Abstract
In this review we propose a broader view of the role of the fibroblast growth factor (FGF) family in modulating brain function. We suggest that some of the FGF ligands together with the FGF receptors are altered in individuals with affective disorder and modulate emotionality in animal models. Thus, we propose that members of the FGF family may be genetic predisposing factors for anxiety, depression or substance abuse; that they play a key organizing role during early development but continue to play a central role in neuroplasticity in adulthood; and that they work not only over extended time frames, but also via rapid signaling mechanisms, allowing them to exert an “on-line” influence on behavior. Therefore, the FGF family appears to be a prototype of “switch genes” that are endowed with organizational and modulatory properties across the lifespan, and that may represent molecular candidates as biomarkers and treatment targets for affective and addictive disorders.
Keywords: hippocampus, drug abuse, stress, anxiety, depression
The Role of Growth Factors in Emotionality
Our understanding of the role of growth factors has evolved significantly over the last quarter century, with increasing appreciation of their pivotal roles in brain function and dysfunction across the life span. Early views emphasized the central role of molecules such as Nerve Growth Factor (NGF) in development, survival and differentiation particularly in embryonic sensory and sympathetic neurons (Levi-Montalcini, 1987). Even following the discovery of several neurotrophins, including Brain-Derived Neurotrophic Factor (BDNF) and the emerging recognition of their coordinate actions as trophic factors in the central nervous system (CNS), much of the emphasis remained on understanding their role in development. For example, a 1993 review concludes that: “In the adult, the roles of the same trophic factors are likely to be more restricted, either activated only in specific neuronal populations or, alternatively, only during very specific physiological states of the nervous tissue” (Knüsel and Hefti, 1993). Nevertheless, that era saw an increasing interest in the ability of neurotrophins to promote cell survival and repair following injury or neurodegeneration, and they were proposed as potential therapeutic targets for neurodegenerative disorders (Snider and Johnson, 1989; Thoenen, 1991).
By the mid 1990′s, additional roles of growth factors in neural function were emerging. For example, NGF was implicated in pain regulation and neuroimmune function (Levi-Montalcini et al., 1995), while neurotrophins were shown to play a role in synapse formation and neuroplasticity (Lu and Figurov, 1997). With the realization that severe and chronic stress can produce significant damage to certain areas of the CNS, such as the hippocampus (Fuchs and Flugge, 1998; Magarinos et al., 1997; McEwen and Magarinos, 1997), the potential role of growth factors in counteracting the effects of stress came into focus. In 1997, it was shown that chronic stress decreases BDNF in conjunction with atrophy of hippocampal neurons (Duman et al., 1997). Given that chronic stress has served as an animal model of clinical depression, the authors suggested that the mode of action of chronic antidepressant therapy might involve activation of neurotrophic factors (Duman et al., 1997; Duman, 1998). This framework represented the first explicit implication of growth factors in a hypothesis related to a psychiatric disorder.
As is the case for other growth factors, our views of the functions of the FGF family in the brain originally revolved primarily around neural development (Gomez-Pinilla et al., 1994; Riedel et al., 1995; Temple and Qian, 1995; Vaccarino et al., 1999). Subsequent observations implicated the FGF family in neurogenesis both during early development and in adulthood (Bartlett et al., 1994; Cheng et al., 2001; Guillemot and Zimmer, 2011; Tao et al., 1996; Zheng et al., 2004). This paved the way to a greater interest in this family's role in neuroplasticity.
In this review, we suggest that the FGF family plays a lifelong neuromodulatory role in the way an organism responds to and copes with the environment. We propose that the fine-tuning of this family of molecules alters the organism's propensity to explore a novel environment and modifies anxiety-like and depression-like behavior. Moreover, the FGF system is involved in fear conditioning and the response to stress and plays a role in the vulnerability to drug-taking behavior.
Why Link the FGF System to Mood and Affect?
Our view on the affective role of the FGF family emerged from studies of postmortem brains of subjects who had died while suffering from severe clinical depression. Major Depressive Disorder (MDD) is the most debilitating mood disorder in the United States, accounting for the single greatest psychiatric cause of disability. Anxiety disorders run a close second, and these two affective diseases are often co-morbid. Thus, relative to the general population, an individual who has one of these disorders has a 25-fold greater chance of expressing the other (Kessler et al., 1994), suggesting highly overlapping if not common etiology. A better understanding of the pathophysiology of these diseases is acutely needed given the high rate of incidence of these diseases (e.g. 25% lifetime incidence of MDD), and only a 33% response rate to first of the line treatments (Robins and Regier, 1991).
In 2004, work in the context of the Pritzker Neuropsychiatric Disorders Research Consortium examined alterations in genome-wide expression profiles in the brains of patients suffering from MDD relative to normal controls (Evans et al., 2004). This “discovery” approach first focused on areas in the frontal cortex. Data mining revealed that members of the FGF family were highly significantly altered in major depression. Moreover, this effect was not dependent on treatment with the selective-serotonin reuptake inhibitors (SSRIs). Indeed, a history of SSRI treatment blunted the dysregulation in FGF gene expression. In that original paper, FGF1, FGF2, FGFR2 and FGFR3 were downregulated in MDD in the anterior cingulate cortex and/or the dorsolateral prefrontal cortex. Conversely, FGF9 and FGF12 were upregulated in these same brain regions. As will be described below, these findings have since been extended to other brain regions using multiple analysis platforms, and have led to a series of studies in animal models that have transformed our understanding of the role of the FGF family in brain function and dysfunction.
In this review, we will focus primarily on the more recent evidence relating to the FGF system, emotionality and mood disorders. We will attempt to answer three main questions regarding FGF signaling and behavior: 1) What is known about the FGF system in mood disorders? 2) What are the effects of the FGF system on other affective behaviors including anxiety, fear, stress responsivity and substance abuse? And, 3) how might the FGF system exert these effects? To this end, we will describe the important ligands and receptors for the FGF family. We will review the various functions of the FGF system with a focus on FGF2, the prototypical ligand. We will end with a discussion of other molecular partners of this system, that suggest pharmacological and clinical strategies with molecules that are not “the usual suspects”
Molecular Components of the FGF Family
Overview of the FGF Family
For a review of the literature on the structure and function of the FGF system prior to 2006, the reader is referred to a previous review (Turner et al., 2006). To summarize, the FGF system is comprised of 18 ligands, of which 10 are expressed in brain. There were four previous members, now termed FGF homologous factors (FHF1-4), that have been removed from the original list of 22 ligands (Goldfarb et al., 2007). These molecules lack functional similarity, although they share structural similarity, and remain intracellular.
There are four membrane-bound receptors and a fifth truncated (soluble) receptor with differing affinities for the various ligands (Reuss and von Bohlen und Halbach, 2003). Many of the ligands lack signal peptides but are secreted nonetheless. The receptors are composed of three extracellular Ig-like domains, a transmembrane domain and two intracellular kinase domains (Reuss and von Bohlen und Halbach, 2003). The acid box region between the first and second Ig-like domain determines the ligand specificity. There are also multiple splice variants of the third Ig-like domain resulting in IIIb or IIIc isoforms. The IIIb isoform is expressed predominantly during early development, while the IIIc isoform is expressed predominantly in adulthood.
The receptors signal primarily through three main pathways, phospholipase Cγ (PLCγ), mitogen-activated protein kinase (MAPK) and AKT to influence gene transcription. This signaling is akin to other growth factors; however, the strength of the signaling may vary between growth factors. This is possible, by analogy, since the strength of the signaling can vary between FGF receptor homodimers. For example, FGF ligands in different subfamilies can induce different FGF receptor 1 (FGFR1) homodimer formations and MAPK signaling (Romero-Fernandez et al., 2011). Moreover, there may be differences in kinase activity depending on which molecule triggered the signal (Ditlevsen et al., 2008). Finally, the receptors can interact with other neurotransmitter receptors, as will be described in more detail below (see Beyond the FGFs: Receptor-Interacting Partners).
FGF Ligands
Each of the FGF ligands has a distinct functional profile. We will focus here on a subset of ligands that are expressed in brain and appear modulated in mood disorders.
FGF2, also known as basic fibroblast growth factor, was the first FGF to be cloned in the rat (Kurokawa et al., 1988). It is the prototypical FGF ligand and has been well-characterized for its roles in cell proliferation, differentiation, growth, survival, as well as angiogenesis in various cell models (Ford-Perriss et al., 2001). This ligand is composed of a beta trefoil motif and has a basic canyon structure allowing heparin sulfate proteoglycans to bind in a 2:2:2 stoichiometry with the receptors (Reuss and von Bohlen und Halbach, 2003). FGF2 exists in multiple molecular weight isoforms of which only the lowest molecular weight (18kD) is secreted. The higher molecular weight isoforms remain in the nucleus and affect nuclear functioning, such as rRNA transcription.
In early brain development, FGF2 is expressed by the neural tube and is involved in neural induction (Ford-Perriss et al., 2001). Later on, FGF2 is expressed in the ventricular region of the developing cortex and the cortical plate. FGF2 is also expressed by neural precursor cells throughout development and promotes the proliferation of neural stem cells (Dono et al., 1998; Vaccarino et al., 1999). Thus, FGF2 knockout mice have alterations in the deep layers of the cortex and the hippocampus compared to wild-type mice (Raballo et al., 2000). These mice also have a lower number of neural progenitor cells in the subventricular zone (SVZ), a decreased ability to proliferate in response to FGF2 in progenitor cells of the subgranular zone (SGZ) and decreased astroglial differentiation (Zheng et al., 2004). Together, the results suggest that FGF2 affects both neuronal and glial output. However, astrocytic expression of FGF2 only becomes apparent starting at postnatal day (PND) 4-6 (Gomez-Pinilla et al., 1994).
In the adult brain, FGF2 is expressed by both neurons and glial cells with astrocytes containing the highest levels of FGF2 (Gonzalez et al., 1995). FGF2 binds with the highest affinity to FGFR1 (Reuss and von Bohlen und Halbach, 2003). Moreover, FGF2 is ubiquitously expressed in the adult brain with the highest expression in the hippocampus and cortical areas (Gomez-Pinilla et al., 1994).
The regulation of FGF2 expression is complex. An antisense transcript regulates its expression (Nudt6), functioning as a repressor (Knee et al., 1997; MacFarlane et al., 2010; MacFarlane and Murphy, 2010). There are also various transcription factors that can bind its promoter elements, such as HoxA10, AP-1 and SP-1 (Shah et al., 2012; Shibata et al., 1991). Moreover, the role of FGF2 in brain development is influenced by the existence of an IRES-dependent mechanism for translation (Audigier et al., 2008). This activity peaks at PND7, remains elevated in neurons during adulthood, and is regulated by itself and by electrical activity. Other mechanisms of regulation of FGF2 expression in the developing brain, be they by epigenetic or microRNA mechanisms, remain to be elucidated. The effects of FGF2 on the adult brain will be discussed below.
FGF1, also known as acidic fibroblast growth factor, was cloned in the rat subsequent to FGF2 (Goodrich et al., 1989). FGF1 is predominantly expressed by neurons and, in stark contrast to FGF2, it is expressed relatively little outside of the nervous system. FGF1 is expressed at low concentrations until E16 when it rises to adult levels (Alam et al., 1996; Elde et al., 1991) Culture experiments demonstrated that FGF1 is involved in the maturation and maintenance of neurons (Ford-Perriss et al., 2001). However, FGF1 knockout mice show no severe deficits (Miller et al., 2000). Finally, not much is known about the effects of FGF1 on the adult brain.
FGF9 is a mitogenic factor expressed predominantly by neurons with high expression in hippocampal and cortical areas. FGF9 also has the highest affinity for the astrocytic receptor, FGFR3, specifically the adult IIIc splice variant (Cinaroglu et al., 2005; Plotnikov et al., 2001). Given the alterations described above in the human post-mortem cortex, the role of FGF9 is of great interest. Unfortunately, not much is known about the in vivo effects of FGF9 in general.
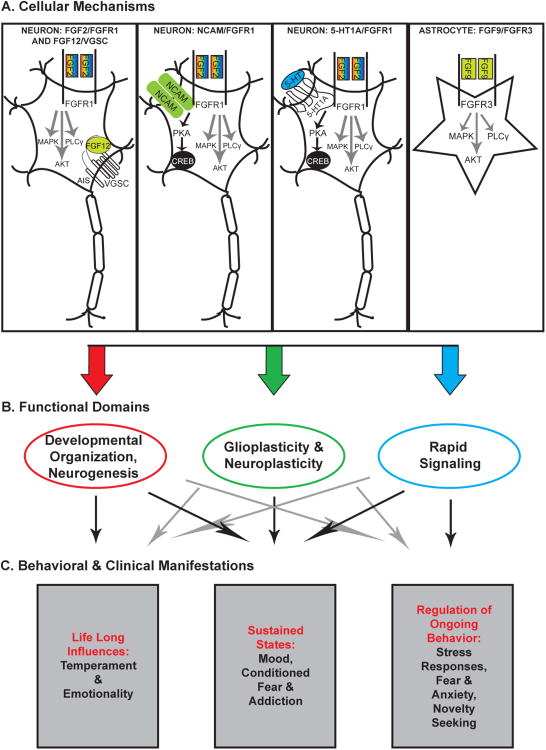
Intracellular fibroblast growth factors (iFGF), also known as FGF homologous factors, may also play a role in emotionality. These molecules share structural, but not functional homology with other FGFs that interact with the extracellular receptors (Olsen et al., 2003). FGF11-14, are also referred to as FHF1-4. By remaining intracellular, FGF12 and FGF14 have the ability to interact directly with and activate voltage-gated sodium channels (VGSC) (Goetz et al., 2009; Goldfarb et al., 2007). This would allow these members of the FGF family to exert rapid effects on numerous intracellular functions. For example, FGF14 is localized to the axon initial segment (Spugnini et al.) and is thereby in a position to strongly influence neuronal excitability (Laezza et al., 2009) (c.f. Fig 2). What effect this has on mood and behavior has yet to be determined.
Figure 2. FGF signaling can have functional effects through different mechanisms in multiple time domains.
Panel A shows that FGF ligands can interact with FGF membrane receptors on both neurons and glial cells. Intracellular FGF ligands can also interact with voltage-gated sodium channels (VGSC), particularly near the initial axon segment. Moreover, FGF receptors can have partners, e.g. they can interact with neural cell adhesion molecules such as NCAM, and can heterodimerize with G Protein Coupled Receptors such as the 5-HT1A receptor. These events take place in neurons and glia, and trigger a host of signaling pathways, with multiple function consequences.
Panel B: depicts the various classes of functional changes in the brain that can be triggered by the actions of FGF signaling.
Panel C: depicts the behavioral and clinical outcomes of the neural mechanisms triggered by FGF signaling and underscores the multiple time domains in which they can occur, from altering lifelong patterns of behavior (through modifying temperamental predisposition and coping styles) to sustained changes during a given period of the organism's life (e.g. an episode of depression) to ongoing regulation of motivated and affective behavior.
Given the complexities of the FGF ligands, it has proven difficult to parse their interactions or precisely define the full range of this family's contribution to brain function and behavior. Beyond the sheer number of ligands, the FGF family exhibits both convergence and divergence. Thus, multiple ligands converge on a smaller number of membrane receptors, and each ligand is capable of activating more than one of these receptors. This is further complicated by the existence of receptor splice variants each with a unique pattern of interactions with the ligands (Zhang et al., 2006). Suffice it to say that each ligand appears to exhibit a unique profile of action, which may be worthy of greater investigation in the context of affective behavior.
FGF Receptors
As previously described, many FGF ligands signal by activating one or more of the four membrane spanning FGF receptors R1-R4 (Turner et al., 2006). As noted above, each of these receptors can be alternatively spliced, resulting in additional variants with distinct profiles of interactions with their various ligands (Zhang et al., 2006). While all of these receptors are present in the brain, FGFR4 is only expressed in the habenula and will not be discussed in this review.
The prototypical receptor, FGFR1, is found mostly on neurons, although its expression has also been demonstrated on neural stem cells (Frinchi et al., 2008). This receptor has been shown to play a predominant role in both the development of the cortex and hippocampus, two key regions in MDD. These two regions are also the output regions of neurogenesis from the subventricular zone and subgranular zone, respectively. This suggests that FGFR1 is likely necessary for the growth and proliferation of neural stem cells. Indeed, FGFR1 dominant negative tyrosine kinase knockout mice exhibit a decrease in the number of pyramidal neurons in layer V of the cortex (Shin et al., 2004). Similarly, conditional knockout of FGFR1 appear to be important in the development and size of the hippocampus (Ohkubo et al., 2004). More recent work has demonstrated the critical role of FGFR1 in hippocampal function, as it modulates: 1) proliferation of neural progenitor cells 2) neurogenesis 3) memory consolidation and 4) long-term potentiation (LTP), a model of learning and memory (Zhao et al., 2007).
On the other hand, FGFR2 exhibits primarily a glial pattern of expression, being expressed predominantly by oligodendrocytes, although its expression has also been demonstrated on neural stem cells. Conditional knockout of FGFR2 in radial glial cells affects the development of the prefrontal cortex, as well as its projection areas (Stevens et al., 2010). Moreover, short-term learning and neurogenesis in the dentate gyrus are dependent on FGFR2 functioning in the adult hippocampus. Conversely, long-term learning and the number of parvalbumin interneurons are dependent on FGFR2 in the embryonic hippocampus (Stevens et al., 2012).
Similarly, FGFR3 is predominantly expressed by astrocytes in the brain. FGFR3 knockout mice exhibit deficits in cortical and hippocampal volumes (Moldrich et al., 2011). These effects appear to be the most extreme on GABAergic neurons of the telencephalon. Moreover, FGFR3 appears to be more important in the formation of the caudal cortex and resultant projections. However, the information on the function of this receptor in the brain is sparse, yet of great interest given its consistent downregulation in human depression (see below).
Interestingly, there are additional endogenous molecules that can bind FGF ligands. One such example is FGF binding protein 3 (FGFBP3), a truncated version of FGFR1 which does not signal but has the ability to bind FGF ligands, likely acting as a local sink towards some ligands (Hanneken et al., 1994). A recent study showed that inactivation of FGFBP3, by a targeted gene deletion increased anxiety behavior in rodents (Yamanaka et al., 2011). However, the relative affinity of FGFBP3 for the different FGF ligands remains under evaluation.
In summary, the predominant receptor subtypes in the rodent and human brain highlight the importance of the hippocampus. Moreover, FGF receptor signaling plays important roles in neurogenesis, cortical and hippocampal development, as well as models of learning and memory. Finally, the development of constructs that can bind FGF ligands will present an intriguing mechanism for further regulation of the available pool of active ligands and their ability to exert functional changes in the CNS.
It should also be mentioned that other ligands, distinct from the classical FGF molecules, have been shown to bind to FGF receptors. One of the best characterized molecules in this class is neural cell adhesion molecule (NCAM) (Christensen et al., 2006; Kiselyov et al., 2005; Williams et al., 1994). Later in this review, we will summarize their role in affective and cognitive behavior.
Affective Functions of the FGF System
FGF System in Human Studies
Alterations in the FGF system were first identified in cortical brain regions in individuals with MDD compared to controls (Evans et al., 2004). Moreover, the FGF family was not altered in individuals with bipolar disorder (BPD). Although differences were observed in FGF1, FGF2, FGF9, FGF12, FGFR2 and FGFR3, no differences were seen in FGF7, FGF13, FGF14 or FGFR1, see Table 1. In these initial studies, gene expression in the anterior cingulate cortex and in the dorsolateral prefrontal cortex was assessed by Affymetrix microarrays in two different cohorts comprising a total of 13 controls and 13 MDDs. In the dorsolateral prefrontal cortex, the results for the FGF system were validated by qRT-PCR for several members of the FGF family. Finally, these effects were found to not be due to treatment with SSRIs, as this treatment tended to normalize values closer to those of controls.
Table 1.
Summary of human post-mortem studies that have assessed gene expression of FGF-related transcripts by region.
| REGION | FGF1 | FGF2 | FGF9 | FGF12 | FGFR1 | FGFR2 | FGFR3 | FGFBP1 | REFERENCE |
|---|---|---|---|---|---|---|---|---|---|
| Dorsolateral Prefrontal Cortex | Down | Down | Up | Down | Down | Evans et al., 2004 | |||
| Anterior Cingulate | Down | Down | Up | Up | Down | Down | Evans et al., 2004 | ||
| Temporal Cortex | Down | Aston et al., 2005 | |||||||
| Hippocampus | Up | Up | Gaughran et al., 2006 | ||||||
| Dorsolateral Prefrontal Cortex | Down | Down | Kang et al., 2007 | ||||||
| Prefrontal Cortex | Up | Tochigi et al., 2008 | |||||||
| Locus Coeruleus | ∼Down | Up | Down | Bernard et al., 2011 |
Subsequent studies also uncovered alterations in the FGF family in other postmortem brain areas, including the locus coeruleus (LC) of individuals with MDD (Bernard et al., 2011). This noradrenergic cell group was dissected by laser capture microscopy, and the resultant RNA was hybridized to Affymetrix microarrays. Gene expression of FGF9 was significantly upregulated, and FGFR3 was significantly downregulated in the LC. Moreover, FGFR3 downregulation was validated by quantitative RT-PCR. It should also be noted that FGF2 exhibited a nonsignificant trend for a decrease, mirroring the observations in the cortex. Therefore, the effects of FGF9 and FGFR3 were replicated in a separate brain region in individuals with MDD. Subsequent studies have extended the findings of dysregulation of the FGF family to multiple other regions including the hippocampus and the amygdala. It should be noted that these studies only used brain samples that have a pH above 6.8, as a low pH is associated with long agonal factors and can significantly alter gene expression profiles (Li et al., 2004).
Following the initial observations, other investigators have assessed members of the FGF family in the postmortem brains of MDD and control subjects. Further studies have confirmed the existence of significant changes in the FGF system associated with depression, a remarkable consistency for human postmortem studies. One study first reported changes in the hippocampus of MDD subjects, and found FGF2 to be decreased and FGFR1 to be increased in MDD brains (Gaughran et al., 2006). One research group has found FGFR1 gene expression to be increased in the prefrontal cortex of individuals with MDD (Tochigi et al., 2008), but this result has not yet been replicated.
Two additional studies examined FGFR2 and FGFR3 in cortical regions of MDD patients relative to controls. In particular, FGFR2 was found to be decreased in the post-mortem temporal cortex of individuals with MDD (Aston et al., 2005). Moreover, FGFR3 and FGFBP1 have been reported to be decreased in the dorsolateral prefrontal cortex of individuals with MDD (Kang et al., 2007). However, this study found no alterations in FGF1, FGF9 or FGFR2. A potential cause for some inconsistencies between studies may relate to the degree to which the issue of brain pH is taken into account. Together, this series of six published reports from postmortem human brains clearly points to the dysregulation of the FGF system across multiple brain areas of depressed subjects, ranging from the brain stem to the temporal and frontal cortex and including the hippocampus. A common theme is a decrease in FGF2 and FGFR3 as well as in FGF2, with more variable findings related to FGFR1.
Even more recently, a few studies have detected alterations of the FGF system in living human subjects suffering from MDD. For example, a single nucleotide polymorphism (SNP) in FGF2 (rs1048201) was found to be associated with side effects and altered responsiveness to antidepressant treatment in individuals with MDD (Kato et al., 2009). Other SNPs in FGF2 (rs1449683 and rs308393) were also associated with differential treatment response to SSRIs. One other study also found serum levels of FGF2 to be increased in individuals with MDD and borderline personality disorder (Kahl et al., 2009). These studies extend the postmortem findings and suggest that the FGF system may offer potentially valuable biomarkers, be they diagnostic or pharmacogenomic, for the diagnosis and treatment of major depression.
In summary, evidence from several independent research groups has shown significant alterations in the FGF family across multiple brain regions of individuals suffering from major depression, see Table 1. More specific hypotheses can now be generated from these discoveries and tested directly in patients or patient samples. These observations prompted studies evaluating the functions of FGF2 in animal models--a case of “reverse translation”.
FGF System in Emotionality in Animals
Animal studies have proven pivotal in validating and extending the discoveries made in human postmortem findings. When changes are observed in gene expression in human brain, the findings, even if fully validated, may not be functionally significant but rather represent mere side effects of other types of dysregulation. To attribute functional import to them, it is critical to manipulate them or test their regulation in the context of animal models.
Indeed the first animal studies, predating the human findings, were pharmacological and suggested a possible role of FGF2 in mediating the actions of antidepressants and anxiolytic dugs. Thus, chronic antidepressant treatment for three weeks resulted in an increase in FGF2 24h later (Mallei et al., 2002). Similarly, FGF2 was increased (six and 12 hours) following acute treatment with an anxiolytic (Gomez-Pinilla et al., 2000). This led to the suggestion that FGF2, like BDNF, may mediate the actions of these drugs, and was consistent with the observation that patients on antidepressants expressed a lower degree of dysregulation in their FGF system relative to untreated MDD's.
Following the observation that FGF2 was decreased in the postmortem brain of MDD subjects, it was important to determine whether FGF2 was altered in an animal model of depression-like behavior. This study relied on a subchronic social defeat stress in the rat as a model of depression, since social stress activates neural circuits in the rodent that parallel those altered in human depression (Kollack-Walker et al., 1997; Kollack-Walker et al., 1999). Moreover, social defeat has ethological validity, in that it mimics some of the physiological and anhedonic aspects of depression in humans. This study focused on the hippocampus, as postmortem studies pointed to this brain region as the most altered by MDD (unpublished observations). Moreover, this is an area that is critical in the biology of “stress-related disorders”, including MDD, anxiety and post-traumatic stress disorder (PTSD). For example, human brain imaging studies have shown that the volume of the human hippocampus is negatively correlated with post-traumatic stress disorder (Gilbertson et al., 2002), consistent with the view that this area is highly responsive to stress-related disorders. This work, in fact, suggested that hippocampal volume may be a predisposing factor in PTSD. Thus, the hippocampal size of the twin who had not been exposed to combat predicted the magnitude of PTSD in the combat twin. Since FGF2 can control the development and size of the hippocampus (Ohkubo et al., 2004), it was logical to assess FGF2 expression in this region follow a social stress animal model. It should also be mentioned that neuroimaging studies have shown a reduced hippocampal volume in depressed subjects (Campbell et al., 2004). However, it remains unclear whether this is a result of stress and an antecedent to depression, or a consequence of having the disorder.
Following repeated social defeat stress, the expression of FGF2, as well as one of its receptors, FGFR1, was decreased in the hippocampus (Turner et al., 2008a), suggesting the hypothesis that the observed decrease in FGF2 both in human MDDs and an animal model may contribute to the affective changes accompanying depression. Could FGF2 be “an endogenous antidepressant”, and could its suppression, therefore, contribute to the negative affect of depressed humans? This hypothesis was tested by administering FGF2 intracerebroventricularly to adult rats to ascertain its potential antidepressant-like effects,. Following both acute and chronic administration and across multiple tests of depression-like behavior, such as the forced swim test and novelty-suppressed feeding, FGF2 proved to have antidepressant properties (Turner et al., 2008c). Surprisingly, FGFR1 expression was also increased by the FGF2 treatment. This suggested that FGF2 can prime its own receptor and further amplify the effects of its administration. Moreover, other ligands known to bind to and activate FGF receptors, such as neural cell adhesion molecule (NCAM), also decreased depression-like behavior following acute intracerebroventricular administration (Turner et al., 2008c).
While the above animal studies implicated FGF2 in response to depression in humans and to stress in animals, and pointed to FGF2 as an “endogenous antidepressant”, they did not address whether FGF2 could be a predisposing factor to vulnerability to anxiety-like behavior. This question was addressed by relying on a selectively-bred rat line of emotionality. Two lines of rat were bred on the basis of novelty seeking in a novel environment and termed bred High Responders (bHR) and bred Low Responders (bLRs). These two lines exhibit many differences across behavior and are proposed as models of externalizing disorders (bHRs) vs. internalizing disorders (bLRs). Thus, bHRs show lower levels of spontaneous anxiety, greater propensity for risk-taking, sign-tracking and drug-taking behavior (Flagel et al., 2008; Flagel et al., 2009; Flagel et al., 2010; Stead et al., 2006). By contrast, bLRs exhibit greater anxiety- and depression-like behaviors and greater responsiveness to stress. It was, therefore, reasonable to use these two lines to investigate whether FGF2 may be a predisposing factor to emotional reactivity.
Indeed, the high anxiety bLRs exibibited lower endogenous levels of FGF2 gene expression in the hippocampus relative to the low anxiety HRs (Perez et al., 2009). Moreover, repeated peripheral administration of FGF2 decreased anxiety-like behavior, and the bLRs benefited more from the treatment than the bHRs. Similarly, environmental complexity, a manipulation known to decrease anxiety in rodents, increased FGF2 expression in the hippocampus and showed a greater effect in bLRs. Perez et al. (2009) also assessed neurogenesis following peripheral FGF2 administration and found that chronic administration did not influence cell proliferation but increased cell survival in the dentate gyrus, especially in the bLR rats that exhibit the greater decrease in anxiety behavior. Although FGF2 increased the survival of both neurons and glia, the increase in the number of astrocytes was particularly prominent. Together, these findings led to the view that FGF2 is both a genetic predisposing factor that affects basal anxiety levels, and a modulator of environmental influences on anxiety behavior in the adult rat.
If FGF2 is indeed not only an endogenous antidepressant but also an endogenous anxiolytic factor, where does it exert this influence on behavior? This question was addressed by using a knockdown strategy to reduce FGF2 expression in the dentate gyrus and CA3 region by RNA interference, and assess its impact on behavior in rats (Eren-Kocak et al., 2011). A lentiviral vector containing a short-hairpin targeting FGF2 was used to knockdown FGF2, and this treatment resulted in an anxiogenic effect without altering other behaviors. This suggests that FGF2 expression in the hippocampus does indeed modulate the level of spontaneous anxiety. Based on this body of work, a model was proposed illustrating the importance of hippocampal levels of FGF2 in the modulation of allostatic load (Salmaso and Vaccarino, 2011).
Having established that FGF2 may modulate both the vulnerability and/or resilience to anxiety-like behavior, as well as mediate environmental changes such as stress and environmental complexity, its role in the development of emotional circuitry became critical. This question was addressed by assessing the effects of early life FGF2, administered the day after birth, on emotionality, hippocampal development and gene expression (Turner et al., 2011). Remarkably, a single injection of FGF2 (20ng/g, s.c.) early in life was able to alter neurogenesis in outbred animals. In adulthood, these animals exhibited a denser dentate gyrus with more neurons, consistent with the idea that neurogenesis precedes gliogenesis in early development (Palmer et al., 1999). Moreover, when the same early life FGF2 treatment was given to high anxiety animals (bLRs), FGF2 decreased their spontaneous anxiety (Turner et al., 2011). This effect was associated with altered gene expression in the dentate gyrus. Laser capture microdissection followed by microarray analyses identified transcripts that differed between bLR-VEH and bLR-FGF2 animals. Specifically, molecules previously associated with anxiety (gad1) were decreased, whereas molecules associated with cell survival (bcl2-like2) were increased in the high anxiety bred rats in conjunction with decreased anxiety by FGF2 treatment. Thus, early life FGF2 treatment altered the developmental trajectory of the dentate gyrus and had long-term effects on emotionality and gene expression.
Most recently, a study by Duman's group extended these findings to mice and to other models of stress (Elsayed et al., 2012). Thus, the authors reported that chronic infusion of FGF2 had antidepressant-like effects in both rats and mice. They also added site-specificity to the antidepressant effects by infusing FGF2 into the medial prefrontal cortex. Moreover, FGF2 blocked the effects of chronic unpredictable stress (CUS) on both depression-like behavior, and the CUS-induced inhibition of glial proliferation. Treatment with an FGF receptor antagonist that targets all FGF receptors blocked the effects of fluoxetine on glial proliferation, as well as the effect of fluoxetine as an antidepressant. These results suggest that not only is FGF2 a sufficient antidepressant, it is also necessary for the antidepressant effects of SSRIs, although the lack of selectivity of the available FGF antagonists requires caution in the interpretation of these latter results.
Moreover, the study by Elsayed et al. (2012) also hinted at relatively rapid effects of FGF2 in animal models of depression and anxiety (five days after administration). We have also observed rapid effects of FGF2 in other paradigms. Indeed, some of the behavior and biochemical effects of FGF2 can be observed within minutes and certainly within hours, but the mechanism of these rapid effects needs further exploration. FGFR1 is required for the electrophysiological correlate of learning and memory, long-term potentiation (Zhao et al., 2007). Is it possible that the same glutamatergic mechanisms may underlie the susceptibility to anxiety and depression? FGFR1, the highest affinity receptor for FGF2, is located on glutamatergic cells in the cortex (Shin et al., 2004). Therefore, it is possible that an increase in glutamate transmission may be responsible for the antidepressant effects of FGF2.
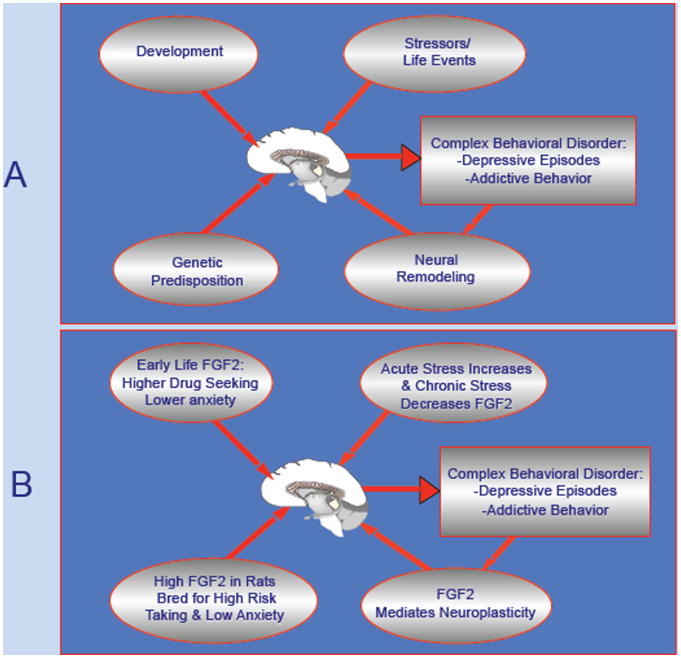
Figure 1 summarizes the body of work that implicates FGF2 in each of the factors thought to modify emotionality. Thus, genetic differences between the bHR and bLR lines implicate FGF2 as a genetic factor. The early FGF2 administration studies demonstrate its critical organizational function in laying down differences in emotional reactivity. And the various studies with stress paradigms and environmental complexity demonstrate its role in mediating changes that result from experience, resulting in altered neurogenesis and other types of neuroplasticity. The hippocampus and prefrontal cortex are two loci of its actions, as shown in both human and animal studies, with other loci yet to be identified.
Figure 1. The Role of FGF2 in the Vulnerability to Mood and Addictive Disorders.
Panel A depicts the major factors that converge on the brain to modify vulnerability to complex behavioral disorders such as major depression or substance abuse- Genes, Development, Stress and other Environmental Events all converge at the level of the brain to alter neural circuits that control behavior. Moreover, the behavior itself, such as a depressive episode or a period of substance abuse, in turn modifies the brain through neuroplasticity mechanisms, and increases the likelihood of relapse.
Panel B illustrates the fact that FGF2 has been implicated in each of these mechanisms, with specific examples of its role in each of these domains. Thus, FGF2 plays multiple roles— genetic, developmental, and experiential, in regulating the propensity to certain behaviors such as mood disorders or drug abuse. In general, low FGF2 levels predispose towards depression, while high FGF2 levels predispose towards substance abuse.
FGF System in Fear Conditioning in Animals
The FGF system has not only been implicated in general anxiety, but also plays a role in emotional learning and fear conditioning, suggesting that it may be involved in another affective disorder-- PTSD. This disorder is based on the inability to extinguish fearful memories under conditions that are presumably “safe”. Initially, FGF2 was implicated in the acquisition phase of fear conditioning (Graham and Richardson, 2009a). When FGF2 was given subcutaneously immediately prior to conditioning, it facilitated contextual fear memory in young rats (PND 16, PND19 or PND22). This group went on to show that systemic FGF2 can also facilitate extinction if it is on-board during consolidation (Graham and Richardson, 2009b). Moreover, when given immediately after extinction, FGF2 reduced reinstatement and relapse (Graham and Richardson, 2010b).
FGF2 was also shown to have effects on fear conditioning when subcutaneously administered to neonatal rats, with testing conducted 11-18 days after the last injection (Graham and Richardson, 2010a). However, unlike the effects on anxiety described above, multiple injections of FGF2 were required (PND1-5) to facilitate both fear conditioning and context-dependent extinction. Taken together, FGF2 may be involved in modulating the conditioning and long-term memory of both the acquisition and the extinction aspects of fear-related responses. However, it remains to be demonstrated how long these effects can last. Would one treatment during extinction be sufficient, and would the effect be permanent? Moreover, would the same treatment have a long-term effect if given in adulthood?
Interestingly, FGF2 appears to alter the way in which memories are erased. Typically, this process proceeds via an NMDA receptor-independent pathway. Subcutaneous administration of FGF2 immediately or 4 hours after extinction required an NMDA-dependent mechanism for reacquisition and re-extinction (Graham and Richardson, 2011a; Graham and Richardson, 2011b). This finding may have profound implications for the PTSD field in that FGF2 may actually erase part of the initial fear memory. This would then lead to the ability of the fear memory to be weakened, rendering it easier to extinguish. Finally, the effects of FGF2 on fear conditioning exhibit site-specificity. When it was administered into the basolateral amygdala, FGF2 enhanced extinction and reduced renewal and reinstatement similar to the peripheral injection findings in adult rats.
In summary, FGF2 plays a role in fear conditioning, extinction and reinstatement, as well as reacquisition and re-extinction. Moreover, FGF2 has both developmental and long-term effects on the memory of fearful events. Glutamate receptors in the amygdala may also play an important role in the functions of FGF2. Thus, the ability of FGF2 to modulate affective behavior includes both spontaneous anxiety as well as conditioned emotional responses, all of which may contribute to long-lasting negative affect as seen in mood disorders. This body of work underscores the role of FGF2 at the interface of affect, learning and memory.
FGF System in Stress Response in Animals
The FGF system plays a role in the cellular and behavioral neuroadaptations to stress. As will be discussed below, these adaptations take place across a wide range of developmental time points ranging from embryonic to adulthood. Moreover, the impact of stress on FGF expression appears to be dynamic within a given developmental window. In general, neuroprotective molecules such as FGF2 are induced by short-term stress or exposure to glucocorticoids, and these FGFs may play an important role in coping with acute stress. Their induction may also buffer against the potential negative impact of high steroid levels (Molteni et al., 2001). However, with repeated or sustained stress, this induction is not sustained, and the expression of the protective FGF molecules and receptors is in fact reduced relative to control levels, likely contributing to the long-term negative sequelae of chronic stress.
Early animal work by the Fuxe laboratory demonstrated that acute and sub-chronic corticosterone administration can increase FGF2 protein levels in the substantia nigra (Chadi et al., 1993). This induction is indeed consistent with a neuroprotective response, as FGF2 can protect neurons from excitotoxic, metabolic and oxidative insults (Mark et al., 1997). Similarly, FGF9 can protect dopaminergic neurons from MPTP-induced cell death (Huang et al., 2009).
In contrast to its induction by acute glucocorticoids in adulthood, FGF2 is typically reduced by early life stress, and this effect is manifested into adulthood. Embryonic stress has been reported to decrease FGF2 expression in the adult hippocampus (Molteni et al., 2001). This manipulation also changed the response of the adult brain to subsequent stress or to corticosterone administration. Furthermore, perinatal anoxia decreased basal levels of FGF2 in the ventral tegmental area in adulthood while simultaneously enhancing the response of FGF2 to an acute stressor (Flores et al., 2002). Similarly, postnatal stress decreased FGF2 expression in the prefrontal cortex, although other areas such as the striatum showed an elevation (Fumagalli et al., 2005). These studies are consistent with the link between early life stress and depression in humans.
Of particular relevance to a link between early stress, depression and the modulatory role of FGF is a fascinating study conducted in human fetal brain aggregates (Salaria et al., 2006). The authors cultured these cells and exposed a subset of them to chronic cortisol for a period of three weeks to model early life stress. They performed microarray analyses to evaluate the global impact of this manipulation, and confirmed key findings with protein analyses. They discovered that the FGF system is among the most altered in response to chronic cortisol. In particular, they found that FGF2 was upregulated, whereas FGF9 was downregulated. These findings are consonant with our observations in the post-mortem brains of individuals with MDD that show complementary changes in these two growth factors in the same directions described by this study.
In adulthood, the pattern of induction of FGF2 and its receptors by acute stress followed by their suppression upon chronic stress is typically manifested. Thus, 24hr following exposure to acute controllable shock in the rat, FGF2 was significantly increased in the dentate gyrus of the hippocampus (Bland et al., 2006). Acute escapable shock produced a similar effect in the prefrontal cortex, suggesting a potential role of FGF2 in the cognitive manifestations of stress (Bland et al., 2007). However, it should be mentioned that BDNF was not altered by stressor controllability in the hippocampus, in contrast to FGF2 (Bland et al., 2007).
By contrast, repeated social defeat decreases FGF2 and FGFR1 in the rat hippocampus (Turner et al., 2008a). Berton et al. have also shown that FGFR3 was decreased in the VTA following a chronic social defeat paradigm in mice (Berton et al., 2006). Finally, FGFR2 gene expression was decreased in the CRF-overexpressing mouse, a model of chronic stress (Peeters et al., 2004). Beyond the acute versus chronic nature of the paradigms, the potential importance of the specific animal model has not been systematically investigated. For example, the relative role of social versus. non-social stress.has not been tested. Nevertheless, the findings with repeated social defeat recapitulate some of the observations in the human postmortem brain from depressed individuals.
A recently published study found alterations in the FGF system following a chronic unpredictable stress paradigm in adult mice (Elsayed et al., 2012). Here, a decrease in FGFR1 expression was also accompanied by a decrease in glial proliferation in the prefrontal cortex, and the latter effect could be prevented by treatment with FGF2. In keeping with the idea of a mechanism for these effects (other than downstream signaling), FGF2 has an antisense transcript (FGF2-AS) that is known to regulate the expression of FGF2 (Knee et al., 1997; MacFarlane et al., 2010; MacFarlane and Murphy, 2010). Furthermore, corticosterone administration can differentially regulate FGF2 and FGF2-AS expression in both escapable and inescapable shock paradigms (Frank et al., 2007). Moreover, inhibiting corticosterone synthesis abrogated the effect of inescapable shock on both transcripts. Thus, glucocorticoids appear to mediate the effects of stress on FGF2 and FGF2-AS.
Much of this work is analogous to the findings with other growth factors, such as BDNF or insulin-like growth factor (IGF-1) (Duman and Monteggia, 2006). For example, animals that have less IGF signaling in the hippocampus due to early life events exhibited a larger stress response in adulthood (Erabi et al., 2007). The interactions between BDNF and stress responsiveness are more complex. While acute stress decreased BDNF in the hippocampus (Pizarro et al., 2004), BDNF in the nucleus accumbens was increased following social defeat and appeared to required for stress susceptibility (Berton et al., 2006) (Krishnan et al., 2007). Interestingly, knocking down BDNF in the mesolimbic system resulted in an increase in FGFR1, suggesting that the two systems may work in concert and that the FGF system may be able to compensate for the BDNF system (Berton et al., 2006).
In summary, FGF2 expression across multiple brain regions, at both the transcript and protein levels is clearly modified by stress and by glucocorticoids. The effects of stress on this system start as early as in utero and are long-lasting. They are also manifest in adulthood, with some transient and controllable stressors enhancing FGF2 while uncontrollable longer stressors inhibit its expression. The hippocampus is particularly susceptible to stress-induced alterations in FGF2 and other FGF family members. Given the above discussion of the role of the FGF family in modulating anxiety, fear and depression, the fact that this family is so clearly responsive to stress and that these responses are so long-lasting makes it a key link between environmental challenges, neuroplasticity and affective behavior.
FGF System in Substance Abuse
While our primary focus in this review is on affective behavior, the role of FGFs in substance abuse is relevant for several reasons: a) addictive behavior is emotional in nature and closely linked not only to reward mechanisms but also to stress, anxiety and coping; b) the study of the neurobiology of temperament and personality and their relation to psychopathology in humans typically contrasts the propensity for internalizing disorders (depression, anxiety) with the propensity for externalizing disorders, such as substance abuse. The bHR/bLR animal model mirrors these differences in temperament in humans. bHR animals have higher basal levels of FGF2 and are, in fact, more prone to drug-taking behavior. It is reasonable to ask whether the high level of FGF2 plays a role in this phenotype; c) like fear conditioning, addiction represents a type of maladaptive neuroplasticity. Given the known role of FGF2 in neuroplasticity, it is reasonable to ascertain its role in both drug-taking behavior and the response to drugs of abuse.
The first reports of the role of FGF2 in drug-related behavior came from Stewart's group (Flores et al., 1998). Repeated amphetamine administration increased the levels of FGF2 in the ventral tegmental area (VTA), and in dopaminergic terminal regions (Flores and Stewart, 2000). In the VTA, this effect was associated with astrocytes and lasted for up to one month following the repeated injections (Flores et al., 1998). While FGF2 altered dopamine release, these effects were believed to be indirect (Forget et al., 2006). The authors went on to show, by using an antibody approach, that endogenous FGF2 in the VTA is required for the induction of amphetamine sensitization (Flores et al., 2000). Further research showed that FGF2 is required for the structural remodeling following administration of drugs of abuse (Mueller et al., 2006). Stewart's group was, therefore, the first to propose that FGF2 may be involved in the neuroplasticity mechanisms underlying sensitization to psychostimulants.
Other investigators have expanded these findings to peripheral administration of other drugs of abuse and other brain regions. Nicotine appears to upregulate FGF2 expression in the striatum by either a D1 or D2 mechanism (Roceri et al., 2001). In terms of dopaminergic agents, apomorphine can increase FGF2 expression via D2 receptors. Conversely, D2 agonists were found to activate FGF2 in the prefrontal cortex and hippocampus (Fumagalli et al., 2003). Cocaine, when administered acutely, can rapidly alter levels of FGF2 in the prefrontal cortex and striatum, with chronic exposure to cocaine resulting in enduring elevations of FGF2, especially in the striatum (Fumagalli et al., 2006). Thus, long-lasting changes take place in regions highly innervated by midbrain dopaminergic neurons, suggesting that FGF2 is not only involved in the initial response to drugs of abuse, but also in the long-term neuroadaptations.
Interestingly, the selectively bred line of rats that shows greater propensity to drug seeking behavior (i.e. bHR rats) exhibit higher basal levels of expression of FGF2 in the hippocampus and nucleus accumbens than their bLRs counterparts that show lower propensity to self administer drugs (Perez et al., 2009) (Clinton et al 2012) . Moreover, a sensitizing treatment with cocaine generally decreased FGFR1 expression in the hippocampus and increased FGFR1 in the prefrontal cortex (Turner et al., 2008b). However, the two selectively bred lines showed a differential effect of the drug. In the hippocampus, cocaine decreased gene expression in bHRs without affecting bLRs, whereas in the prefrontal cortex cocaine increased gene expression in bLRs without affecting bHRs. Thus, cocaine interacted with the novelty-seeking trait to alter gene expression differentially depending on brain region, furthering the idea that the FGF system may be involved in the individual differences in the response to drugs of abuse.
A single administration of FGF2 on PND1 increased cocaine self-administration in adulthood (Turner et al., 2009). This effect is selective as there were no associated differences in spatial or appetitive learning. Moreover, there were no sustained changes in gene expression in the dopaminergic system seen in the adult animal. This does not preclude the possibility that early exposure to FGF2 primed the dopaminergic system, which in turn led to increased drug-taking behavior in adulthood. Whether the actions of early life FGF2 are mediated via dopamine or other mechanisms, the ability of this growth factor to enhance drug-taking behavior identifies it as a molecular antecedent of vulnerability for substance abuse.
Given the fact that drugs of abuse interact with stress, it is notable that both stress and drugs of abuse converge to modulate FGF2 expression. Thus, in the prefrontal cortex, acute stress potentiated the cocaine-induced increase in FGF2 expression, whereas prolonged stress prevented the response of FGF2 to cocaine (Fumagalli et al., 2008). In the striatum, the cocaine-induced FGF2 response was only increased following repeated stress.
In summary, FGF2 appears to promote both the initial vulnerability and the sequelae of substance abuse. Its administration in early life enhances the propensity for self-administration of drugs of abuse in adulthood. In turn, repeated exposure to drugs of abuse induces FGF2 expression especially in the dopaminergic system, and this induction is required for the development of sensitization.
Overall, FGF2, along with FGFR1, can be construed as molecular factors that modulate emotional reactivity-- higher FGF2 levels render animals more prone to novelty and drug taking behavior, while lower FGF2 levels render animals less prone to drug seeking but more prone to anxiety- and depression-like behaviors.
Beyond the FGFs: The Role of Other Interacting Ligands
Other molecules, such as NCAM, can also interact with the FGF receptors and appear to play a role in the control of emotionality. NCAM polymorphisms have been observed in conjunction with mood disorders in humans (Atz et al., 2007; Vawter, 2000). In animal models, NCAM responds to stress system activation, with upregulation of its expression in the cortex following acute corticosterone injections and downregulation following chronic corticosterone (Sandi and Loscertales, 1999) a pattern that mirrors the regulation of FGF2 by this stress hormone. However, the isoform of NCAM is also important. For example, exposure to a stressful situation decreased NCAM-180 levels in the hippocampus without affecting the levels of NCAM-140 or NCAM-120 (Sandi et al., 2005). Finally, post-translational modifications of NCAM (polysialylation) can also be affected by stress (Cordero et al., 2005).
Similar to FGF2, FGL, a fragment of the NCAM structure (Carafoli et al., 2008; Ditlevsen et al., 2008), has antidepressant effects when acutely administered intracerebroventricularly to rodents (Turner et al., 2008c). Conversely, NCAM-deficient mice exhibit an increase in anxiety- and depression-like behavior. The latter effect, along with FGFR signaling deficits, can be restored by treatment with FGL (Aonurm-Helm et al., 2008; Aonurm-Helm et al., 2010). Similarly, FGL was able to reverse the chronic stress, as well as NCAM-deficiency induced cognitive impairments (Bisaz et al., 2011). The effects of FGL on fear conditioning and spatial learning have also been assessed, whereby both the positive and negative effects were enhanced (Cambon et al., 2004). Additionally, FGL can enhance presynaptic function, promote synaptogenesis and facilitate memory (Cambon et al., 2004). Not surprisingly, FGL can also prevent stress-induced impairments in cognitive function (Borcel et al., 2008). Two other NCAM-derived peptides, dennexin and plannexin, have been shown to have effects in vivo, modulating neuroplasticity and learning (Kohler et al., 2010) (Kraev et al., 2011). For a more thorough discussion of the role of NCAM in cognition and stress, the reader is referred to other reviews (Conboy et al., 2010; Sandi and Bisaz, 2007).
Other ligands, such as N-cadherin and pentraxin, are cell adhesion molecules that can bind to FGF receptors as well as the cytoskeleton (Hansen et al., 2008; Sanchez-Heras et al., 2006). Similar to NCAM, N-cadherin binds to the acid box region of the FGF receptor, which is different than the binding site for FGF2. Interestingly, peptide moieties of N-cadherin have been identified that can act as agonists, and one of the main functions of N-cadherin is to induce neurite outgrowth (Williams et al., 2002).
In general, non-FGF ligands that interact with the FGF receptors have been identified for the treatment of cognitive deficits. Given the relevance of the FGF system to fear, anxiety, depression and addiction, it will be important to ascertain their potential as targets for affective disorders.
Beyond the FGFs: Receptor-Interacting Partners
The complexity and the potential functions of the FGF system are augmented not only by a host of binding molecules but also by the potential for receptor-receptor interactions. Recently, FGFR1 has been shown to directly interact with two different neurotransmitter receptors. The first is the adenosine 2A receptor (Flajolet et al., 2008). Activation of this G-protein coupled receptor along with FGFR1 resulted in activation of MAPK/ERK pathway and enhanced corticostriatal plasticity. The direct physical interaction allows FGF ligands to function as co-transmitters at adenosine 2A receptors.
More recently, FGFR1 has also been shown to heterodimerize with the 5-HT1A receptor in both the hippocampus and the raphe (Borroto-Escuela et al., 2012a; Borroto-Escuela et al., 2012b). The heteroreceptor complex has been co-immunoprecipitated in cultured cells, and in neurons in the dorsal rat hippocampal formation and in the dorsal raphe. Moreover, the investigators co-administered FGF2 and serotonin and uncovered a heightened response in an antidepressant test, as compared to the effect of either ligand alone. Moreover, combining FGF-2 and 5-HT1A agonist synergistically enhanced both receptor signaling and cell differentiation, suggesting a trophic role in the serotonergic neurons (Borroto-Escuela et al., 2012b). Given the large literature on 5HT1A receptor signaling (Hannon and Hoyer, 2008), and its role in mediating the mode of action of antidepressants and in the regulation of emotional responsiveness (Blier and Abbott, 2001; Blier and Ward, 2003), the molecular interaction between these two systems opens up exciting avenues for understanding the biology and pathophysiology of affect and mood. In addition, since both FGFR1 and 5HT1A receptors are known to be present on neural stem cells, their interplay in modulating neurogenesis, e.g. upon antidepressant treatment or with environmental complexity or exercise, is of great interest.
These two examples of interaction with G-Protein coupled receptors greatly expand the range of potential influence of the FGF system on neuronal signaling and the control of growth and differentiation. Such interactions might exist in other brain regions, and possibly with other G-protein coupled receptors, and couple the FGF control of neuroplasticity more directly to the actions of specific neurotransmitters.
Discussion
The body of work summarized here underscores the surprising role of the FGF family not only in controlling neural development and neuroplasticity, but also in modulating many facets of emotional and motivated behavior. Equally notable is the fact that this modulation occurs in multiple time domains, with early effects lasting into adult life, but also with evidence for “on-line” control of signaling and behavioral responsiveness during adulthood.
It should be mentioned that other growth factors, such as BDNF and IGF-1, have similar neuromodulatory effects as FGF2. For example, both molecules promote neurogenesis and act as antidepressants (Anderson et al., 2002; Hoshaw et al., 2005; Schmidt and Duman, 2010). BDNF is also upregulated following antidepressant drug treatment and has long-lasting effects on hippocampal function (Monteggia et al., 2004; Nibuya et al., 1995). However, FGF2 has effects on glial cells, specifically astrocytes, which have not been shown for BDNF or IGF-1 (Numakawa et al., 2011). One of these functions includes upregulating microRNAs, where BDNF and IGF-1 failed to do so. Given that depression may be related to a perturbation in glia, this may represent a significant difference between growth factor families (Bernard et al., 2011; Choudary et al., 2005). Finally, FGF receptors can interact with other neurotransmitters, and this has the potential for FGF ligands to have multiple and rapid cellular and behavioral effects.
The FGF family appears to reside at the interface of genetic, developmental, environmental and experiential regulation of mood, affect and addiction. As depicted in Figure 1, endogenous levels of FGF molecules are predisposing factors that regulate stress responsiveness and the vulnerability or resilience to anxiety, depression, fear conditioning and substance abuse. In turn, as depicted in Figure 2, FGF molecules are effectors of the impact of experience on brain morphology, neurogenesis, cell survival and neuronal signaling. They rely on a host of mechanisms to alter every phase of neuronal organization and function, to modify stable patterns of reactivity, and to control ongoing behavior.
In the context of mood disorders, the role of the FGF family combines two distinct hypotheses regarding the biological causes of severe depression—a neurotransmitter based hypothesis such as the dysregulation of serotonin signaling (Sharp and Cowen, 2011) and a stress hypothesis (Akil, 2005), focusing on early developmental adversity, enhanced vulnerability to stressors and a disrupted neuroendocrine dysregulation, resulting in a range of negative consequences on brain structure and function. Our view of the FGF family synthesizes these hypotheses by placing FGFs at the very interface of stress regulation, neurotransmitter signaling and neural remodeling.
In particular, FGF molecules appear to interact with classical neurotransmitter molecules at the level of heteroreceptor complexes, or by direct physical interaction, to control both cellular morphology and signaling, as shown in Figure 2. In addition, a host of other molecules modulate this system including cell adhesion molecules and endogenous molecules. These factors operate in both neurons and glia and in different combinations across distinct neural circuits. Clearly, much remains to be learned about the role of the various members of this complex family in the regulation of affect, motivation and mood. But the research to date has already illuminated previously unsuspected roles and pointed to exciting new targets for the treatment of affective and addictive disorders.
Acknowledgments
This work was supported by NIMH Conte Center Grant P50 MH60398, NIDA P01 DA021633, The Office of Naval Research (ONR) Grants N00014-09-1-0598 and N00014-12-1-0366, the Pritzker Neuropsychiatric Disorders Research Consortium Fund LLC (http://www.pritzkerneuropsych.org), the Hope for Depression Research Foundation, NCRR Grant UL1RR024986, as well as a Rachel Upjohn Clinical Scholars Award to CT.
Footnotes
Financial Disclosures: The authors are members of the Pritzker Neuropsychiatric Disorders Research Consortium, which is supported by the Pritzker Neuropsychiatric Disorders Research Fund L.L.C. A shared intellectual property agreement exists between this philanthropic fund and the University of Michigan, Stanford University, the Weill Medical College of Cornell University, the University of California at Irvine, and the HudsonAlpha Institute for Biotechnology to encourage the development of appropriate findings for research and clinical applications.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil H. Stressed and depressed. Nat Med. 2005;11:116–118. doi: 10.1038/nm0205-116. [DOI] [PubMed] [Google Scholar]
- Alam KY, Frostholm A, Hackshaw KV, Evans JE, Rotter A, Chiu IM. Characterization of the 1B promoter of fibroblast growth factor 1 and its expression in the adult and developing mouse brain. J Biol Chem. 1996;271:30263–30271. doi: 10.1074/jbc.271.47.30263. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134:115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- Aonurm-Helm A, Jurgenson M, Zharkovsky T, Sonn K, Berezin V, Bock E, Zharkovsky A. Depression-like behaviour in neural cell adhesion molecule (NCAM)-deficient mice and its reversal by an NCAM-derived peptide, FGL. Eur J Neurosci. 2008;28:1618–1628. doi: 10.1111/j.1460-9568.2008.06471.x. [DOI] [PubMed] [Google Scholar]
- Aonurm-Helm A, Berezin V, Bock E, Zharkovsky A. NCAM-mimetic, FGL peptide, restores disrupted fibroblast growth factor receptor (FGFR) phosphorylation and FGFR mediated signaling in neural cell adhesion molecule (NCAM)-deficient mice. Brain Res. 2010;1309:1–8. doi: 10.1016/j.brainres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatr. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiat Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audigier S, Guiramand J, Prado-Lourenco L, Conte C, Gonzalez-Herrera IG, Cohen-Solal C, Recasens M, Prats AC. Potent activation of FGF-2 IRES-dependent mechanism of translation during brain development. RNA. 2008;14:1852–1864. doi: 10.1261/rna.790608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett PF, Dutton R, Likiardopoulos V, Brooker G. Regulation of neurogenesis in the embryonic and adult brain by fibroblast growth factors. Alcohol Alcohol Suppl. 1994;2:387–394. [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatr. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bisaz R, Schachner M, Sandi C. Causal evidence for the involvement of the neural cell adhesion molecule, NCAM, in chronic stress-induced cognitive impairments. Hippocampus. 2011;21:56–71. doi: 10.1002/hipo.20723. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–597. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Abbott FV. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. J Psychiat Neurosci. 2001;26:37–43. [PMC free article] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiat. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Mudo G, Perez-Alea M, Ciruela F, Tarakanov AO, Narvaez M, Di Liberto V, Agnati LF, Belluardo N, Fuxe K. Fibroblast growth factor receptor 1- 5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol Psychiat. 2012a;71:84–91. doi: 10.1016/j.biopsych.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Romero-Fernandez W, Perez-Alea M, Narvaez M, Tarakanov AO, Mudo G, Agnati LF, Ciruela F, Belluardo N, Fuxe K. The existence of FGFR1-5-HT1A receptor heterocomplexes in midbrain 5-HT neurons of the rat: relevance for neuroplasticity. J Neurosci. 2012b;32:6295–6303. doi: 10.1523/JNEUROSCI.4203-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cambon K, Hansen SM, Venero C, Herrero AI, Skibo G, Berezin V, Bock E, Sandi C. A synthetic neural cell adhesion molecule mimetic peptide promotes synaptogenesis, enhances presynaptic function, and facilitates memory consolidation. J Neurosci. 2004;24:4197–4204. doi: 10.1523/JNEUROSCI.0436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiat. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chadi G, Rosen L, Cintra A, Tinner B, Zoli M, Pettersson RF, Fuxe K. Corticosterone increases FGF-2 (bFGF) immunoreactivity in the substantia nigra of the rat. Neuroreport. 1993;4:783–786. doi: 10.1097/00001756-199306000-00047. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Tao Y, Black IB, DiCicco-Bloom E. A single peripheral injection of basic fibroblast growth factor (bFGF) stimulates granule cell production and increases cerebellar growth in newborn rats. J Neurobiol. 2001;46:220–229. doi: 10.1002/1097-4695(20010215)46:3<220::aid-neu1004>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, Myers RM, Bunney WE, Jr, Akil H, Watson SJ, Jones EG. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci U S A. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen C, Lauridsen JB, Berezin V, Bock E, Kiselyov VV. The neural cell adhesion molecule binds to fibroblast growth factor receptor 2. FEBS Lett. 2006;580:3386–3390. doi: 10.1016/j.febslet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Cinaroglu A, Ozmen Y, Ozdemir A, Ozcan F, Ergorul C, Cayirlioglu P, Hicks D, Bugra K. Expression and possible function of fibroblast growth factor 9 (FGF9) and its cognate receptors FGFR2 and FGFR3 in postnatal and adult retina. J Neurosci Res. 2005;79:329–339. doi: 10.1002/jnr.20363. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Turner CA, Flagel SB, Simpson DN, Watson SJ, Akil H. Neonatal fibroblast growth factor treatment enhances cocaine sensitization. Pharmacol Biochem Behav. 2012;103(1):6–17. doi: 10.1016/j.pbb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Bisaz R, Markram K, Sandi C. Role of NCAM in emotion and learning. Adv Exp Med Biol. 2010;663:271–296. doi: 10.1007/978-1-4419-1170-4_18. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Rodriguez JJ, Davies HA, Peddie CJ, Sandi C, Stewart MG. Chronic restraint stress down-regulates amygdaloid expression of polysialylated neural cell adhesion molecule. Neuroscience. 2005;133:903–910. doi: 10.1016/j.neuroscience.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Ditlevsen DK, Owczarek S, Berezin V, Bock E. Relative role of upstream regulators of Akt, ERK and CREB in NCAM- and FGF2-mediated signalling. Neurochem Int. 2008;53:137–147. doi: 10.1016/j.neuint.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Dono R, Texido G, Dussel R, Ehmke H, Zeller R. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. Embo J. 1998;17:4213–4225. doi: 10.1093/emboj/17.15.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiat. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiat. 1998;44:324–335. doi: 10.1016/s0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiat. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Elde R, Cao YH, Cintra A, Brelje TC, Pelto-Huikko M, Junttila T, Fuxe K, Pettersson RF, Hokfelt T. Prominent expression of acidic fibroblast growth factor in motor and sensory neurons. Neuron. 1991;7:349–364. doi: 10.1016/0896-6273(91)90288-b. [DOI] [PubMed] [Google Scholar]
- Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS. Antidepressant Effects of Fibroblast Growth Factor-2 in Behavioral and Cellular Models of Depression. Biol Psychiat. 2012;72:258–265. doi: 10.1016/j.biopsych.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erabi K, Morinobu S, Kawano K, Tsuji S, Yamawaki S. Neonatal isolation changes the expression of IGF-IR and IGFBP-2 in the hippocampus in response to adulthood restraint stress. Int J Neuropsychopharmacol. 2007;10:369–381. doi: 10.1017/S1461145706006675. [DOI] [PubMed] [Google Scholar]
- Eren-Kocak E, Turner CA, Watson SJ, Akil H. Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiat. 2011;69:534–540. doi: 10.1016/j.biopsych.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A. 2004;101:15506–15511. doi: 10.1073/pnas.0406788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacol. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajolet M, Wang Z, Futter M, Shen W, Nuangchamnong N, Bendor J, Wallach I, Nairn AC, Surmeier DJ, Greengard P. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11:1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Rodaros D, Stewart J. Long-lasting induction of astrocytic basic fibroblast growth factor by repeated injections of amphetamine: blockade by concurrent treatment with a glutamate antagonist. J Neurosci. 1998;18:9547–9555. doi: 10.1523/JNEUROSCI.18-22-09547.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J Neurosci. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Stewart J. Changes in astrocytic basic fibroblast growth factor expression during and after prolonged exposure to escalating doses of amphetamine. Neuroscience. 2000;98:287–293. doi: 10.1016/s0306-4522(00)00115-9. [DOI] [PubMed] [Google Scholar]
- Flores C, Stewart J, Salmaso N, Zhang Y, Boksa P. Astrocytic basic fibroblast growth factor expression in dopaminergic regions after perinatal anoxia. Biol Psychiat. 2002;52:362–370. doi: 10.1016/s0006-3223(02)01363-x. [DOI] [PubMed] [Google Scholar]
- Ford-Perriss M, Abud H, Murphy M. Fibroblast growth factors in the developing central nervous system. Clin Exp Pharm P. 2001;28:493–503. doi: 10.1046/j.1440-1681.2001.03477.x. [DOI] [PubMed] [Google Scholar]
- Forget C, Stewart J, Trudeau LE. Impact of basic FGF expression in astrocytes on dopamine neuron synaptic function and development. Eur J Neurosci. 2006;23:608–616. doi: 10.1111/j.1460-9568.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- Frank MG, Der-Avakian A, Bland ST, Watkins LR, Maier SF. Stress-induced glucocorticoids suppress the antisense molecular regulation of FGF-2 expression. Psychoneuroendo. 2007;32:376–384. doi: 10.1016/j.psyneuen.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Frinchi M, Bonomo A, Trovato-Salinaro A, Condorelli DF, Fuxe K, Spampinato MG, Mudo G. Fibroblast growth factor-2 and its receptor expression in proliferating precursor cells of the subventricular zone in the adult rat brain. Neurosci Lett. 2008;447:20–25. doi: 10.1016/j.neulet.2008.09.059. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23:295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Maragnoli ME, Gennarelli M, Perez J, Racagni G, Riva MA. Dopaminergic D2 receptor activation modulates FGF-2 gene expression in rat prefrontal cortex and hippocampus. J Neurosci Res. 2003;74:74–80. doi: 10.1002/jnr.10733. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Slotkin TA, Racagni G, Riva MA. Prenatal stress elicits regionally selective changes in basal FGF-2 gene expression in adulthood and alters the adult response to acute or chronic stress. Neurobiol Dis. 2005;20:731–737. doi: 10.1016/j.nbd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Pasquale L, Racagni G, Riva MA. Dynamic regulation of fibroblast growth factor 2 (FGF-2) gene expression in the rat brain following single and repeated cocaine administration. J Neurochem. 2006;96:996–1004. doi: 10.1111/j.1471-4159.2005.03627.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Stress and cocaine interact to modulate basic fibroblast growth factor (FGF-2) expression in rat brain. Psychopharmacology (Berl) 2008;196:357–364. doi: 10.1007/s00213-007-0966-x. [DOI] [PubMed] [Google Scholar]
- Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz R, Dover K, Laezza F, Shtraizent N, Huang X, Tchetchik D, Eliseenkova AV, Xu CF, Neubert TA, Ornitz DM, Goldfarb M, Mohammadi M. Crystal structure of a fibroblast growth factor homologous factor (FHF) defines a conserved surface on FHFs for binding and modulation of voltage-gated sodium channels. J Biol Chem. 2009;284:17883–17896. doi: 10.1074/jbc.M109.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D'Angelo E. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55:449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Lee JW, Cotman CW. Distribution of basic fibroblast growth factor in the developing rat brain. Neuroscience. 1994;61:911–923. doi: 10.1016/0306-4522(94)90412-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, Choi J, Ryba EA. Diazepam induces FGF-2 mRNA in the hippocampus and striatum. Brain Res Bull. 2000;53:283–289. doi: 10.1016/s0361-9230(00)00342-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- Goodrich SP, Yan GC, Bahrenburg K, Mansson PE. The nucleotide sequence of rat heparin binding growth factor 1 (HBGF-1) Nucleic Acids Res. 1989;17:2867. doi: 10.1093/nar/17.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term memory in developing rats. Neurobiol Learn Mem. 2009a;91:424–430. doi: 10.1016/j.nlm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term extinction of fear and reduces reinstatement in rats. Neuropsychopharmacol. 2009b;34:1875–1882. doi: 10.1038/npp.2009.14. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Early-life exposure to fibroblast growth factor-2 facilitates context-dependent long-term memory in developing rats. Behav Neurosci. 2010a;124:337–345. doi: 10.1037/a0019582. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Fibroblast growth factor-2 enhances extinction and reduces renewal of conditioned fear. Neuropsychopharmacol. 2010b;35:1348–1355. doi: 10.1038/npp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Fibroblast growth factor-2 alters the nature of extinction. Learn Mem. 2011a;18:80–84. doi: 10.1101/lm.2006511. [DOI] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Memory of fearful events: the role of fibroblast growth factor-2 in fear acquisition and extinction. Neuroscience. 2011b;189:156–169. doi: 10.1016/j.neuroscience.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron. 2011;71:574–588. doi: 10.1016/j.neuron.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Hanneken A, Ying W, Ling N, Baird A. Identification of soluble forms of the fibroblast growth factor receptor in blood. Proc Natl Acad Sci U S A. 1994;91:9170–9174. doi: 10.1073/pnas.91.19.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hansen SM, Berezin V, Bock E. Signaling mechanisms of neurite outgrowth induced by the cell adhesion molecules NCAM and N-cadherin. Cell Mol Life Sci. 2008;65:3809–3821. doi: 10.1007/s00018-008-8290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Huang JY, Hong YT, Chuang JI. Fibroblast growth factor 9 prevents MPP+-induced death of dopaminergic neurons and is involved in melatonin neuroprotection in vivo and in vitro. J Neurochem. 2009;109:1400–1412. doi: 10.1111/j.1471-4159.2009.06061.x. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, Schweiger U. Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrino. 2009;34:353–357. doi: 10.1016/j.psyneuen.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, Overholser JC, Meltzer HY, Jurjus GJ, Konick LC, Newton SS, Duman RS. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Okugawa G, Wakeno M, Takekita Y, Nonen S, Tetsuo S, Nishida K, Azuma J, Kinoshita T, Serretti A. Effect of basic fibroblast growth factor (FGF2) gene polymorphisms on SSRIs treatment response and side effects. Eur Neuropsychopharm. 2009;19:718–725. doi: 10.1016/j.euroneuro.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiat. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kiselyov VV, Soroka V, Berezin V, Bock E. Structural biology of NCAM homophilic binding and activation of FGFR. J Neurochem. 2005;94:1169–1179. doi: 10.1111/j.1471-4159.2005.03284.x. [DOI] [PubMed] [Google Scholar]
- Knee R, Li AW, Murphy PR. Characterization and tissue-specific expression of the rat basic fibroblast growth factor antisense mRNA and protein. Proc Natl Acad Sci U S A. 1997;94:4943–4947. doi: 10.1073/pnas.94.10.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knüsel B, Hefti F. Multiple and interactive responses of central neurons to neurotrophic factors. Sem Neurosci. 1993;5:259–267. [Google Scholar]
- Kohler LB, Christensen C, Rossetti C, Fantin M, Sandi C, Bock E, Berezin V. Dennexin peptides modeled after the homophilic binding sites of the neural cell adhesion molecule (NCAM) promote neuronal survival, modify cell adhesion and impair spatial learning. Eur J Cell Biol. 2010;89:817–827. doi: 10.1016/j.ejcb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Kraev I, Henneberger C, Rossetti C, Conboy L, Kohler LB, Fantin M, Jennings A, Venero C, Popov V, Rusakov D, Stewart MG, Bock E, Berezin V, Sandi C. A peptide mimetic targeting trans-homophilic NCAM binding sites promotes spatial learning and neural plasticity in the hippocampus. PLoS One. 2011;6:e23433. doi: 10.1371/journal.pone.0023433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Seno M, Igarashi K. Nucleotide sequence of rat basic fibroblast growth factor cDNA. Nucleic Acids Res. 1988;16:5201. doi: 10.1093/nar/16.11.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Lampert A, Kozel MA, Gerber BR, Rush AM, Nerbonne JM, Waxman SG, Dib-Hajj SD, Ornitz DM. FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels. Mol Cell Neurosci. 2009;42:90–101. doi: 10.1016/j.mcn.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Dal Toso R, della Valle F, Skaper SD, Leon A. Update of the NGF saga. J Neurol Sci. 1995;130:119–127. doi: 10.1016/0022-510x(95)00007-o. [DOI] [PubMed] [Google Scholar]
- Li JZ, Vawter MP, Walsh DM, Tomita H, Evans SJ, Choudary PV, Lopez JF, Avelar A, Shokoohi V, Chung T, Mesarwi O, Jones EG, Watson SJ, Akil H, Bunney WE, Jr, Myers RM. Systematic changes in gene expression in postmortem human brains associated with tissue pH and terminal medical conditions. Hum Mol Genet. 2004;13:609–616. doi: 10.1093/hmg/ddh065. [DOI] [PubMed] [Google Scholar]
- Lu B, Figurov A. Role of neurotrophins in synapse development and plasticity. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- MacFarlane LA, Gu Y, Casson AG, Murphy PR. Regulation of fibroblast growth factor-2 by an endogenous antisense RNA and by argonaute-2. Mol Endocrinol. 2010;24:800–812. doi: 10.1210/me.2009-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane LA, Murphy PR. Regulation of FGF-2 by an endogenous antisense RNA: effects on cell adhesion and cell-cycle progression. Mol Carcinogen. 2010;49:1031–1044. doi: 10.1002/mc.20686. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Keller JN, Kruman I, Mattson MP. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain Res. 1997;756:205–214. doi: 10.1016/s0006-8993(97)00196-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich RX, Mezzera C, Holmes WM, Goda S, Brookfield SJ, Rankin AJ, Barr E, Kurniawan N, Dewar D, Richards LJ, Lopez-Bendito G, Iwata T. Fgfr3 regulates development of the caudal telencephalon. Dev Dyn. 2011;240:1586–1599. doi: 10.1002/dvdy.22636. [DOI] [PubMed] [Google Scholar]
- Molteni R, Fumagalli F, Magnaghi V, Roceri M, Gennarelli M, Racagni G, Melcangi RC, Riva MA. Modulation of fibroblast growth factor-2 by stress and corticosteroids: from developmental events to adult brain plasticity. Brain Res Brain Res Rev. 2001;37:249–258. doi: 10.1016/s0165-0173(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Chapman CA, Stewart J. Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor. Neuroscience. 2006;137:727–735. doi: 10.1016/j.neuroscience.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Yamamoto N, Chiba S, Richards M, Ooshima Y, Kishi S, Hashido K, Adachi N, Kunugi H. Growth factors stimulate expression of neuronal and glial miR-132. Neurosci Lett. 2011;505:242–247. doi: 10.1016/j.neulet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Uchida AO, Shin D, Partanen J, Vaccarino FM. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. J Neurosci. 2004;24:6057–6069. doi: 10.1523/JNEUROSCI.1140-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SK, Garbi M, Zampieri N, Eliseenkova AV, Ornitz DM, Goldfarb M, Mohammadi M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J Biol Chem. 2003;278:34226–34236. doi: 10.1074/jbc.M303183200. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters PJ, Fierens FL, van den Wyngaert I, Goehlmann HW, Swagemakers SM, Kass SU, Langlois X, Pullan S, Stenzel-Poore MP, Steckler T. Gene expression profiles highlight adaptive brain mechanisms in corticotropin releasing factor overexpressing mice. Brain Res Mol Brain Res. 2004;129:135–150. doi: 10.1016/j.molbrainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Plotnikov AN, Eliseenkova AV, Ibrahimi OA, Shriver Z, Sasisekharan R, Lemmon MA, Mohammadi M. Crystal structure of fibroblast growth factor 9 reveals regions implicated in dimerization and autoinhibition. J Biol Chem. 2001;276:4322–4329. doi: 10.1074/jbc.M006502200. [DOI] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Riedel B, Friauf E, Grothe C, Unsicker K. Fibroblast growth factor-2-like immunoreactivity in auditory brainstem nuclei of the developing and adult rat: correlation with onset and loss of hearing. J Comp Neurol. 1995;354:353–360. doi: 10.1002/cne.903540305. [DOI] [PubMed] [Google Scholar]
- Robins LN, Regier DA. psychiatric disorders in america: the epidemiologic catchment area study. New York: The Free Press; 1991. [Google Scholar]
- Roceri M, Molteni R, Fumagalli F, Racagni G, Gennarelli M, Corsini G, Maggio R, Riva M. Stimulatory role of dopamine on fibroblast growth factor-2 expression in rat striatum. J Neurochem. 2001;76:990–997. doi: 10.1046/j.1471-4159.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- Romero-Fernandez W, Borroto-Escuela DO, Tarakanov AO, Mudo G, Narvaez M, Perez-Alea M, Agnati LF, Ciruela F, Belluardo N, Fuxe K. Agonist-induced formation of FGFR1 homodimers and signaling differ among members of the FGF family. Biochem Biophys Res Comm. 2011;409:764–768. doi: 10.1016/j.bbrc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Salaria S, Chana G, Caldara F, Feltrin E, Altieri M, Faggioni F, Domenici E, Merlo-Pich E, Everall IP. Microarray analysis of cultured human brain aggregates following cortisol exposure: implications for cellular functions relevant to mood disorders. Neurobiol Dis. 2006;23:630–636. doi: 10.1016/j.nbd.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Vaccarino FM. Toward a novel endogenous anxiolytic factor, fibroblast growth factor 2. Biol Psychiat. 2011;69:508–509. doi: 10.1016/j.biopsych.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Heras E, Howell FV, Williams G, Doherty P. The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J Biol Chem. 2006;281:35208–35216. doi: 10.1074/jbc.M608655200. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M. Opposite effects on NCAM expression in the rat frontal cortex induced by acute vs. chronic corticosterone treatments. Brain Res. 1999;828:127–134. doi: 10.1016/s0006-8993(99)01346-3. [DOI] [PubMed] [Google Scholar]
- Sandi C, Woodson JC, Haynes VF, Park CR, Touyarot K, Lopez-Fernandez MA, Venero C, Diamond DM. Acute stress-induced impairment of spatial memory is associated with decreased expression of neural cell adhesion molecule in the hippocampus and prefrontal cortex. Biol Psychiat. 2005;57:856–864. doi: 10.1016/j.biopsych.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Sandi C, Bisaz R. A model for the involvement of neural cell adhesion molecules in stress-related mood disorders. Neuroendocrinology. 2007;85:158–176. doi: 10.1159/000101535. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacol. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah CA, Bei L, Wang H, Platanias LC, Eklund EA. HoxA10 protein regulates transcription of gene encoding fibroblast growth factor 2 (FGF2) in myeloid cells. J Biol Chem. 2012;287:18230–18248. doi: 10.1074/jbc.M111.328401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp T, Cowen PJ. 5-HT and depression: is the glass half-full? Curr Opin Pharmacol. 2011;11:45–51. doi: 10.1016/j.coph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Shibata F, Baird A, Florkiewicz RZ. Functional characterization of the human basic fibroblast growth factor gene promoter. Growth Factors. 1991;4:277–287. doi: 10.3109/08977199109043913. [DOI] [PubMed] [Google Scholar]
- Shin DM, Korada S, Raballo R, Shashikant CS, Simeone A, Taylor JR, Vaccarino F. Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci. 2004;24:2247–2258. doi: 10.1523/JNEUROSCI.5285-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, Johnson EM., Jr Neurotrophic molecules. Ann Neurol. 1989;26:489–506. doi: 10.1002/ana.410260402. [DOI] [PubMed] [Google Scholar]
- Spugnini EP, Citro G, Fais S. Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res. 2010;29:44. doi: 10.1186/1756-9966-29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanabrough M, Horvath TL, Vaccarino FM. Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J Neurosci. 2010;30:5590–5602. doi: 10.1523/JNEUROSCI.5837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens HE, Jiang GY, Schwartz ML, Vaccarino FM. Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol Psychiat. 2012;71:1090–1098. doi: 10.1016/j.biopsych.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Black IB, DiCicco-Bloom E. Neurogenesis in neonatal rat brain is regulated by peripheral injection of basic fibroblast growth factor (bFGF) J Comp Neurol. 1996;376:653–663. doi: 10.1002/(SICI)1096-9861(19961223)376:4<653::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Temple S, Qian X. bFGF, neurotrophins, and the control or cortical neurogenesis. Neuron. 1995;15:249–252. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, Kato T. Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci Res. 2008;60:184–191. doi: 10.1016/j.neures.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiat. 2006;59:1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Turner CA, Calvo N, Frost DO, Akil H, Watson SJ. The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett. 2008a;430:147–150. doi: 10.1016/j.neulet.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Flagel SB, Clinton SM, Akil H, Watson SJ. Cocaine interacts with the novelty-seeking trait to modulate FGFR1 gene expression in the rat. Neurosci Lett. 2008b;446:105–107. doi: 10.1016/j.neulet.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008c;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Capriles N, Flagel SB, Perez JA, Clinton SM, Watson SJ, Akil H. Neonatal FGF2 alters cocaine self-administration in the adult rat. Pharmacol Biochem Be. 2009;92:100–104. doi: 10.1016/j.pbb.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Clinton SM, Thompson RC, Watson SJ, Jr, Akil H. Fibroblast growth factor-2 (FGF2) augmentation early in life alters hippocampal development and rescues the anxiety phenotype in vulnerable animals. Proc Natl Acad Sci U S A. 2011;108:8021–8025. doi: 10.1073/pnas.1103732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:848. doi: 10.1038/12226. [DOI] [PubMed] [Google Scholar]
- Vawter MP. Dysregulation of the neural cell adhesion molecule and neuropsychiatric disorders. Eur J Pharmacol. 2000;405:385–395. doi: 10.1016/s0014-2999(00)00568-9. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Williams G, Williams EJ, Doherty P. Dimeric versions of two short N-cadherin binding motifs (HAVDI and INPISG) function as N-cadherin agonists. J Biol Chem. 2002;277:4361–4367. doi: 10.1074/jbc.M109185200. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Kitano A, Takao K, Prasansuklab A, Mushiroda T, Yamazaki K, Kumada T, Shibata M, Takaoka Y, Awaya T, Kato T, Abe T, Iwata N, Miyakawa T, Nakamura Y, Nakahata T, Heike T. Inactivation of fibroblast growth factor binding protein 3 causes anxiety-related behaviors. Mol Cell Neurosci. 2011;46:200–212. doi: 10.1016/j.mcn.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiat. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]