Abstract
Context
Progressive brain volume changes in schizophrenia are thought to be due principally to the disease. However, recent animal studies indicate that antipsychotics, the mainstay of treatment for schizophrenia patients, may also contribute to brain tissue volume decrement. Because antipsychotics are prescribed for long periods for schizophrenia patients and have increasingly widespread use in other psychiatric disorders, it is imperative to determine their long-term effects on the human brain.
Objective
To evaluate relative contributions of 4 potential predictors (illness duration, antipsychotic treatment, illness severity, and substance abuse) of brain volume change.
Design
Predictors of brain volume changes were assessed prospectively based on multiple informants.
Setting
Data from the Iowa Longitudinal Study.
Patients
Two hundred eleven patients with schizophrenia who underwent repeated neuroimaging beginning soon after illness onset, yielding a total of 674 high-resolution magnetic resonance scans. On average, each patient had 3 scans (≥2 and as many as 5) over 7.2 years (up to 14 years).
Main Outcome Measure
Brain volumes.
Results
During longitudinal follow-up, antipsychotic treatment reflected national prescribing practices in 1991 through 2009. Longer follow-up correlated with smaller brain tissue volumes and larger cerebrospinal fluid volumes. Greater intensity of antipsychotic treatment was associated with indicators of generalized and specific brain tissue reduction after controlling for effects of the other 3 predictors. More antipsychotic treatment was associated with smaller gray matter volumes. Progressive decrement in white matter volume was most evident among patients who received more antipsychotic treatment. Illness severity had relatively modest correlations with tissue volume reduction, and alcohol/illicit drug misuse had no significant associations when effects of the other variables were adjusted.
Conclusions
Viewed together with data from animal studies, our study suggests that antipsychotics have a subtle but measurable influence on brain tissue loss over time, suggesting the importance of careful risk-benefit review of dosage and duration of treatment as well as their off-label use.
Schizophrenia, a common mental illness affecting 1% of the worldwide population, remains a leading cause of chronic disability among young adults.1 Antipsychotic medications are the mainstay of treatment because there is strong empirical evidence that these drugs reduce psychotic symptoms and relapse rates in schizophrenia patients.2 Even though the majority of patients receive antipsychotics and benefit from reduction in psychotic symptoms, many patients continue to have negative symptoms, cognitive impairments, and progressive brain tissue loss.3–13 The causes underlying these brain abnormalities are unclear and have been a focus of much debate14,15 and many literature reviews.16,17
In a previous study comprising 119 schizophrenia patients,18 we found that brain volume reductions on magnetic resonance imaging (MRI) were related to a common genetic variant within the brain-derived neurotrophic factor gene and to antipsychotic treatment, such that more antipsychotic treatment correlated with greater frontal gray matter (GM) volume reductions. When viewed in conjunction with controlled antipsychotic treatment studies in animals,19–21 our previous findings suggest that antipsychotic treatment may contribute to brain tissue volume loss. More recent literature reviews have highlighted the potential role of antipsychotics in influencing brain volume deficits in schizophrenia and its implications.22–26
The goal of the current study was to comprehensively evaluate the contributions of 4 potential causative factors that may mediate progressive brain volume decrement in schizophrenia: illness duration, long-term antipsychotic treatment, illness severity, and substance abuse. Extending our previous work,18 the present study has the largest available cohort of schizophrenia patients who have undergone longitudinal MRI assessments. We examined 211 patients and collected 674 high-resolution MRI brain scans (averaging 3 scans per patient; at least 2 and up to 5 per patient) over an extended period (mean, 7 years; up to 14 years). Furthermore, multiple within-patient MRI scans coupled with an extensive clinical database provide for more robust estimates of brain volume trajectories.
Understanding the long-term effects of antipsychotics on the brain has wider clinical ramifications beyond treatment of patients with schizophrenia. Given the sharp rise in antipsychotic utilization,27 especially among pediatric and geriatric populations,27–30 examining the possibility of antipsychotic-associated brain tissue loss has important implications for assessing the risk-benefit ratio in a large number of psychiatric patients.
METHODS
Study participants were obtained through the Iowa Longitudinal Study (ILS).31 To be eligible, participants must have met DSM-III or DSM-IV criteria for schizophrenia-spectrum disorders and have been presenting for treatment of their first psychotic episode. At intake, patients underwent an extensive evaluation, including standardized clinical rating scales (Scale for Assessment of Negative Symptoms,32 Scale for Assessment of Positive Symptoms,33 Comprehensive Assessment of Symptoms and History,34 and Psychiatric Symptoms You Currently Have [PSYCH]35) and MRI. After intake, patients were examined at 6-month intervals by means of longitudinal follow-up versions of the Comprehensive Assessment of Symptoms and History and PSYCH, which included illness severity measures, alcohol and illicit drug use, and detailed information regarding antipsychotic treatment. Follow-up assessments were completed by experienced research personnel who have undergone interrater and test-retest reliability training.36 At follow-up assessments after 2, 5, and 9 years and every 3 years thereafter, MRI was repeated. Participant retention in the ILS is 63%. Sociodemographic and illness characteristics of patients who remained in the study are comparable to those of patients who dropped out.37
PATIENTS
The 211 patients (152 men and 59 women) in this report were selected from the larger ILS sample on the basis of having (1) a DSM-IV diagnosis of schizophrenia (n= 192) or schizoaffective disorder (n= 19) (verified at follow-up by psychiatrists’ consensus), and (2) undergone 2 or more MRI brain scans. There were 674 MRI scans (211 patients each had 2 scans, 139 had 3, 82 had 4, and 31 had 5) covering a mean follow-up period of 7.2 years (SD, 3.9 years; range, 1.9–14.0 years), and inter-scan intervals were approximately 3 years. At the initial MRI, mean (SD) age was 26.3 (7.6) years, and most patients had received minimal antipsychotic treatment (as detailed later).
MRI ACQUISITION AND ANALYSIS
High-resolution brain anatomic MRI data were collected by means of 1 of 2 imaging protocols on two 1.5-T MR scanners (General Electric Medical Systems, Milwaukee, Wisconsin). The type of imaging protocol was dependent on when the patient first enrolled in the ILS. For patients who entered the study before calendar year 2000, their initial and follow-up MRI scans were collected with the first imaging protocol (termed MR5). In patients who were enrolled in 2000 or later, all MRI scans were obtained with the second imaging protocol (termed MR6; see the supplementary “Methods” section and eTable 1 [http://www.archgenpsychiatty.com] regarding imaging parameters, data processing, and comparability). Of the 674 MRI brain scans, 570 were MR5 scans derived from 168 patients. Patients in the MR5 group had been followed up longer (mean, 8.05 years vs 4.06 years for the 43 patients in the MR6 group). Otherwise, there were no significant differences between the MR5 and MR6 groups on socio-demographics or illness characteristics (t ≤ 1.27, P ≥ .21).
In this study, we examined the following regions of interest: total cerebral tissue volume, total GM and white matter (WM), and GM:WM subdivided by Talairach atlas–based cerebral lobes (frontal, temporal, and parietal), lateral ventricles, sulcal cerebrospinal fluid (CSF), caudate, putamen, thalamus, and cerebellum (see the supplementary “Methods” section regarding region of interest measurements and the eFigure showing schematic representation of regions of interest).
ANTIPSYCHOTIC TREATMENT, ILLNESS SEVERITY, AND SUBSTANCE MISUSE
At each 6-month follow-up assessment, detailed information during the preceding 6 months was obtained from all available informants (ie, patient, family members, significant others, and medical records) and summarized in a timeline that records specific antipsychotic dose, treatment duration and medication adherence, illness severity, and alcohol/illicit drug misuse.
Antipsychotic treatment is naturalistic given that the long-term nature of the study precludes a random assignment design. Patients received “treatment as usual” in the community. Antipsychotic choice and dosages were left to the patient and his or her treating psychiatrist. Although it can be difficult to make precise measurements of lifetime antipsychotic exposure by using retrospective methods, our assessments every 6 months combining multiple information sources provide the most accurate treatment data possible in a long-term, large-sample naturalistic study. In this report, we use lifetime antipsychotic treatment up until the time of each MRI scan (expressed as mean daily antipsychotic dose [chlorpromazine (CPZ) milligram equivalents per day]) to assess relationships between antipsychotic treatment and brain volumes. To derive mean daily (total) antipsychotic dose, each antipsychotic was first converted to CPZ milligram equivalent units,38,39 and then all antipsychotic doses were summed and divided by the number of treatment days (see eTable 2 for CPZ equivalencies of individual antipsychotics).
Because intensity of treatment may be closely related to symptom severity and because we wished to examine its potential effect on brain change independently, we also examined the impact of illness severity on brain change. Since there is no single measure that comprehensively captures illness severity in schizophrenia, we explored multiple alternative approaches (eTables 3 and 4): Global Assessment Scale (GAS),40 symptom severity (mean of psychotic, negative, and disorganized symptoms [global ratings on the Scale for Assessment of Negative Symptoms and Scale for Assessment of Positive Symptoms] or as 3 separate symptom domains), global psychosocial functioning (rating scale within PSYCH), mean daily clozapine dose, and a composite score derived from the 4 preceding illness severity measures (weighted sum based on principal component analysis eigenvalues). Only results using GAS scores (mean score during follow-up period; lower score means greater illness severity) are presented herein. The GAS score is widely used in clinical studies, provides anchors to enhance interrater reliability, and has good psychometric properties. Mean GAS scores, negative/positive symptom ratings, and global psychosocial functioning scores were highly intercorrelated with one another (Pearson |r|≥0.82). Mean daily clozapine dose was less strongly correlated with the other 3 measures of illness severity (Pearson |r|≤0.14). Furthermore, regardless of which individual illness severity measure or the weighted sum composite score was used, results (eg, eTable 4) were similar to those using mean GAS score. Research personnel assessing GAS scores showed good agreement and reliability on their ratings (interrater and test-retest intraclass correlation coefficients, 0.79 and 0.62, respectively).
Substance abuse is another potential confounder for the study of change in brain measures. At follow-up assessments every 6 months, severity of alcohol use and severity of illicit drug use were each assessed by a 6-point ordinal scale: 0, no use; 1, occasional use (weekend binges) without social or occupational impairment; 2, occasional heavy use without impairment; 3, frequent use (≥3 times per week) with mild impairment; 4, daily use with moderate impairment; and 5, daily use with severe impairment leading to inability to function in social or occupational roles. Severity of alcohol/illicit drug misuse was derived by averaging both scores.
STATISTICAL ANALYSIS
Analyses were performed with SAS statistical software (version 9.2; SAS Institute, Inc, Cary, North Carolina). Random regression coefficient mixed models were used to evaluate the relationships between MRI brain volume changes and the 4 predictor variables: follow-up duration (time between MRI scan and initial scan), antipsychotic treatment (mean daily antipsychotic dose), illness severity (mean GAS score), and alcohol/ illicit drug misuse (mean severity score). For each region of interest, within-patient repeated measures of brain volumes were the dependent variables in each mixed model. Follow-up duration and an intercept term were specified as random effects to model within-patient correlations in brain volumes across time. The 4 predictor variables were entered concurrently as fixed effects, allowing us to examine the influence of one predictor variable on brain volume changes independent of the other 3 predictors. An antipsychotic treatment × follow-up duration interaction term was also included in the statistical models to further assess the effects of antipsychotic treatment on within-patient changes in brain volumes over time. Mean daily antipsychotic dose was mildly to moderately correlated with mean GAS score and with follow-up duration (Spearman r=−0.21 and 0.42, respectively; P< .001), Otherwise, there were weak intercorrelations between these predictor variables (Spearman r ≤ |0.12|). Furthermore, there was no evidence that these 4 predictor variables were highly collinear in the mixed models (tolerance values ≥0.74). Intracranial volume at initial MRI scan, sex, imaging protocol (MR5 vs MR6), and age at initial MRI scan were included as covariates. A 2-sided P<.05 was used to determine statistical significance.
RESULTS
ANTIPSYCHOTIC TREATMENT BEFORE AND DURING LONGITUDINAL FOLLOW-UP
The sample had minimal antipsychotic treatment before study enrollment (Table 1); there were 31 anti-psychotic-naive patients, and median treatment duration was 0.43 year. The types of antipsychotics patients received reflect prevailing medication prescribing patterns in the United States at the time (initial MRI scan, 1991–2006; last scan, 1995–2009). Typical antipsychotics were the predominant treatment before the initial MRI scan. Nonclozapine atypical antipsychotics became the main choice (in approximately two-thirds of the sample) during subsequent interscan intervals. About 25% of patients received clozapine treatment. Patients received adequate antipsychotic dosages, and treatment adherence was good (mean [SD] of 1.90 [0.82] on a 5-point clinical rating scale in which 1 is excellent; 2, good [ie, patient takes all psychiatric medications as prescribed; rarely, if ever, forgets or chooses not to take medications]; 3, fair; 4, poor; and 5, nonadherent).
Table 1.
APS Treatment Before Initial MRI Scan and Interval Preceding Each Follow-up Scan
| Initial Scan (N=211) | 1st Follow-up (n=211) | 2nd Follow-up (n=139) | 3rd Follow-up (n=82) | 4th Follow-up (n=31) | |
|---|---|---|---|---|---|
| Interscan interval, mean (SD), Ya | NA | 3.07 (1.57) | 3.31 (0.92) | 3.89 (1.21) | 3.00 (0.36) |
| No treatment, No. (%) | 31(14.7) | 8 (3.8) | 8 (5.8) | 6 (7.3) | 2 (6.5) |
| Clozapine treatment, No. (%) | 15(7.1) | 37 (17.5) | 34 (24.5) | 23(28.0) | 10 (32.3) |
| APS dose, mean (SD), CPZ mg equivalents/d | 245.3 (350.9) | 348.9 (323.3) | 438.9 (333.6) | 519.9(320.5) | 579.9 (336.3) |
| Type of APS treatment, mean (SD) % of total CPZ dose-yearsb | |||||
| Typical APSs | 58.2 (45.6) | 31.9 (39.3) | 19.3(30.4) | 16.4(30.0) | 16.5 (31.7) |
| Nonclozapine atypical APSs | 38.8(46.1) | 57.4 (41.7) | 62.2(40.6) | 60.9 (43.2) | 60.7 (44.9) |
| Clozapine | 3.0(13.7) | 10.7(26.9) | 18.5(36.0) | 22.7(39.3) | 22.8 (39.0) |
Abbreviations: APS, antipsychotic; CPZ, chlorpromazine; MRI, magnetic resonance imaging; NA, not applicable.
First follow-up is the time interval between the initial and first follow-up images; second follow-up, the time interval between the first and second follow-up images; etc.
One CPZ dose-year=100 mg of CPZ per day for 1 year.
INDEPENDENT EFFECTS OF FOLLOW-UP DURATION AND ANTIPSYCHOTIC TREATMENT ON BRAIN VOLUMES
Follow-up duration provides an indication of whether progressive brain changes occur over time. It had significant main effects on all brain volumes (Table 2; F ≥ 5.39, P ≤ .02) except for total cerebral WM, frontal WM, temporal WM, and cerebellum (F ≤ 2.03, P ≥ .16). Longer duration of follow-up was significantly associated with total cerebral tissue, GM, and subcortical brain tissue volume reductions (Table 2; b ≤ −0.01 cm3/y), and with parietal WM, lateral ventricles, and sulcal CSF volume enlargements (≥0.18 cm3/y).
Table 2.
Random Regression Coefficient Mixed Models: Fixed Effects of Follow-up Duration, APS Treatment, Illness Severity, and Substance Misuse on MRI Brain Volumes in 211 Schizophrenia Patientsa
| Regions of Interest | Follow-up Durationb
|
APS Treatmentc
|
Illness Severityd
|
Substance Misusee
|
APS × Timef
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| bb(SE) | F(P) | b(SE) | F(P) | b(SE) | F(P) | b(SE) | F(P) | b(SE) | F(P) | |
| Total cerebral tissue | −1.62 (0.37) | 16.80 (<.001) | −0.11 (0.07) | 2.39(.12) | 0.49 (0.26) | 3.65 (.08) | 4.81 (4.67) | 1.06 (.30) | −0.03 (0.01) | 4.29 (.04) |
| Total cerebral GM | −1.80(0.26) | 46.61 (<.001) | −0.15(0.05) | 8.11 (.005) | 0.39 (0.18) | 4.38 (.04) | 1.56 (3.32) | 0.22 (.64) | 0.008 (0.01) | 0.74 (.39) |
| Frontal GM | −1.04(0.13) | 62.44 (<.001) | −0.07 (0.03) | 6.67 (.01) | 0.27 (0.10) | 6.94 (.01) | 0.99 (1.83) | 0.29 (.59) | 0.0005 (0.005) | 0.01 (.93) |
| Temporal GM | −0.15 (0.06) | 6.43 (.01) | −0.03 (0.01) | 4.33 (.04) | 0.07 (0.05) | 1.86 (.17) | −1.00 (0.88) | 1.29 (.26) | 0.001 (0.002) | 0.18 (.67) |
| Parietal GM | −0.47 (0.07) | 45.48 (<.001) | −0.03(0.01) | 4.75 (.03) | 0.09 (0.06) | 2.20 (.14) | 0.56 (1.07) | 0.27 (.60) | 0.003 (0.003) | 1.17 (.28) |
| Total cerebral WM | 0.16 (0.32) | 0.27 (.61) | 0.05 (0.06) | 0.66 (.42) | 0.19 (0.24) | 0.68 (.41) | 2.98 (4.27) | 0.49 (.49) | −0.04 (0.01) | 10.34 (.001) |
| Frontal WM | −0.18 (0.13) | 2.03(.16) | 0.01 (0.03) | 0.13 (.72) | 0.07 (0.11) | 0.37 (.54) | 1.16 (1.97) | 0.34 (.56) | −0.01 (0.005) | 6.11 (.01) |
| Temporal WM | 0.05 (0.05) | 1.17 (.28) | 0.01 (0.01) | 1.50 (.22) | 0.02 (0.04) | 0.30 (.58) | −0.16 (0.76) | 0.05 (.83) | −0.006 (0.002) | 9.08 (.005) |
| Parietal WM | 0.18 (0.08) | 5.39 (.02) | 0.03 (0.02) | 3.19 (.08) | 0.06(0.07) | 0.65 (.42) | 1.70 (1.31) | 1.67 (.20) | −0.01 (0.003) | 13.40 (<.001) |
| Lateral ventricles | 0.27 (0.06) | 24.27 (<.001) | −0.01 (0.01) | 0.68 (.41) | 0.00 (0.06) | 0.01 (.94) | 2.44 (1.03) | 5.60 (.02) | 0.003 (0.002) | 3.79 (.05) |
| Sulcal CSF | 2.01 (0.23) | 78.78 (<.001) | −0.02 (0.04) | 0.27 (.61) | −0.16 (0.21) | 0.56 (.15) | 0.14 (3.77) | 0.00 (.97) | 0.02 (0.01) | 6.35 (.01) |
| Caudate | −0.01 (0.00) | 8.92 (.003) | 0.00 (0.00) | 0.47 (.49) | 0.00 (0.00) | 0.69 (.41) | 0.00 (0.07) | 0.17 (.68) | −0.0003 (0.0001) | 4.27 (.04) |
| Putamen | −0.03(0.01) | 7.73(<.01) | 0.01 (0.00) | 21.32 (<.001) | −0.01 (0.01) | 0.70 (.40) | 0.06 (0.14) | 0.20 (.66) | 0.0006 (0.0004) | 5.63 (.02) |
| Thalamus | −0.05(0.01) | 30.86 (<.001) | 0.00 (0.00) | 0.44 (.51) | 0.00 (0.01) | 0.14 (.71) | 0.13 (0.12) | 1.08 (.30) | −0.0006 (0.0003) | 3.67 (.06) |
| Cerebellum | 0.00(0.04) | 0.00 (35) | 0.01 (0.01) | 0.31 (.58) | −0.10 (0.08) | 1.54 (.22) | −3.25 (1.39) | 5.48 (.02) | −0.0053 (0.0017) | 9.28 (.002) |
Abbreviations: APS, antipsychotic; CSF, cerebrospinal fluid; GM, gray matter; MRI, magnetic resonance imaging; WM, white matter.
Covariates: intracranial volume at intake scan, sex, imaging protocol, and age at intake scan; random effects: follow-up duration and an intercept term to model within-patient correlations in brain volumes across time (unstructured covariance structure).
Interscan interval since initial MRI brain scan (days).
Lifetime APS treatment up to the time of MRI scan acquisition (mean daily APS treatment; chlorpromazine milligram equivalents per day).
Mean Global Assessment Scale score during follow-up period.
Mean severity of alcohol and illicit substance misuse during follow-up period (6-point rating scale: 0, none; 1, occasional use; 2, occasional heavy use; 3, mild impairment; 4, moderate impairment; and 5, severe impairment).
Antipsychotic treatment × follow-up duration interaction term.
Estimate of regression coefficient or slope.
Because they may be confounders, we adjusted our analysis of treatment effects by statistically correcting for the effects of follow-up duration, illness severity, and substance misuse. Antipsychotic treatment still had significant main effects on total cerebral and lobar GM and putamen volumes (Table 2; F ≥ 4.33, P ≤.04). Higher antipsychotic dose was associated with smaller GM volumes (b ≤ −0.03) and larger putamen (b=0.01). Antipsychotic treatment effects on GM volumes were independent of follow-up duration (Table 2; antipsychotic treatment × follow-up duration interaction term F ≤ 1.17, P≥ .28). On the other hand, there were statistically significant treatment × time interaction effects on total cerebral tissue volumes, total cerebral and lobar WM, lateral ventricles, and sulcal CSF, caudate, putamen, and cerebellar volumes (Table 2; F ≥ 3.79, P ≤ .05). Higher doses of antipsychotic treatment were associated with greater reductions in WM, caudate, and cerebellar volumes over time (b ≤ −0.0003), and with greater CSF volume and putamen enlargements (b ≥ 0.0008).
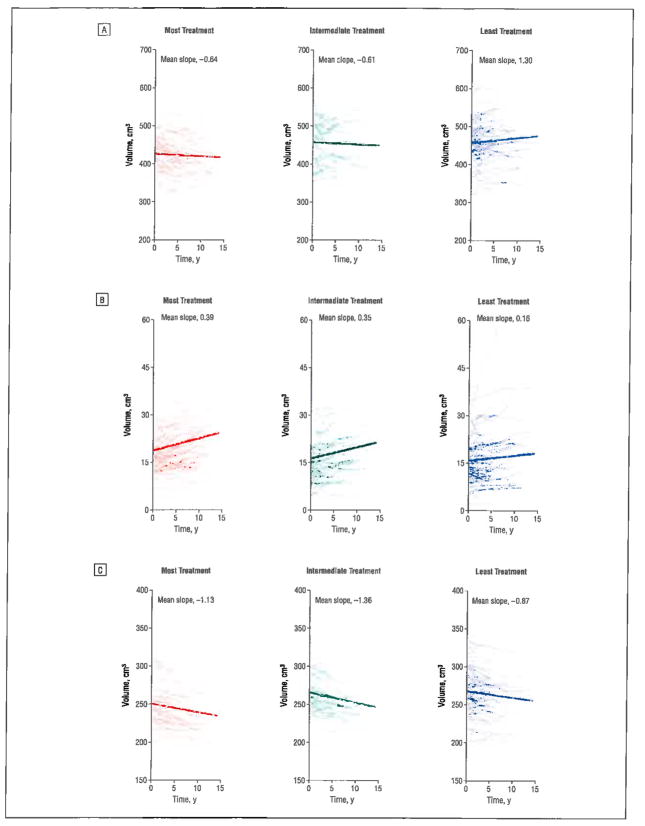
To further illustrate how brain volume trajectories may differ according to the amount of antipsychotic treatment, patients were grouped into tertiles of mean daily antipsychotic dose: most treatment (70 patients; mean dose, 929.4 CPZ mg equivalents/d), intermediate treatment (70 patients; mean dose, 391.7 CPZ mg equivalents/d), and least treatment (71 patients; mean dose, 111.5 CPZ mg equivalents/d). The Figure shows individual subject brain volume trajectories and treatment tertile group mean brain volume trajectories for total cerebral WM, lateral ventricles, and frontal GM volumes. We then conducted an extreme group comparison to contrast brain volume changes between the most and least treatment groups. Random regression coefficient mixed model analyses were duplicated replacing antipsychotic dose with tertile group membership (most vs least treatment) and included a tertile group × follow-up duration interaction term.
Figure.
Comparison of magnetic resonance imaging brain volume trajectories between tertiles of antipsychotic treatment. Tertiles were categorized as those who received the most treatment (70 patients; mean [SD] dose, 929.4 [47.7] chlorpromazine [CPZ] mg equivalents/d), intermediate treatment (70 patients; mean [SD] dose, 391.7 [77.2] CPZ mg equivalents/d), and the least treatment (71 patients; mean [SD] dose, 111.5 [87.7] CPZ mg equivalents/d). Individual patient brain volume trajectories (thin lines) and treatment tertile group mean brain volume trajectories (thick lines) are shown for total cerebral white matter (A), lateral ventricles (B), and frontal gray matter volumes (C).
For total cerebral WM and lateral ventricles, there were statistically significant main effects of tertile group × time interaction (Figure, A and B; t ≥ 2.28, P ≤ .02), indicating that brain volume trajectories differed significantly across tertile groups of antipsychotic treatment. The least-treatment group showed increased total cerebral WM over time in contrast to WM volume reductions among patients in the most-treatment group (mean regression slopes, 1.30 vs −0.64, respectively). Similarly, patients in the most-treatment group had greater enlargement of lateral ventricles than those in the least-treatment group (Figure, B; mean regression slopes, 0.39 and 0.16, respectively). Consistent with the previous random coefficient regression mixed-model analyses where antipsychotic treatment was entered as a continuous measure, extreme tertile group contrast found significant main effects of tertile grouping on frontal GM volumes (t = 2.19, P = .03). Patients who received the most antipsychotic treatment had smaller frontal GM volumes, and this difference was independent of follow-up duration (group × time interaction, t = 1.32, P = .18).
Patients who received the most antipsychotic treatment had smaller baseline total cerebral tissue and larger lateral ventricles than the other 2 tertile subgroups (F ≥ 5.30, P ≤ .006). There were no statistically significant differences between the treatment tertile groups regarding the other baseline brain volumes (F ≤ 2.95, P ≥ .06).
INDEPENDENT EFFECTS OF ILLNESS SEVERITY AND SUBSTANCE ABUSE ON BRAIN VOLUMES
After controlling for the other 3 predictors, mean GAS score had significant main effects on total cerebral GM and frontal GM volumes (Table 2) (F ≥ 4.38, P ≤ .04). Less illness severity was associated with increased brain tissue volumes (b ≥ 0.27). There were no statistically significant main effects of mean GAS score on the other brain volumes (F ≤ 3.65, P ≥ .06).
The majority of the sample (68.3%) did not meet criteria for alcohol abuse/dependence or illicit drug abuse/dependence. Seven patients had alcohol abuse/dependence only, 13 marijuana abuse/dependence only, 8 alcohol and marijuana abuse/dependence only, 19 alcohol abuse/dependence and nonmarijuana illicit drug abuse/dependence, and 30 nonmarijuana illicit drug abuse/dependence only. Severity of alcohol/illicit substance misuse had no significant main effects on brain volumes (Table 2) (F ≤ 1.69, P ≥ .20) except on lateral ventricles (F = 5.60, P = .02; b = 2.44) and on cerebellar volumes (F = 5.48, P = .02; b = −3.25).
INDEPENDENT EFFECTS OF ANTIPSYCHOTIC CLASS ON BRAIN VOLUMES
To explore whether typical antipsychotics, nonclozapine atypical antipsychotics, and clozapine may have differential effects on brain volumes in schizophrenia, we repeated the mixed-models analyses in Table 2 by replacing mean daily (total) antipsychotic dose with lifetime mean daily doses of typical antipsychotics, nonclozapine atypical antipsychotics, and clozapine up until the time of each MRI scan (covariates: initial intracranial volume, sex, imaging protocol, and age at initial scan; other fixed effects: follow-up duration, mean GAS score, and substance misuse severity; and random effects: follow-up duration and intercept term).
There were significant main effects of typical antipsychotic dose, nonclozapine atypical antipsychotic dose, and clozapine dose on GM brain volumes (Table 3). Higher typical antipsychotic doses were associated with smaller total cerebral GM and frontal GM volumes (F ≥ 4.82, P ≤ .03). Higher doses of nonclozapine atypical antipsychotics were associated with lower frontal and parietal GM volumes (F ≥ 6.74, P = .01), and higher clozapine doses were associated with smaller total cerebral and lobar GM volumes (F ≥ 10.90, P ≤.001). For WM volumes, higher nonclozapine atypical antipsychotic doses were significantly associated with larger parietal WM volumes (F = 4.34, P = .04). There were no statistically significant main effects of typical antipsychotic class or nonclozapine atypical antipsychotic class on the remaining WM brain volume measures or on lateral ventricles. Higher clozapine doses were associated with larger sulcal CSF volumes and smaller caudate, putamen, and thalamic volumes (F ≥ 4.70, P ≤.03). Enlarged putamen was associated with higher doses of both typical and nonclozapine atypical antipsychotics. Treatment with higher doses of nonclozapine atypical antipsychotics was also associated with caudate volume enlargement.
Table 3.
Random Regression Coefficient Mixed Models: Fixed Effects of Typical APSs, Nunclozapine Atypical APSs, and Clozapine on MRI Brain Volumes in 211 Schizophrenia Patientsa
| Regions of Interest | Typical APSsb
|
NonclozapiriB Atypicalsc
|
Clozapined
|
|||
|---|---|---|---|---|---|---|
| bg(SE) | F(P) | b(SE) | F(P) | b(SE) | F(P) | |
| Total cerebral tissue | −0.09 (0.07) | 1.51 (.22) | −0.31 (0.28) | 1.26 (.26) | −1.31 (0.35) | 14.83 (<.001) |
| Total cerebral GM | −0.15 (0.05) | 7.89 (.01) | −0.26 (0.20) | 1.61 (.21) | −1.13 (0.25) | 19.87 (<.001) |
| Frontal GM | −0.06 (0.03) | 4.82 (.03) | −0.26 (0.10) | 7.13 (.01) | −0.57 (0.12) | 21.82 (<.001) |
| Temporal GM | −0.02 (0.01) | 2.74 (.10) | −0.07 (0.05) | 1.87 (.17) | −0.29 (0.06) | 20.98 (<.001) |
| Parietal GM | −0.03 (0.01) | 3.11 (.08) | −0.14 (0.06) | 6.74 (.01) | −0.24 (0.07) | 10.90 (.001) |
| Total cerebral WM | 0.07 (0.07) | 1.08 (.30) | −0.07 (0.25) | 0.07 (.79) | −0.20 (0.32) | 0.39 (.53) |
| Frontal WM | 0.02 (0.03) | 0.46 (.50) | −0.07 (0.10) | 0.40 (.53) | −0.14 (0.13) | 1.14 (.29) |
| Temporal WM | 0.01 (0.01) | 1.17 (.28) | 0.06 (0.04) | 1.80 (.18) | 0.03 (0.05) | 0.28 (.60) |
| Parietal WM | 0.02 (0.02) | 2.03 (.16) | 0.13 (0.06) | 4.34 (.04) | 0.01 (0.08) | 0.01 (.32) |
| Lateral ventricles | −0.01 (0.01) | 0.89 (.35) | 0.03 (0.03) | 1.01 (.32) | 0.06 (0.04) | 1.90 (.17) |
| Sulcal CSF | −0.01 (0.04) | 0.12 (.73) | −0.13 (0.16) | 0.73 (.39) | 0.45 (0.20) | 5.07 (.03) |
| Caudate | 0.000 (0.000) | 0.07 (.79) | 0.01 (0.003) | 17.08 (<.001) | −0.01 (0.003) | 12.00 (<.001) |
| Putamen | 0.01 (0.002) | 9.46 (.002) | 0.06 (0.01) | 71.95 (<.001) | −0.03 (0.01) | 8.00 (.01) |
| Thalamus | 0.001 (0.002) | 0.23 (.63) | −0.002 (0.01) | 0.07 (.80) | −0.02(0.01) | 4.70 (.03) |
| Cerebellum | 0.002 (0.01) | 0.03 (.86) | 0.06 (0.04) | 1.75 (.19) | −0.01(0.05) | 0.06 (.81) |
Abbreviations: APSs, antipsychotics; CSF, cerebrospinal fluid; GM, gray matter; MRI, magnetic resonance imaging; WM, white matter.
Covariates: intracranial volume at intake scan, sex, imaging protocol, age at intake scan, follow-up duration, illness severity (mean Global Assessment Scale score), and substance misuse; random effects: follow-up duration and an intercept term to model within-patient correlations in brain volumes across time (unstructured covariance structure).
Lifetime typical antipsychotic treatment up to the time of MRI scan (mean daily APS treatment; chlorpromazine milligram equivalents per day).
Lifetime nonclozapine atypical APS treatment up to the time of MRI scan (mean daily APS treatment; chlorpromazine milligram equivalents per day).
Lifetime clozapine treatment up to the time of MRI scan (mean daily APS treatment; chlorpromazine milligram equivalents per day).
Estimate of regression coefficient or slope.
COMMENT
In this large longitudinal cohort of patients with schizophrenia (211 patients with 674 MRI scans) who were in their first episode and had received minimal treatment at the time of entry into the study, we examined the independent effects of 4 variables on progressive brain change during an extended period: illness duration, antipsychotic treatment, illness severity, and substance misuse. We found that longer follow-up was associated with a greater decrease in brain tissue volumes. Antipsychotic treatment also had a significant influence on brain volumes even after accounting for the potential confounds of the other 3 variables. More antipsychotic treatment was associated both with generalized tissue volume reduction involving multiple subregions and with a specific increase in putamen. The other 2 variables, severity of illness and substance abuse, had minimal or no effects. Progressive brain volume changes during the lifelong course of schizophrenia, including GM and WM volume reductions, CSF volume expansions, and basal ganglia volume enlargements, appeared in part to be related to antipsychotics. These findings may potentially have clinical implications for the use of long-term antipsychotic treatment.
The plausibility of long-term antipsychotic treatment leading to global brain volume reductions is further supported by recent controlled studies in macaque monkeys.19–21 Animal studies provide an additional perspective on possible causative links because they permit postmortem neuropathological examination of the brain. Dorph-Petersen et al19 administered haloperidol, olanzapine, or sham medication to macaque monkeys in doses that produced plasma levels equivalent to those observed in treatment of schizophrenia patients. After 17 to 27 months of treatment, both haloperidol- and olanzapine-treated monkeys had an equivalent and highly significant 8% to 11% decrease in fresh brain weight and volume when compared with the sham group. These decreases affected all major brain regions but were most robust in frontal and parietal lobes. The neuropathological manifestations of antipsychotic-related frontoparietal volume reductions in macaque monkeys involves decreased astrocyte numbers, decreased dendritic arborization, decreased dendritic spine density, and increased neuronal density with no neuronal loss.20,21 Although there have been some conflicting findings in the nonhuman primate literature (eg, studies by Lidow et al41 and Sweet et al42), these neuropathological changes are strikingly similar to those described in the schizophrenia postmortem literature (eg, studies by Pakkenberg43 and Selemon et al44).
These findings are also consistent with previous MRI studies suggesting that antipsychotics produce changes in the human brain that are measurable by in vivo neuroimaging techniques. The earliest work with morphometric MRI found increased basal ganglia size in schizophrenia patients, typically in the putamen.45,46 Subsequent studies have shown that this may be a medication effect and that typical antipsychotics in particular play a causal role in basal ganglia enlargement.47–51 Positron emission tomography studies measure cerebral blood flow and, by inference, cellular metabolism. Previous positron emission tomography studies conducted by our group52–54 confirm that both typical and atypical antipsychotics increase putamen cerebral blood flow. In addition, antipsychotics reduce frontal cerebral blood flow, suggesting that chronic frontal hypoperfusion could be a mechanism underlying smaller brain tissue volumes. However, the available studies that have used morphometric MRI to examine the effects of antipsychotics on cortical GM have yielded ambiguous results,10,55–57 possibly due to small sample sizes, differing duration of treatment assessment, variation in brain regions measured, and discrepant measurement techniques.
In the present study, WM but not GM volumes showed significant time × antipsychotic treatment interaction effects. Although higher antipsychotic doses were associated with a decrease in GM volumes, this relationship did not appear to change during the course of longitudinal follow-up in our study. A change in WM volume trajectories, on the other hand, was associated with antipsychotic treatment. As illustrated by the extreme tertile treatment group comparisons, patients in the most-treatment group had longitudinal WM volume reductions. As a group, patients with the least treatment showed WM volume increases over time that would be expected of individuals during their third and fourth decades of life.58 Bartzokis and colleagues59 previously found that patients with schizophrenia do not show the normal age-related WM volume expansion during early to mid-adulthood. Thus, the treatment × time interaction effects in the present study suggest that WM volume deficits in schizophrenia may, in part, be related to antipsychotic treatment. Our study found that all 3 classes of antipsychotics (ie, typical, nonclozapine atypical, and clozapine) were associated with deceases in GM brain volumes. The only differential effects on brain volumes between typical and nonclozapine atypical antipsychotics were in parietal WM and caudate measures. These typical-atypical differential effects on brain volumes in our study differ somewhat from a recent randomized treatment study.10 Lieberman and colleagues found that haloperidol treatment was associated with progressive GM volume reductions during that 2-year study. In contrast, olanzapine-treated patients did not show GM volume decrement. This raises concerns regarding the possibility of typical antipsychotic-associated neurotoxic effects. Our study suggests that atypical antipsychotic treatment may mitigate parietal WM volume loss in patients with schizophrenia; this finding needs to be interpreted with caution and will require support from additional well-designed clinical trials in first-episode patients.
Our results must be interpreted in the context of additional limitations. Identifying an association does not necessarily indicate a causal relationship. Furthermore, observational studies involving long durations such as ours inevitably preclude use of the “gold standard”: a random-assignment controlled trial. The current study could have been strengthened by having control groups, eg, schizophrenia patients assigned to deferred or no antipsychotic treatment or healthy volunteers treated with antipsychotics for comparable periods. However, ethical standards in human subject research prohibit such comparison groups. The small number of schizophrenia patients in our sample who received no antipsychotic treatment did not allow for meaningful statistical analyses. Illness severity and antipsychotic dosages were modestly correlated (Spearman r=−0.21), and patients who received the most treatment had smaller baseline cerebral tissue volumes. Associations between smaller brain tissue volumes and more antipsychotic treatment may still be moderated via illness severity despite our inclusion of illness severity as a covariate and obtaining similar results from different measures of illness severity. Even with the most sophisticated statistical methods, we may not be able to fully distinguish the potential confounding influences that illness severity or other sources of unmeasured variance could still have on the relationships between progressive brain volume reductions and antipsychotic treatment. Last, although our Talairach atlas–based lobar measures have well-established reliability and validity, these cerebral brain volumes lack precision to delineate abnormalities within smaller subregions implicated in schizophrenia (eg, superior temporal gyrus and prefrontal cortex).
Findings from the present study raise several clinical questions. Are antipsychotic-associated GM and WM volume reductions “bad” for patients? The implicit assumption is that brain volume reductions are probably undesirable because patients with schizophrenia already have diffuse brain volume deficits at the time of illness onset. Schizophrenia patients with poor outcomes are also more likely to have smaller brain volumes. However, the neurobiological changes that underlie MRI measurements of antipsychotic-associated brain volume decrement remain poorly understood. Some studies indicate that antipsychotic-induced changes mimic the neuropathologycal changes of schizophrenia,20,21 while others suggest otherwise.41,42 If antipsychotics do indeed result in deleterious brain tissue volume reductions, how does this influence the risk-benefit ratio of antipsychotic treatment? Given that these medications have substantially improved the long-term prognosis of schizophrenia and that schizophrenia is a disease with significant morbidity, continued use of antipsychotics is clearly still necessary. However, our findings point toward the importance of prescribing the lowest doses necessary to control symptoms. They also imply the need for rethinking the underlying pathological processes in schizophrenia,47,48 the target at which treatment is aimed, and the possibility that antipsychotic treatment may improve psychotic symptoms but also contribute to progressive brain tissue volume deficits. Antipsychotics were designed for the purpose indicated by their name, ie, to arrest psychosis. Not only is it probable that antipsychotics do not treat the fundamental pathophysiologic mechanism of schizophrenia (ie, the brain disease), but we perhaps must also entertain the possibility that they might have potentially undesirable effects of brain tissue volume reductions. In conjunction with neuroscientists and clinical investigators, pharmaceutical companies must continue the vigorous search for agents that are genuinely neuroprotective.
The second-generation antipsychotics are also now widely used for people who do not have schizophrenia, including children, the elderly, and patients with bipolar disorder or depressive disorders.27–30 They are also used in adolescents who have been identified to be at high risk for schizophrenia. Our findings may lead to heightened concerns regarding potential brain volume changes associated with the sharp rise in atypical antipsychotic use in non-schizophrenia psychiatric disorders. Even though no studies have assessed the long-term effects of antipsychotics on brain volumes in nonschizophrenia patients, our results suggest that antipsychotics should still be used with caution in these patient groups after careful risk-benefit assessment. Because typical antipsychotics are off patent and less expensive than atypical ones, there is also a growing trend to prescribe them preferentially for patients with schizophrenia. Given that these older medications carry a greater risk of producing extrapyramidal adverse effects and tardive dyskinesia, such a shift in clinical practice may produce deleterious effects on the primary diseased organ in schizophrenia: the brain.
CONCLUSIONS
Antipsychotics are effective medications for reducing some of the target clinical symptoms of schizophrenia: psychotic symptoms. In medicine we are aware of many instances in which improving target symptoms worsens other symptoms. Hormone therapy relieves menopausal symptoms but increases stroke risk. Nonsteroidal anti-inflammatory drugs relieve pain but increase the likelihood of duodenal ulcers and gastrointestinal tract bleeding. It is possible that, although antipsychotics relieve psychosis and its attendant suffering, these drugs may not arrest the pathophysiologic processes underlying schizophrenia and may even aggravate progressive brain tissue volume reductions.
Acknowledgments
Funding/Support: This research was supported in part by grants MH68380, MH31593, MH40856, and MH43271 from the National Institute of Mental Health.
Role of the Sponsor: The National Institute of Mental Health financially supported the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation of the manuscript, but was not involved in manuscript review or approval.
Footnotes
Financial Disclosure: Dr Ho receives grant support from Ortho-McNeil Janssen Scientific Affairs. Dr Andreasen has served on the Ortho-McNeil Janssen Advisory Board and receives grant support from Ortho-McNeil Janssen Scientific Affairs.
Author Contributions: Drs Ho and Andreasen had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Online-Only Material: The supplementary Methods and References and the eFigure and eTables are available at http://www.archgenpsychiatry.com.
Additional Contributions: Dawei Liu, PhD, provided valuable advice and assistance in statistical analysis.
References
- 1.Murray CJL, Lopez AD, editors. The Global Burden of Disease; A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- 2.Gilbert PL, Harris MJ, McAdams LA, Jeste DV. Neuroleptic withdrawal in schizophrenic patients: a review of the literature. Arch Gen Psychiatry. 1995;52(3):173–188. doi: 10.1001/archpsyc.1995.03950150005001. [DOI] [PubMed] [Google Scholar]
- 3.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74(3):129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 4.Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC. A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55(2):145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman J, Chakos M, Wu H, Atvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49(6):487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 6.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(2):148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 7.Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 8.Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 9.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jalesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M HGDH Study Group. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 11.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CD, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160(1):156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32(10):2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 13.Cahn W, Rais M, Stigter FP, van Haren NE, Caspers E, Hulshoff Pol HE, Xu Z, Schnack HG, Kahn RS. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19(2):147–151. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Mathalon DH, Rapoport JL, Davis KL, Krystal JH. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry [letter] Arch Gen Psychiatry. 2003;60(8):846–848. doi: 10.1001/archpsyc.60.8.846. [DOI] [PubMed] [Google Scholar]
- 15.Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry [reply] Arch Gen Psychiatry. 2003;60(8):848–849. doi: 10.1001/archpsyc.60.8.846. [DOI] [PubMed] [Google Scholar]
- 16.Hulshoff Pol HE, Kahn RS. What happens after the first episode? a review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34(2):354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantelis C, Yücel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31 (3):672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 18.Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007;164(12):1890–1899. doi: 10.1176/appi.ajp.2007.05111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 20.Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32 (6):1216–1223. doi: 10.1038/sj.npp.1301233. [DOI] [PubMed] [Google Scholar]
- 21.Konopaske GT, Dorph-Petersen K-A, Sweet RA, Pierri JN, Zhang W, Sampson AR, Lewis DA. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63(8):759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? a systematic and critical review of MRI findings. Psychol Med. 2009;39(11):1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- 23.Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rössler A, Borgwardt SJ. The effects of antipsychotics on the brain; what have we learnt from structural imaging of schizophrenia? a systematic review. Curr Pharm Des. 2009;15(22):2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- 24.Borgwardt SJ, Smieskova R, Fusar-Poli P, Bendfeldt K, Riecher-Rössler A. The effects of antipsychotics on brain structure: what have we learnt from structural imaging of schizophrenia? Psychol Med. 2009;39(11):1781–1782. doi: 10.1017/S0033291709006060. [DOI] [PubMed] [Google Scholar]
- 25.Lewis DA. Brain volume changes in schizophrenia: how do they arise? what do they mean? Psychol Med. 2009;39(11):1779–1780. doi: 10.1017/S003329170900573X. [DOI] [PubMed] [Google Scholar]
- 26.Vita A, De Peri L. The effects of antipsychotic treatment on cerebral structure and function In schizophrenia. Int Rev Psychiatry. 2007;19(4):429–436. doi: 10.1080/09540260701486332. [DOI] [PubMed] [Google Scholar]
- 27.Domino ME, Swartz MS. Who are the new users of antipsychotic medications? Psychiatr Serv. 2008;59(5):507–514. doi: 10.1176/appi.ps.59.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63(6):679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 29.Castle NG, Hanlon JT, Handler SM. Results of a longitudinal analysis of national data to examine relationships between organizational and market characteristics and changes in antipsychotic prescribing in US nursing homes from 1996 through 2006. Am J Geriatr Pharmacother. 2009;7(3):143–150. doi: 10.1016/j.amjopharm.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Governale L, Mehta H. Outpatient use of atypical antipsychotic agents in the pediatric population: years 2004–2008. US Food and Drug Administration; [Accessed January 28, 2010]. Web site, http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/PediatricAdvisoryCommittee/UCM193204.pdf. Published December 8, 2009. [Google Scholar]
- 31.Flaum MA, Andreasen NC, Arndt S. The Iowa prospective longitudinal study of recent-onset psychoses. Schizophr Bull. 1992;18(3):481–490. doi: 10.1093/schbul/18.3.481. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC. The Scale for Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 33.Andreasen NC. The Scale for Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- 34.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psyctiopathology. Arch Gen Psychiatry. 1982;49(8):615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen NC. PSYCH-BASE. Iowa City: University of Iowa; 1989. [Google Scholar]
- 36.Ho BC, Flaum M, Hubbard W, Arndt S, Andreasen NC. Validity of symptom assessment in psychotic disorders: information variance across different sources of history. Schizophr Hes. 2004;68(2–3):299–307. doi: 10.1016/j.schres.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive neural change in schizophrenia: a prospective longitudinal study of first episode schizophrenia. Biol Psychiatry. doi: 10.1016/j.biopsych.2011.05.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis JM. Dose equivalence of the antipsychotic drugs. J Psychiatr Res. 1974;11:65–69. doi: 10.1016/0022-3956(74)90071-5. [DOI] [PubMed] [Google Scholar]
- 39.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 41.Lidow MS, Song Z-M, Castner SA, Allen PB, Greengard P, Goldman-Rakic PS. Antipsychotic treatment induces alterations in dendrite- and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biol Psychiatry. 2001;49(1):1–12. doi: 10.1016/s0006-3223(00)01058-1. [DOI] [PubMed] [Google Scholar]
- 42.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34(2):374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pakkenberg B. Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical disectors. Biol Psychiatry. 1993;34(11):768–772. doi: 10.1016/0006-3223(93)90065-l. [DOI] [PubMed] [Google Scholar]
- 44.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52(10):805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 45.Jemigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48(10):881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- 46.Swayze VW, II, Andreasen NC, Alllger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures In affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31(3):221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- 47.Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345(8947):456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- 48.Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156(8):1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- 49.Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL. Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry. 1996;153(4):564–566. doi: 10.1176/ajp.153.4.564. [DOI] [PubMed] [Google Scholar]
- 50.Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroieptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155(12):1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- 51.Keshavan MS, Rosenberg D, Sweeney JA, Pettegrew JW. Decreased caudate volume in neuroleptic-naive psychotic patients. Am J Psychiatry. 1998;155(6):774–778. doi: 10.1176/ajp.155.6.774. [DOI] [PubMed] [Google Scholar]
- 52.Miller DD, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Comparison of the effects of risperidone and halcperidol on regional cerebral blood flow in schizophrenia. Biol Psychiatry. 2001;49(8):704–715. doi: 10.1016/s0006-3223(00)01001-5. [DOI] [PubMed] [Google Scholar]
- 53.Miller DD, Andreasen NC, O’Leary DS, Rezal K, Watkins GL, Ponto LL, Hichwa RD. Effect of antipsychotics on regional cerebral blood flow measured with positron emission tomography. Neuropsychopharmacology. 1997;17(4):230–240. doi: 10.1016/S0893-133X(97)00042-0. [DOI] [PubMed] [Google Scholar]
- 54.Corson PW, O’Leary DS, Miller DD, Andreasen NC. The effects of neuroleptic medications on basal ganglia blood flow in schizophreniform disorders: a comparison between the neuroleptic-naïve and medicated states. Biol Psychiatry. 2002;52(9):855–862. doi: 10.1016/s0006-3223(02)01421-x. [DOI] [PubMed] [Google Scholar]
- 55.Crespo-Facorro B, Klm J-J, Chemerinski E, Magnotta V, Andreasen NC, Nopoulos P. Morphometry of the superior temporal plane in schizophrenia: relationship to clinical correlates. J Neuropsychiatry Clin Neurosci. 2004;16(3):284–294. doi: 10.1176/jnp.16.3.284. [DOI] [PubMed] [Google Scholar]
- 56.McCormick L, Decker L, Nopoulos P, Ho BC, Andreasen N. Effects of atypical and typical neuroleptics on anterior cingulate volume in schizophrenia. Schizophr Res. 2005;80(1):73–84. doi: 10.1016/j.schres.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 57.Pressler M, Nopoulos P, Ho BC, Andreasen NC. Insular cortex abnormalities in schizophrenia: relationship to symptoms and typical neuroleptic exposure. Biol Psychiatry. 2005;57(4):394–398. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 58.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6 (3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 59.Bartzokis G, Nuechterlein KH, Lu PH, Gltlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53(6):412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]