terminal kinase) (Junttila et al., 2008). Moreover, other important kinases appear to be modulated by heat stress: Akt (Gabai and Sherman, 2002), AMPK (AMP-activated protein kinase) (Spasić et al., 2009) and PKC (protein kinase C) (Joyeux-Faure et al., 2003). These kinases are activated by the dual phosphorylation of neighbouring threonine and tyrosine residues in response to various extracellular stimuli affecting cell fate, as an adaptation to environmental stress (Rattan, 2004; Nadeau and Landry, 2007).
According to previous studies, most cells activate these intracellular signalling pathways to recover from heat damage. The aim of the present study was to analyse the phosphorylation of MAPKs as a measure of cellular responsiveness to mild heat stress and to heat shift in different cell lines, in order to indicate possible sources of error due to different experimental temperature conditions.
2. Materials and methods
2.1. Cell lines and treatments
GH3, HeLa, PC12, 3T3-L1 and CHO (Chinese-hamster ovary) cell lines were obtained from A.T.T.C. (Bethesda, MA, U.S.A.) and cultured according to general methods. Primary cultures of CGCs (cerebellar granule cells) were obtained as described previously (D’Mello et al., 1993). In addition, βTC and INS-1 cells were originally obtained from Dr C. B. Wollheim (Department of Cell Physiology and Metabolism, University Medical Center, Geneva, Switzerland) (Possenti et al., 1999), and N38 (a hypothalamic cell line) were purchased from Cellution Biosystems.
Cells were plated into 35-mm poly-l-lysine-treated culture dishes at 70–80% confluence and cultured in complete medium, as recommended for each cell line, at 37°C in a humidified incubator containing 95% air and 5% CO2. After overnight complete medium incubation, cells were shifted to the appropriate medium without serum and left in the incubator at 37°C for 1 h. Following starvation, cells were kept on the bench at room temperature (25°C) for 15 min. After this period, cells were exposed for 5, 15 or 30 min at different temperatures (room temperature was 25°C, 37°C to induce a heat shift or 40°C to induce a mild HS).
Rabbit polyclonal antibodies against the phosphorylated kinases: ERK (p-ERK), PKC (p-PKC), AMPK (p-AMPK), Akt (p-Akt), JNK (p-JNK) and p38 (p-p38) were from Cell Signaling Technologies. The other reagents were from Sigma.
2.2. Western blot analysis
To assess kinase activation after the different temperature conditions used, the reaction was quickly stopped by placing on ice, the medium was removed and ice-cold lysis buffer (4°C) was added [50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 10 mM EDTA, 10 mM EGTA, 50 μM NaF, 2.5 μM sodium pyrophosphate, 1 μM 2-glycerophosphate and 1 μM sodium orthovanadate] containing 1×protease inhibitor mixture (Sigma). Equivalent amounts of whole-cell lysates were mixed with SDS-reducing sample buffer according to the manufacturer’s instructions (Invitrogen). Following heating at 70°C for 10 min, samples were placed at 4°C and proteins were subjected to SDS/PAGE (NuPAGE) and transferred electrophoretically on to PVDF membrane (Amersham). After staining with Ponceau S to verify uniformity of protein loading/transfer, the membranes were analysed for immunoreactivity. Incubation with primary antibodies was performed overnight at 4°C (rabbit anti-p-ERK1/2, -p-AMPK, -p-AKT, -p-JNK, -p-p38 or -p-PKC; at a dilution of 1:1000). Incubation with horseradish peroxidase-coupled secondary antibodies (Amersham; a 1:5000 dilution) was performed for 1 h at room temperature. Immunoreactivity was developed by ECL (enhanced chemiluminescence; Amersham). To normalize the loaded sample, the blots were stripped (Restore Western Blot Stripping, Pierce) and reprobed with antibodies against α-tubulin or β-actin (Sigma; 1:10000 dilution, overnight at 4°C).
3. Results and discussion
3.1. ERK1/2 phosphorylation is modified by experimental temperature conditions
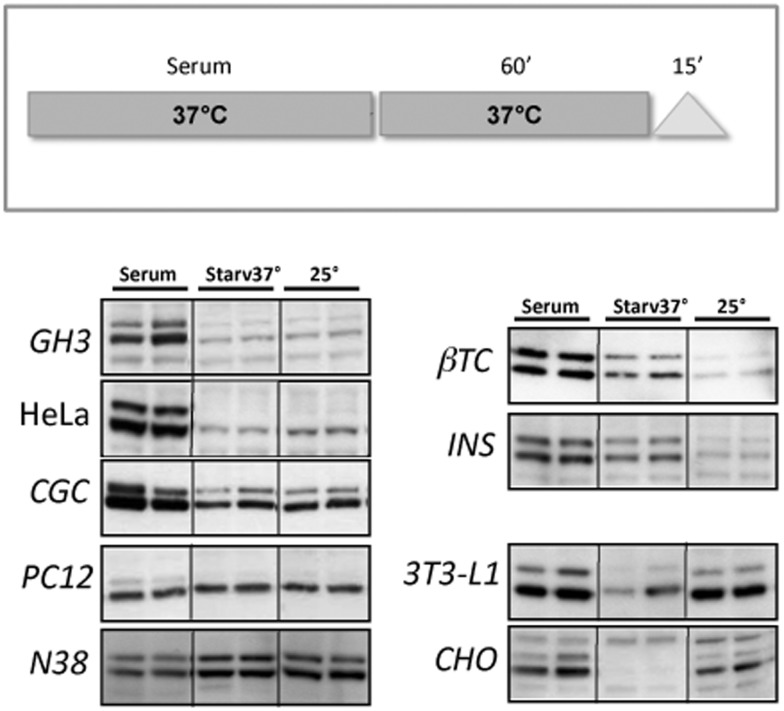
The best described, and most susceptible to stress, MAPK signalling pathway in mammalian cells is the ERK1/2 pathway (Lee et al., 2005). To examine the role of ERK1/2 in the heat-shift response, we analysed the phosphorylation state of ERK1/2 of different cell lines at three different conditions: overnight complete medium incubation (serum), starvation with the appropriate medium without serum for 1 h (Starv 37°C), and maintainance at room temperature for 15 min (25°C). As shown in Figure 1, serum deprivation generally caused a decrease in ERK1/2 phosphorylation (42–44 kDa bands). However, the amount of phosphorylated state in several cell lines is strictly dependent on the experimental temperature conditions. Indeed, βTC and INS-1 cell lines showed a significant decrease of p-ERK1/2 if treated at room temperature, whereas in 3T3 and CHO cell lines the same treatment caused an increase in the amount of p-ERK1/2. Although some fluctuations can be seen, no major changes were observed in the levels of p-ERK1/2 by either high or low temperature in GH3, HeLa, CGC, PC12 or N38 cells.
Figure 1. ERK1/2 phosphorylation.
Upper panel: scheme showing cell culture experimental conditions. Cells were grown in normal conditions (with serum) at 37°C overnight, starved for 1 h at 37°C (without serum) and then maintained for 15 min at room temperature (25°C). Lower panels: representative Western blots from at least three experiments, run in duplicate, showing ERK1/2 phosphorylation of different cell lines subjected to the experimental conditions described. To assess equal loading of samples, blots were stained with Ponceau S and probed with an antibody against α-tubulin (data not shown).
3.2. Activation of different kinases after heat-shift and mild HS
In view of the observed temperature-induced alterations in the phosphorylated state of ERK1/2, we examined the roles of other kinases in the temperature stress responses, considering that, usually, pharmacological administrations are carried out under a hood at room temperature (25°C) before replacing the plates in the incubator, whereas corresponding control cells are generally left in the incubator (at 37°C).
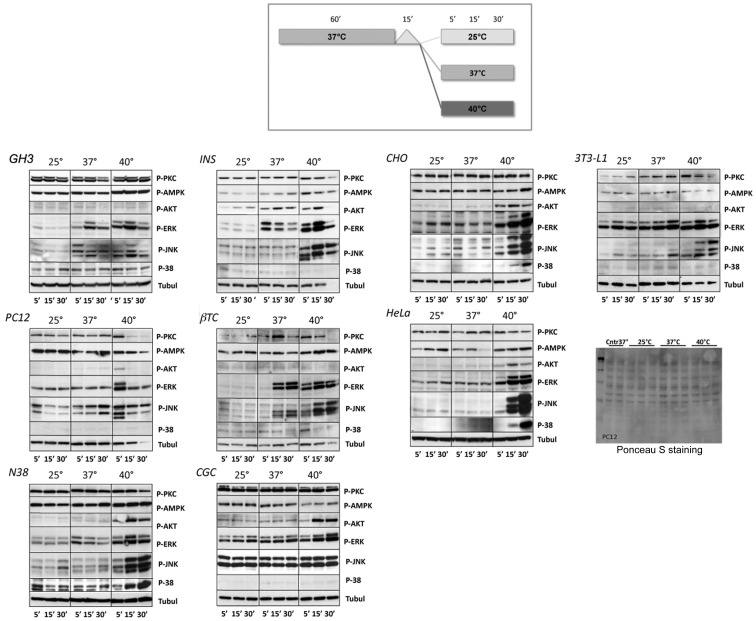
We analysed, in addition to p-ERK1/2, the phosphorylation state of PKC, AMPK, Akt, JNK and p38 in various cell lines treated as reported in the scheme shown in Figure 2. After 60 min of starvation, cells were transferred on to the worktable at room temperature (25°C) for 15 min, mimicking the experimental condition procedures (as changing medium or drug administrations). After this time, cells were kept at different temperatures: (i) room temperature 25°C, (ii) in a warm room at 37°C to induce a heat shift, or (iii) on a hot plate set to 42° (internal temperature of 40°C) to induce a mild HS, and cells were harvested after 5, 15 and 30 min.
Figure 2. Different MAPK activations.
Upper panel: scheme showing cell culture experimental conditions. Cells were grown under normal conditions (with serum) at 37°C overnight, starved for 1 h at 37°C (without serum) and then maintained for 15 min at room temperature (25°C). After this time, cells were kept for 5, 15 or 30 min at different temperatures (room temperature 25°C, 37°C to induce a heat shift or 40°C to induce a mild HS). Lower panels: representative Western blots from at least three experiments, showing phosphorylation of different kinases by various cell lines subjected to the experimental conditions described. Temperature conditions are indicated above the blots, whereas times of exposure are indicated below the blots. To assess that equal amounts of protein were present in each lane, the same blots were probed with an antibody against α-tubulin. To verify, eventually, different loading, gel blots were stained with Ponceau S or probed with an anti-α-tubulin antibody (data not shown).
As shown in Figure 2, phospho-kinase bands obtained after 5, 15 and 30 min at room temperature (25°), as expected, did not show significant changes over time and can be considered as a basal level of phosphorylation. The transfer into the warm room (37°C), which represents the usual working temperature and is able to induce a heat shift, generally caused an increase in ERK1/2 phosphorylation, mainly evident in GH3, INS-1, βTC and 3T3-L1 cell lines. Although some oscillations can be seen, no major alterations were observed in the levels of p-ERK1/2 induced by heat shift in PC12, HeLa, CGC, CHO, CGC or N38 cells.
Besides ERK1/2, another kinase appeared to be significantly regulated by temperature variations. Indeed, the temperature of 37°C (heat shift) was able to increase JNK phosphorylation in GH3, PC12 and βTC cell lines, with no marked changes in the other cell lines.
As shown in the same Figure, a further increase in temperature (40°C), able to induce a mild HS, demonstrated a strict increase in the ERK1/2 and JNK phosphorylation state in all of the cell lines examined, whereas HS differently regulated the other kinases in different cell lines.
Indeed, a significant increase of p-p38 was shown in N38, CHO, HeLa and βTC cells and of p-AMPK in GH3, N38, CHO, HeLa, INS-1 and βTC cells. In contrast, p-AMPK appeared to be down-regulated in PC12, CGC and 3T3-L1 cells. In addition, after HS, p-Akt activation was evident in N38, CHO, HeLa and CGC cells, whereas no significant changes were observed in the other cell lines. PKC phosphorylation decreased in PC12, N38, HeLa, INS-1 and βTC cells. In 3T3-L1 cells, after an early increase (5 and 15 min), a down-regulation of p-PKC was seen.
Furthermore, in some cell lines (PC12, INS-1, βTC and 3T3-L1 cells), the α-tubulin signal, used to normalize loading, decreased over time during mild HS exposure (Figure 2; 40°C for 30 min). To verify this result, PVDF blots were stained with Ponceau S (Figure 2) and then probed with anti-β-actin (data not shown). We noticed a normal protein loading for PC12, βTC and 3T3-L1 cells, whereas a decrease of protein content was observed in INS-1 cells treated for 30 min at 40°C. These findings indicate that α-tubulin could also be subjected to a rapid turnover in HS conditions.
Results of the present study show that there is not a homogeneous response in activating specific intracellular pathways to recover from heat damage, and that this activation is specific for each cell line. Moreover, simply moving cell cultures from the incubator (37°C) to the bench at room temperature (25°C), mimicking procedures usually used to add compounds to be tested, can significantly alter the phosphorylation level of some kinases.
One should take into account that pharmacological treatments are usually carried out at room temperature under a hood, before replacing cell cultures into the incubator, whereas the corresponding control cells are generally mantained in the incubator over time. These results suggest the need to consider carefully parallel-matched control points of kinase phosphorylation levels with cells treated under the same experimental conditions.
The present results may be of technical relevance, indicating possible sources of error in the result interpretation owing to the influence of different experimental temperature conditions.
Acknowledgements
This work was supported by the Fondazione Sovena, Rome, Italy (fellowship to P.P.).
Author contribution
Roberta Possenti designed the research and analysed data. Pamela Petrocchi, Stefania Quaresima and Maria Patrizia Mongiardi performed the research. Cinzia Severini and Roberta Possenti wrote the paper.
References
- D’Mello SR, Galli C, Ciotti T, Calissano P. Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci USA. 1993;90:10989–93. doi: 10.1073/pnas.90.23.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY. Interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol. 2002;92:1743–8. doi: 10.1152/japplphysiol.01101.2001. [DOI] [PubMed] [Google Scholar]
- Joyeux-Faure M, Arnaud C, Godin-Ribuot D, Ribuot C. Heat stress preconditioning and delayed myocardial protection: what is new? Cardiovasc Res. 2003;60:469–77. doi: 10.1016/j.cardiores.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–65. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Preheating accelerates mitogen-activated protein (MAP) kinase inactivation post-heat shock via a heat shock protein 70-mediated increase in phosphorylated MAP kinase phosphatase-1. J Biol Chem. 2005;280:13179–86. doi: 10.1074/jbc.M410059200. [DOI] [PubMed] [Google Scholar]
- Nadeau SI, Landry J. Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. Adv Exp Med Biol. 2007;594:100–13. doi: 10.1007/978-0-387-39975-1_10. [DOI] [PubMed] [Google Scholar]
- Possenti R, Rinaldi AM, Ferri GL, Borboni P, Trani E, Levi A. Expression, processing, and secretion of the neuroendocrine VGF peptides by INS-1 cells. Endocrinology. 1999;140:3727–35. doi: 10.1210/endo.140.8.6920. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Mechanisms of hormesis through mild heat stress on human cells. Ann N Y Acad Sci. 2004;1019:554–8. doi: 10.1196/annals.1297.103. [DOI] [PubMed] [Google Scholar]
- Spasić MR, Callaerts P, Norga KK. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist. 2009;15:309–16. doi: 10.1177/1073858408327805. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–7. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]