Summary
Chronic dietary restriction (DR) is considered among the most robust life-extending interventions, but several reports indicate that DR does not always extend and may even shorten lifespan in some genotypes. An unbiased genetic screen of the lifespan response to DR has been lacking. Here we measured the effect of one commonly used level of dietary restriction (DR: 40% reduction in food intake) on mean lifespan of virgin males and females in 41 recombinant inbred (RI) strains of mice. Mean strain-specific lifespan varied 2- to 3-fold under ad libitum (AL) feeding and 6- to 10-fold under DR, in males and females, respectively. Notably, DR shortened lifespan in more strains than those in which it lengthened life. Food intake and female fertility varied markedly among strains under AL feeding, but neither predicted DR survival: therefore, strains in which DR shortened lifespan did not have low food intake or poor reproductive potential. Finally, strain-specific lifespans under DR and AL feeding were not correlated, indicating that the genetic determinants of lifespan under these two conditions differ. These results demonstrate that the lifespan response to a single level of DR exhibits wide variation amenable to genetic analysis. They also show that DR can shorten lifespan in inbred mice. Although strains with shortened lifespan under 40% DR may not respond negatively under less stringent DR, the results raise the possibility that life extension by DR may not be universal.
Keywords: calorie restriction, lifespan, food restriction, longevity, nutrition
In 1935 McCay et al. (1935) reported that underfed rats “attained extreme ages beyond those of either sex that grew normally.” Since then, chronic reduction of food intake (dietary restriction or DR) has become the most common environmental intervention used to extend lifespan and probe mechanisms specifying longevity. DR extends lifespan across a variety of taxa (Weindruch & Walford, 1988; Finch, 1990; Masoro, 2003) and is considered to be among the most robust life-extending interventions (Weindruch & Walford, 1988; Masoro, 2005). Clinical studies are underway to test the effect of DR on various mortality risk factors in humans (Holloszy & Fontana, 2007), and members of one organization, the Calorie Restriction Society, practice self-imposed DR in an effort to extend their lives (Fontana et al., 2008).
However, life extension by DR may not be universal (Carey et al., 2002; Cooper et al., 2004). Several reports indicate that DR does not extend lifespan or has minimal effects in some rodent strains (Weindruch & Walford, 1988; Harper et al., 2006; Turturro et al., 1999). Others even report that DR shortens lifespan in some strains (Barrow & Roeder, 1965; Fernandes et al., 1976; Harrison & Archer, 1987; Forster et al., 2003), but these studies have not been conclusive given that other studies have shown lifespan extension under different conditions (Weindruch & Walford, 1988; Turturro et al., 1999). A systematic, unbiased screen to determine the efficacy of moderate DR across a range of genotypes is lacking. Here, we undertook such a study -- testing the hypothesis that the lifespan response to DR is subject to naturally-occurring genetic variation encompassing null or even negative effects.
This study used 41 ILSXISS recombinant inbred (RI) mouse strains (Williams et al., 2004) (formerly called LXS) originally developed to analyze genetic variation in alcohol sensitivity (Bennett et al., 2006). Mice were typically maintained 5/cage (Supplementary Table S1) and started at 2–5 months of age fed ad libitum (AL) or DR diets (60% of strain-specific AL intake) in a specific-pathogen-free vivarium dedicated to murine aging research (Ikeno et al., 2005). The DR rations, which were not implemented gradually, were calculated on the basis of AL food intake measured weekly for each strain, adjusted for wastage (Ikeno et al., 2005), and the rations were given daily just before lights out. At 12 months of age, the DR rations were fixed to avoid tracking the reduction of food intake that occurs during aging. We have followed this DR protocol at 60% of AL intake for over 30 years (Ikeno et al., 2005; Yu et al., 1982; McCarter et al., 2007). This level of restriction is one of the most common (Turturro et al., 1999; de Cabo et al., 2005), although DR levels from 40% to 80% of AL intake have been used to achieve life extension (Weindruch & Walford, 1988).
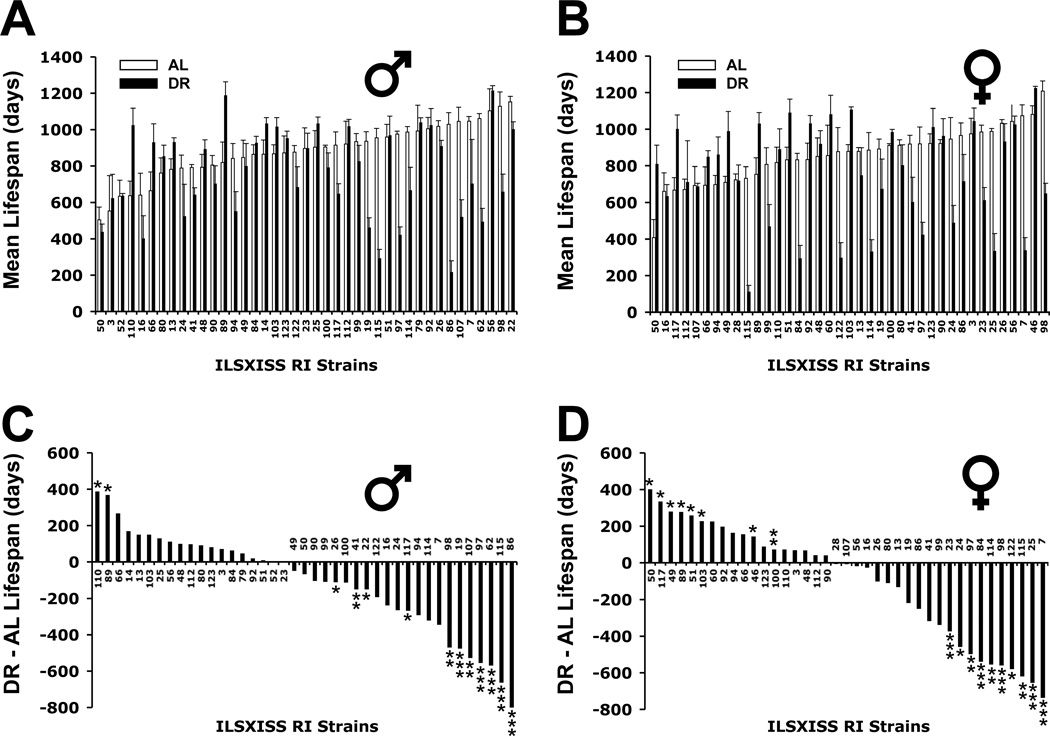
We found that the RI strains exhibited marked genetic variation in lifespan under both AL and DR conditions (Figs. 1 A, B; Supplementary Table S1). Mean lifespan under AL feeding ranged two- to three-fold: 504 to 1152 days in males and 407 to 1208 days in females. This variation in AL lifespan is comparable to that of 31 inbred strains selected for their genetic diversity (Yuan et al., 2009) (Supplementary Fig. S1). Strain variation of mean lifespan in mice under DR was even greater, ranging six- to ten-fold: 217 to 1215 days in males and 113 to 1225 days in females. Effect of strain on lifespan was significant for both sexes under both feeding conditions (p < 1×10−6, ANOVA). Heritability of lifespan under AL feeding was 28% (males) and 36% (females) and under DR was 55% (males) and 53% (females).
Fig. 1.
Strain variation in mean lifespan of ILSXISS recombinant inbred (RI) mice under ad libitum (AL) and dietary restriction (DR) diets. Lifespans were typically obtained from 10 AL and 10 DR mice from each strain (5 males & 5 females per treatment group); sample sizes in a few strains were either greater or less than 10 (details in Supplementary Table S1). The mean lifespans in the upper two panels are shown for each strain [AL (□) and DR (■)], ranked in ascending order according to the AL means (A: males, 41 strains; B: females, 39 strains). The lower two panels illustrate the deviation (positive and negative) of the mean DR lifespan from the mean AL for the same strains, ranked from the strain with the greatest increase in lifespan under DR to the strain with the greatest decrease (C: males; D: females). Error bars represent SEM. * p < 0.05, ** p < 0.01, *** p < 0.001 by t-test (no experiment-wise Bonferroni correction).
Strikingly, the majority of strains showed no extension of lifespan under the level of DR used in this study (Figs. 1C, D). Only 5% of the strains for males and 21% of the strains for females showed statistically significant life extension under DR, using single strain p values < 0.05. DR shortened lifespan in more strains (27% and 26%; males and females, respectively; p < 0.05 – 0.001). Although sample sizes were small, mean lifespans of males and females were significantly correlated under both AL (r = 0.50, p = 0.002) and DR (r = 0.42. p = 0.012) conditions. In addition, doubling sample size by combining the two sexes yielded a similar result: DR shortened life in more strains than showed lengthened life (Supplementary Fig. S2). Maximum lifespan (age at death of oldest mouse) was highly correlated with mean lifespan across strains under both AL and DR regimens (AL males, r = 0.81; AL females, r = 0.82; DR males, r = 0.92; DR females, r = 0.94; all p < 1×10−9), indicating that the strain variation in mean lifespan was not disproportionately affected by early deaths that can arise in DR mice. That early deaths in DR mice contributed to lifespan shortening is not supported by the finding that exclusion of deaths occurring before 12 months of age had negligible effect on the frequency of lifespan shortening (Supplementary Fig. S3). These results, using a large genetic screen, buttress previous but often overlooked results showing no extension or shortening of lifespan by DR (Weindruch & Walford, 1988; Harper et al., 2006; Turturro et al., 1999; Barrow & Roeder, 1965; Fernandes et al., 1976; Harrison & Archer, 1987; Forster et al., 2003). However, whether strains showing no increase in lifespan under 40% or other fixed level of DR show no increase in lifespan under less stringent level of DR remains to be determined.
Of note, the longest lifespans achieved under DR did not exceed the longest achieved under AL feeding (Figs. 1A, B). The average of the mean lifespans of the five longest-lived strains under DR (1103±40 and 1108±32 days in males and females) did not exceed that of the five longest-lived, albeit different, strains under AL feeding (1098±20 and 1088±31 days). Future studies are needed to determine why DR cannot further extend the lifespan of long-lived strains in this RI panel. One testable hypothesis is that the lifespan extending biochemical pathways modulated by DR are already maximally modulated in strains that are long-lived under AL conditions.
The biological basis for the strikingly different responses of lifespan to the commonly used level of DR, including life shortening, is important to determine. For example, some lines in this study may have unusual nutritional needs, and thus 40% DR could cause nutritional deficiencies that might outweigh the beneficial effects of DR. However, the possibility that some strains are vulnerable to a mineral or vitamin deficiency under DR is unlikely because, with the exception of selenium and choline, the diet used (Harlan-Teklad 7912) exceeded by several fold the minimum requirements established by the National Research Council (Nutrient Requirements of Laboratory Animals, 1995) (Supplementary Table S2). Also, even with diets supplemented with vitamins, the lifespan of male DBA/2J mice was either not extended (Forster et al., 2003) or minimally lengthened (Turturro et al., 1999). There also was no correlation between DR lifespan and the large strain variation in absolute food intake (Table 1), suggesting that the strains most likely to encounter deficiency were not more likely to have reduced survival under DR.
Table 1.
Absence of correlation between lifespan under dietary restriction (DR) and lifespan, food consumption, fertility and ethanol sensitivity under ad libitum (AL) feeding.f
| AL lifespana |
AL food intakeb |
AL fertilityc |
AL fertilityd |
AL LORRe |
||
|---|---|---|---|---|---|---|
| DR lifespana |
Males |
r = 0.09 p = 0.56 |
r = −0.02 p = 0.91 |
r = 0.21 p = 0.19 |
r = −0.21 p = 0.19 |
r = 0.13 p = 0.39 |
| Females |
r = −0.03 p = 0.86 |
r = 0.30 p = 0.06 |
r = 0.18 p = 0.27 |
r = −0.12 p = 0.48 |
||
Strain-specific mean lifespan (days).
The food intake (g/mouse/day) was measured on a weekly basis. The values are the average of food intake from 3–5 months to 12 months of age.
Fertility (litter size) was measured in the generation of mice preceding the lifespan cohort and defined as the average litter size of the first three litters.
Fertility (litter/female) defined as the average number of litters per strain.
The sedative-hypnotic response to a high-dose, intraperitoneal injection of ethanol, defined as time to regain loss of righting reflex (LORR). The LORR data are from Bennett et al., 2006.
The p values of the Pearson correlation coefficients (r) are all from 2-tailed tests.
Considering the derivation of the ILSXISS strains, we tested whether the lifespan variation in response to DR might be related to the segregation of alleles for extreme differences in ethanol sensitivity, which could potentially reflect differences in vitality or stress resistance. However, there was no correlation between sensitivity to this stressor and lifespan in DR mice (Table 1). Another potential measure of vigor, female fertility, also showed no correlation with DR lifespan (Table 1). These results argue against the notion that strains in which DR shortened lifespan lacked overall vitality.
Many other testable possibilities exist to explain life-shortening of some strains under DR. These include vulnerability a) to stresses requiring energy expenditure, such as cold stress; b) to inbreeding depression (recessive alleles) not reflected by the variation in AL lifespan or fertility; and c) to a 40% reduction in food intake that would not be present at a 30% or 20% reduction. Nevertheless, the variable response of these strains to DR provides a valuable tool for identifying quantitative trait loci (genes) that modulate DR’s mechanism of action. In addition, mechanistic traits hypothesized to underlie the lifespan modulating effect of DR should correlate positively with the variation in the lifespan response to DR.
In summary, these findings, coupled with earlier reports, show that even though DR extends lifespan across a variety of taxa, a prolongevity effect may not be a foregone conclusion for many genotypes. The marked genetic variation among RI strains provides a tool for identifying genes and biochemical pathways that mediate lifespan modulation by DR. Finally, the results raise a cautionary note concerning the application of DR to humans and a critical need for predictors of efficacy.
Supplementary Material
Acknowledgements
This work was funded by the National Institute on Aging (R01 AG024354), the Ellison Medical Foundation (JFN, TEJ, BAR) and the Glenn Foundation (JFN). We thank Drs. Jonathan Gelfond and Alex McMahon for statistical consultation, and the staff of the Nathan Shock Center Aging Animal Core for expert treatment and monitoring of the mice. Brad Rikke is acknowledged for his seminal role in formulating the idea of using recombinant inbred mice to probe DR mechanisms.
References
- Barrows CH, Jr, Roeder LM. The Effect of Reduced Dietary Intake on Enzymatic Activities and Life Span of Rats. J Gerontol. 1965;20:69–71. doi: 10.1093/geronj/20.1.69. [DOI] [PubMed] [Google Scholar]
- Bennett B, Carosone-Link P, Zahniser NR, Johnson TE. Confirmation and fine mapping of ethanol sensitivity quantitative trait loci, and candidate gene testing in the LXS recombinant inbred mice. J Pharmacol Exp Ther. 2006;319:299–307. doi: 10.1124/jpet.106.103572. [DOI] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Harshman L, Zhang Y, Muller H-G, Partridge L, Wang J-L. Life history response of Mediterranean fruit flies to dietary restriction. Aging Cell. 2002;1:140–148. doi: 10.1046/j.1474-9728.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly, Musca domestica. Faseb J. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Anson RM, Jones B. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. AGE. 2005;27:17–25. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. Longevity, Senescence, and the Genome. University Of Chicago Press; 1990. [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. Faseb J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Genetic differences in effects of food restriction on aging in mice. J Nutr. 1987;117:376–382. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Richardson A, Strong R, Diaz V, Nelson JF. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Subfield history: caloric restriction, slowing aging, and extending life. Sci Aging Knowl Environ. 2003;2003:RE2. doi: 10.1126/sageke.2003.8.re2. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- McCarter R, Mejia W, Ikeno Y, Monnier V, Kewitt K, Gibbs M, McMahan A, Strong R. Plasma glucose and the action of calorie restriction on aging. J Gerontol A Biol Sci Med Sci. 2007;62:1059–1070. doi: 10.1093/gerona/62.10.1059. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life and upon the ultimate body size. J Nutr. 1935:63–79. [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford R. The Retardation of Aging and Disease by Dietary Restriction. Springfield, III: Charles C Thomas Publisher; 1988. [Google Scholar]
- Williams RW, Bennett B, Lu L, Gu J, DeFries JC, Carosone-Link PJ, Rikke BA, Belknap JK, Johnson TE. Genetic structure of the LXS panel of recombinant inbred mouse strains: a powerful resource for complex trait analysis. Mamm Genome. 2004;15:637–647. doi: 10.1007/s00335-004-2380-6. [DOI] [PubMed] [Google Scholar]
- Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol. 1982;37:130–141. doi: 10.1093/geronj/37.2.130. [DOI] [PubMed] [Google Scholar]
- Yuan R, Tsaih S-W, Petkova SB, Evsikoca CMd, Xing S, Marion MA, Bogue MAB, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutrient Requirements of Laboratory Animals. 4th revised edition. Washington, DC: National Academy Press; 1995. pp. 80–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.