Abstract
Lignocellulosic biomass, upon pretreatment and enzymatic hydrolysis, generates a mixture of hexose and pentose sugars such as glucose, xylose, arabinose and galactose. While Escherichia coli utilizes all these sugars it lacks the ability to produce ethanol from them. Recombinant ethanologenic E. coli strains have been created with a goal to produce ethanol from both hexose and pentose sugars. Herein, we review the current state of the art on the production of ethanol from lignocellulosic hydrolyzates by an ethanologenic recombinant E. coli strain (FBR5). The bacterium is stable without antibiotics and can tolerate ethanol up to 50 gL-1. It produces up to 45 g ethanol per L and has the potential to be used for industrial production of ethanol from lignocellulosic hydrolyzates.
Keywords: ethanol fermentation, lignocellulosic biomass, recombinant ethanologenic Escherichia coli, separate hydrolysis and fermentation, simultaneous saccharification and fermentation
Introduction
Ethanol is a renewable oxygenated fuel. In the USA, about 13.23 billion gallons of fuel ethanol was produced in 2010, replacing the gasoline produced from some 445 million barrels of imported oil.1 Various agricultural residues (corn stover, wheat straw, barley straw, rice straw and sugarcane bagasse), processing byproducts (corn fiber and rice hulls), and energy crops (switchgrass and miscanthus) are available as low cost feedstocks for conversion to fuel ethanol. The production of ethanol from lignocellulosic biomass generally involves four steps—feedstock pretreatment, enzymatic saccharification, fermentation and product recovery. The pretreatment of any lignocellulosic biomass is crucial before enzymatic hydrolysis.2 Furthermore, any lignocellulosic biomass, upon pretreatment and enzymatic saccharification, produces a mixture of sugars such as glucose, xylose, arabinose and galactose.
The utilization of all the sugars generated from lignocellulosic biomass is essential for the economic production of ethanol.3 The conventional ethanol fermenting yeast (Saccharomyces cerevisiae) or bacterium (Zymomonas mobilis) cannot ferment pentose sugars to ethanol.4 One major technical hurdle to converting any lignocellulosic feedstock to ethanol is the development of an appropriate microorganism for fermentation of both hexose and pentose sugars. A number of recombinant microorganisms such as Escherichia coli, Klebsiella oxytoca, Z. mobilis and S. cerevisiae have been developed over the past 25 y with the express goal of fermenting both hexose and pentose sugars to ethanol simultaneously.4 Herein, we review the production of ethanol by a recombinant E. coli strain (FBR5) from various lignocellulosic biomass substrates.
Construction of Recombinant E. coli Strain FBR5
The ethanol pathway in S. cerevisiae and Z. mobilis requires only two enzymes, pyruvate decarboxylase and alcohol dehydrogenase. Ingram et al.5 inserted the genes encoding these two enzymes from Z. mobilis into E. coli under the control of a single enteric promoter to produce an artificial operon, designated the pet operon, for the production of ethanol. The resultant recombinant E. coli strain produced high levels of ethanol from glucose. Later Ohta et al.6 integrated the pyruvate decarboxylase (pdc) and alcohol dehydrogenase II (adhB) genes into the E. coli chromosome within or near the pyruvate formate-lyase gene (pfl)). Strains carrying the pet operon in plasmid (e.g., E. coli B/pLOI297) or in chromosomal sites (e.g., E. coli KO11) require antibiotics in the medium to maintain genetic stability and high ethanol productivity.7 To overcome this requirement, Hespell et al.8 used the conditionally lethal E. coli strain FMJ39, which carries mutations for lactate dehydrogenase and pyruvate formate lyase and grows aerobically but is incapable of anaerobic growth unless these mutations are complemented.9 E. coli strains FBR1 and FBR2 were created by transforming E. coli FMJ39 with the pet (production of ethanol) operon plasmids pLOI295 and pLOI297, respectively. Both strains continued to produce high levels of ethanol from glucose in the absence of antibiotics. However, the strain FMJ39 does not metabolize xylose. Dien et al.10 was able to isolate FMJ39 mutants that do metabolize xylose and then transformed one of these mutants with plasmid pLOI297 which resulted in the new E. coli strain FBR3. The strain FBR3 selectively maintained the plasmid pLOI297 when grown anaerobically. Following 10 serial transfers of this strain in aerobic and anaerobic cultures containing either glucose or xylose with no selective antibiotics led to loss of the plasmid from the aerobic cultures. An average of 97.4 ± 3.5% of the cells maintained the plasmid in anaerobic cultures. The strain utilized 10% (w/v) each of glucose, xylose, arabinose or a mixture of these sugars [4% (w/v) glucose, 4% (w/v) xylose and 2% (w/v) arabinose] and produced 4.38–4.66% (w/v) ethanol. Dien et al.11 used this approach to construct two additional E. coli strains with different genetic backgrounds. They introduced the plasmid pLOI297 into two new E. coli strains (DC1368 and NZN111) that also carry pfl and ldhA mutations.12 Unlike the parent strain (FMJ39x) for FBR3, the pfl mutation in these strains was introduced by genetic recombination and included an antibiotic resistance marker which is the gene for chloramphenicol acetyl transferase (cat) responsible for conferring resistance to chroramphenicol (Cm). These two recombinant E. coli strains were named FBR4 and FBR5, respectively. Serial transfer experiments demonstrated that the ethanol plasmid was stably maintained in the absence of antibiotics.11 E. coli FBR5 performed better than FBR4 in ethanol production from corn fiber hydrolyzate. Figure 1 shows an illustration outlining the construction of the recombinant E. coli strain FBR5. Nichols et al.13 constructed ethanologenic E. coli strains with the glucose phospho-transferase (ptsG) mutation. The mutants (FBR14, FBR16) had an altered pattern of mixed sugar utilization—xylose and arabinose were simultaneously consumed as fast as glucose, suggesting that the catabolic pathways for xylose and arabinose were fully activated despite the presence of glucose in the medium (FBR16). The fumarate dehydrogenase gene (⊗frdABCD) was further deleted in the mutant FBR16, preventing the production of succinic acid to minimize byproduct formation. However, ptsG- also disables active glucose transport in E. coli strain FBR16. For this reason, the mutants grew slower on glucose and were more sensitive to inhibitors present in corn fiber hydrolyzate than FBR5.14
Figure 1.
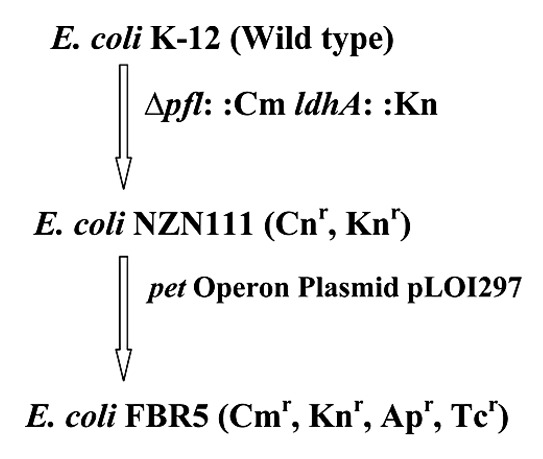
Construction of ethanologeic Escherichia coli strain FBR5. Details are available in references 12 and 11. Cm, chloroamphenicol; Kn, kanamycin; Ap, ampicilin; Tc, tetracycline.
Fermentation of Mixed Sugars
Dien et al.11 investigated the fermentation of xylose (7.62%, w/v) and mixed sugars (7.62%, w/v) containing glucose (3.05%), xylose (3.05%) and arabinose (1.52%) by the recombinant E. coli FBR5; revealing that it produced 3.34 ± 0.04 and 3.40 ± 0.05% (w/v) ethanol from xylose and mixed sugars with the ethanol productivity of 0.66 ± 0.01 and 0.92 ± 0.04 gL-1h-1, respectively. The strain produced 39.2 to 41.5 g ethanol from 95 g xylose per L with ethanol productivity of 0.59 gL-1h-1. Qureshi et al.15 investigated substrate and product inhibition and kinetic parameters for ethanol production from xylose by the strain. The culture could tolerate very high xylose concentrations (up to 250 gL-1) in the medium. The maximum ethanol produced from xylose was 43.5 gL-1 and the bacterium tolerated a maximum of 50 g ethanol per L. In pH controlled experiments, the maximum ethanol productivity of 0.90 gL-1h-1 was obtained from xylose (90 gL-1). The strain tolerated up to a maximum 40 g salt (sodium chloride) per L of fermentation broth but showed inhibition of growth at above 10 g salt per L.15
Fermentation of Lignocellulosic Hydrolyzates
The ethanol production from wheat straw by various pretreatments (hydrothermal, dilute acid, concentrated acid, lime, alkaline peroxide and microwave), enzymatic saccharification and batch fermentations of both non-detoxified and detoxified (by overliming) hydrolyzates by both separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF) using the recombinant E. coli strain FBR5 has been investigated in detail.16-19 Integration of process steps is important in lowering the cost of the production of ethanol from any lignocellulosic feedstock.3 SSF is an option that is considered a necessary step in this regard.20 The optimal pH and temperature for the enzymatic hydrolysis of pretreated wheat straw and rice hulls were at 5.0 and 45–50°C.17,21 However, the recombinant E. coli FBR5 performed well at pH 6.5 and 35°C.11 In SSF experiments, a compromi+sed pH of 6.0 and temperature at 35°C were used. The minimum and maximum ethanol produced from pretreated wheat straw (86 gL-1) in these studies were 13.0 ± 2.0 and 22.5 ± 0.6 gL-1, respectively. The ethanol yields varied between 0.37 to 0.50 gg-1 available sugars depending on the type of pretreatment used. The fermentation time also varied greatly from 17 to 136 h which was also highly dependent of the type of pretreatment and the inhibitory compounds present in the hydrolyzate. Figure 2 shows the patterns of utilization of glucose, xylose, arabinose and total sugars and production of ethanol by the strain from alkaline peroxide pretreated and enzymatically saccharified wheat straw hydrolyzate.18 It is evident that the strain utilized glucose first, then arabinose and finally xylose at a slower rate, even though it did completely utilize xylose. Similar patterns of mixed sugar utilization were also observed in other pretreated hydrolyzates16,17,19,21-24 and with another ethanologenic recombinant E. coli ATCC 11,303 carrying plasmid pLOI297.25 Saha et al.21-24 obtained a maximum ethanol production of 18.7 ± 0.6 gL-1 in 64 h from dilute acid pretreated rice hulls (150 gL-1) and 11.9 ± 0.0 g ethanol per L in 17 h from alkaline peroxide pretreated barley straw (100 gL-1) by SHF. Recently, Saha et al.26 studied ethanol production by recombinant E. coli strain FBR5 from dilute acid pretreated wheat straw by SHF and SSF in detail. The yield of total sugars from dilute acid (0.5% H2SO4) pretreated (160°C, 10 min) and enzymatically saccharified (pH 5.0, 45°C, 72 h) wheat straw (86 gL-1) was 50.0 ± 1.4 gL-1. The hydrolyzate contained 1,184 ± 19 mg furfural and 161 ± 1 mg hydroxymethyl furfural (HMF) per L which are toxic byproducts. The recombinant E. coli FBR5 showed no growth at pH controlled at 4.5 to 6.5 in the non-abated wheat straw hydrolyzate at 35°C. However, it produced 21.9 ± 0.3 g ethanol per L from non-abated wheat straw hydrolyzate (total sugars, 44.1 ± 0.4 gL-1) in 90 h including the lag time of 24 h when pH was controlled at pH 7.0 and 35°C (SHF). It appears that this E. coli strain was able to metabolize furfural, HMF and acetic acid at pH 7.0. This finding is both important and encouraging because it provides an option of running the fermentation at pH 7.0 for SHF process without detoxifying the hydrolyzate. The bacterium produced 21.6 ± 0.5 g ethanol per L in 40 h from the bioabated and enzymatically saccharified wheat straw hydrolyzate (total sugars, 44.1 ± 0.4 g) at pH 6.0 and 35°C. The bioabatement of wheat straw was performed by growing Coniochaeta ligniaria NRRL 30616,27 in the liquid portion of the pretreated wheat straw aerobically at pH 6.5 and 30°C for 15 h. The recombinant E. coli strain FBR5 produced 24.9 ± 0.3 g ethanol per L in 96 h and 26.7 ± 0.0 g ethanol per L in 72 h from bioabated wheat straw hydrolyzate by batch and fed-batch SSF, respectively. SSF offered a distinct advantage over SHF with respect to reducing the total time required to produce ethanol from the bioabated wheat straw by the recombinant E. coli strain FBR5. However, mixing is one of the major problems of using SSF for ethanol production from lignocellulosic feedstocks. By feeding the pretreated wheat straw 3 times in the reactor (fed-batch approach), Saha et al.26 were able to complete the fermentation of bioabated wheat straw within 72 h. Fed-batch SSF performed better than the batch SSF with respect to shortening the time requirement and increase in ethanol yield.
Figure 2.
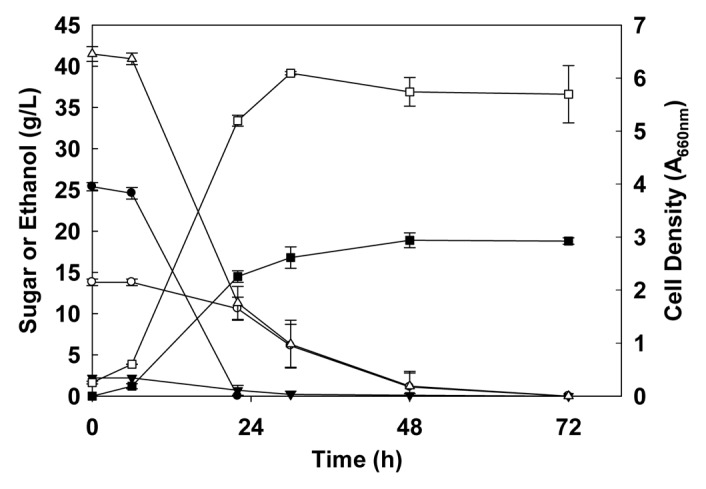
Time course of ethanol production by recombinant Escherichia coli FBR5 from alkaline H2O2 pretreated (2.15%, w/v, pH 11.5, 3 h) and enzymatically saccharified (45°C, pH 5.0, 120 h) wheat straw (8.6%, w/v) hydrolyzate at pH 6.5 and 35°C. The data presented are averages of two individual experiments. Symbols: ●, glucose; ○, xylose; ▼, arabinose; ∅, total sugars; ■, ethanol; □, cell density. From reference 18.
Dien et al.11 reported that the recombinant E. coli strain FBR5 produced a maximum of 30–42 g ethanol per L in 60 h from xylose (95 gL-1) with ethanol yields of 86–92% of theoretical in batch culture. Qureshi et al.15 showed that the maximum ethanol that could be produced by the strain from xylose in batch culture was 43.5 gL-1. Increasing the ethanol titer in the fermentation broth is crucially important for cost reduction of cellulosic ethanol production due to the high energy demand for ethanol recovery by distillation.28 An ethanol concentration of 40 gL-1 or above in the fermentation broth could be considered as a bench mark for economically viable distillation.29 Saha et al.30 studied the ethanol production by the recombinant bacterium from wheat straw at high solids loading. The yield of total sugars from dilute acid (0.75% H2SO4, v/v) pretreated (160°C, 10 min) wheat straw (150 gL-1) after enzymatic saccharification at pH 5.0 and 45°C for 72 h was 86.3 ± 1.5 gL-1. The pretreated wheat straw was bio-abated by growing C. ligniaria NRRL 30616,27 aerobically in the liquid portion for 16 h. The recombinant E. coli strain FBR5 produced 41.1 ± 1.1 g ethanol per L from non-abated wheat straw hydrolyzate (total sugars, 86.6 ± 0.3 gL-1) in 168 h at pH 7.0 and 35°C (SHF). The bacterium produced 41.8 ± 0.0 g ethanol per L in 120 h from the bioabated wheat straw by SHF. It produced 41.6 ± 0.7 g ethanol per L in 120 h from bioabated wheat straw by fed-batch SSF. The fed-batch SSF was started with one-fourth of the pretreated solid material in the fermentation broth and the remaining three portions were added at 16, 21 and 24 h. This approach helped significantly with the high solids (150 gL-1) mixing problem. This is the first report of the production of > 40 g ethanol per L from a lignocellulosic hydrolyzate by this recombinant bacterium. A summary of the fermentation activity of recombinant E. coli FBR5 from dilute acid pretreated wheat straw hydrolyzate is presented in Table 1.
Table 1. Summary of fermentation of dilute acid pretreated wheat straw by recombinant Escherichia coli FBR5 at 35oC.26,30.
| Fermentation type |
Fermentation time (h) |
Total sugars (g L-1) |
Ethanol (g L-1) |
Ethanol productivity (g L-1 h-1) |
Ethanol yield (g g-1 sugar) |
Ethanol (g g-1 straw) |
|---|---|---|---|---|---|---|
| Wheat straw (86 g L-1) |
|
|
|
|
|
|
| Non-abated | ||||||
| SHF (pH 7.0) |
90 |
44.1 ± 0.4 |
21.9 ± 0.3 |
0.24 |
0.50 |
0.25 |
| SSF (pH 7.0) | 96 | - | 17.4 ± 1.8 | 0.18 | 0.39 | 0.20 |
| Bioabated | ||||||
|---|---|---|---|---|---|---|
| SHF (pH 6.5) |
66 |
44.1 ± 0.4 |
21.6 ± 0.5 |
0.33 |
0.49 |
0.25 |
| SSF (pH 6.0) |
96 |
- |
24.9 ± 0.3 |
0.26 |
- |
0.29 |
| Fed-batch SSF (ph 6.0) | 72 | - | 26.7 ± 0.0 | 0.37 | - | 0.31 |
| Washed solids | ||||||
|---|---|---|---|---|---|---|
| SSF (pH 6.0) | 96 | - | 12.2 ± 0.3 | 0.13 | - | 0.14 |
| Wheat straw (150 g L-1) |
|
|
|
|
|
|
|---|---|---|---|---|---|---|
| SHF | ||||||
| Non-abated (pH 7.0) |
168 |
86.6 ± 0.3 |
41.1 ± 1.1 |
0.24 |
0.47 |
0.27 |
| Bioabated (pH 6.5) | 120 | 86.6 ± 0.3 | 41.8 ± 0.0 | 0.35 | 0.48 | 0.28 |
| SSF | ||||||
|---|---|---|---|---|---|---|
| Non-abated (pH 7.0) |
- |
- |
0.0 |
- |
- |
- |
| Bioabated (pH 6.0) | 104 | 41.6 ± 0.7 | 0.40 | - | 0.28 |
The dilute acid pretreatment of wheat straw (86 g L-1, 0.5% H2SO4, v/v; 150 g L-1, 0.75% H2SO4, v/v) was performed at 160oC for 10 min. Enzymatic saccharification was performed at pH 5.0 and 45oC for 72 h with a cocktail of 3 commercial enzyme (cellulase, β-glucosidase, and hemicellulase) preparations. For wheat straw (86 g/L), fed-batch SSF was performed by adding the substrate 3 times (0, 16, and 24 h) in 3 equal portions. Washed solid was prepared by separating the liquid portion of pretreated wheat straw and washing the residue with water. For wheat straw (150 g L-1), fed-batch SSF was performed by adding the substrate 4 times (0, 16, 21, and 24 h) in 4 equal portions. SHF, separate hydrolysis and fermentation; SSF, simultaneous saccharification and fermentation.
Liu et al.31,32 evaluated the performance of E. coli FBR5 for ethanol production from dilute acid hydrolyzate of hot water wood extract. Its growth was strongly inhibited in the dilute acid hydrolyzate of hot-water wood extract. The strain was then challenged by hot water wood extract (containing 59.1 g reducing sugars per L). After repeated strain adaptation, an improved strain FBHW was obtained. This E. coli strain FBHW was resistant to toxicity of the hydrolyzate in the fermentation medium containing concentrated hydrolyzate and xylose was completely utilized by the strain to produce ethanol. The strain FBHW was grown in the concentrated hydrolyzate without any detoxification. The yield of ethanol was 36.8 gL-1 of fermentation broth in 96 h. Sanny et al.33 further engineered E. coli FBR5 using three different contructs, to contain and express Vitreoscilla hemoglobin gene (vgh). The three resulting strains expressed Viteoscilla hemoglobin (VHb) at various levels, and the production of ethanol was inversely proportional to the expressed VHb level. High levels of VHb were correlated with an inhibition of ethanol production. However, the strain with the lowest VHb expression (approximately the normal induced level in Vitreoscilla), produced more ethanol than the parental strain FBR5 with glucose, xylose or corn stover hydrolyzate as the predominant carbon source under microaerobic condition in shake flasks.
Stability of Recombinant E. coli FBR5
Martin et al.34 studied the stability of the ethanologenic E. coli strain FBR5 during continuous culture on glucose or xylose. They obtained stable ethanol yields of about 80–85% of the theoretical on glucose (50 gL-1) or xylose (50 gL-1) over 26 d at dilution rates of 0.075 h-1 for glucose and 0.045 h-1 for xylose under chemostat conditions using this strain. Recently, Saha and Cotta35 studied the long-term performance of this recombinant bacterium in a series of continuous culture runs (16–105 d) using alkaline peroxide pretreated and enzymatically saccharified wheat straw hydrolyzate as a feedstock without using any antibiotics. The average ethanol produced from the available sugars (21.9 to 47.8 gL-1) ranged from 8.8 to 17.3 gL-1 (0.28 to 0.45 gg-1 available sugars, 0.31 to 0.48 gg-1 sugar consumed) with ethanol productivity of 0.27 to 0.78 gL-1h-1 in a set of 14 continuous culture runs (16–105 d). During these studies, no loss of ethanol productivity was observed which indicates that the strain showed robustness in performance. The time courses of the production of ethanol and succinic acid, growth (cell mass) and residual xylose concentration are shown in Figure 3 for a set of two continuous cultures run up to 105 d. On average, about 11.2% of total sugars (22.8% of xylose) were left unutilized. Glucose was almost completely utilized. A disadvantage of recombinant E. coli strain FBR5 is that the fumarate reductase enzyme apparently functions in strain FBR5, since none of the four subunit genes were disrupted allowing the production of considerable quantities of succinic acid.11,34 The fumarate reductase is only expressed anaerobically. Relatively stable succinic acid production (1.4–3.1 gL-1) was observed in all these continuous culture experiments performed. Similar quantities (1.0–3.0 gL-1) of succinic acid were produced by the recombinant E. coli FBR5 over the course of continuous culture experiments using 50 g glucose or xylose per L as feedstock.34
Figure 3.
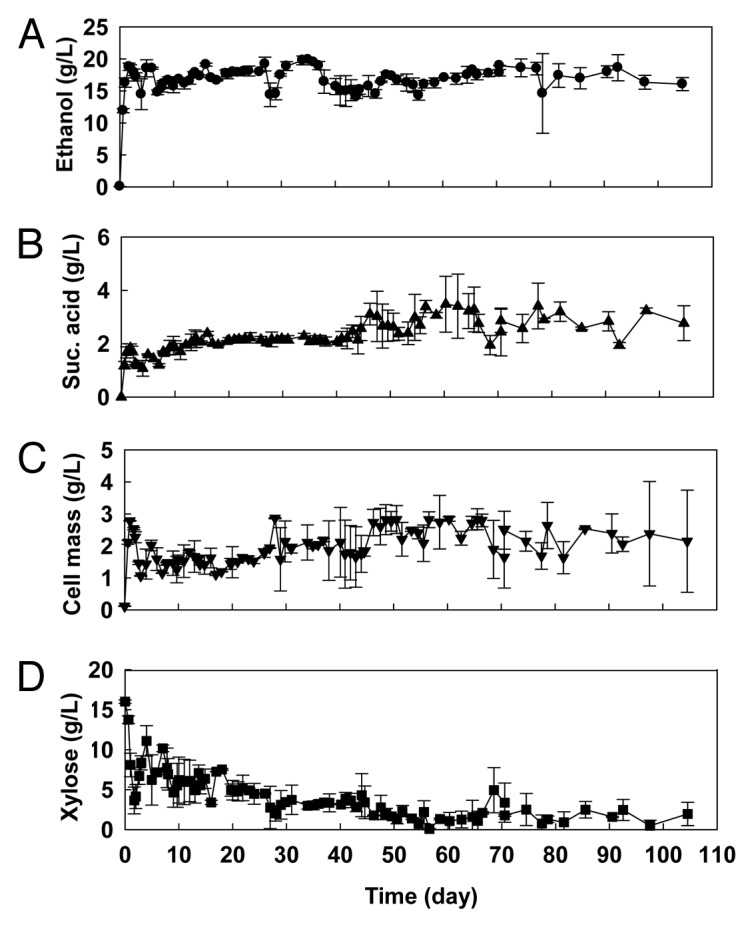
pH-Controlled continuous culture of recombinant Escherichia coli FBR5 on alkaline H2O2 pretreated (2.15%, w/v, pH 11.5, 3 h) and enzymatically saccharified (45°C, pH 5.0, 120 h) wheat straw hydrolyzate at pH 6.5 and 35°C. Dilution rate, 0.04 h-1. Total sugars in feedstock, 44.1 gL-1. Reactor volume, 240 ml. The data presented are averages of two parallel experiments. (A) Ethanol produced; (B) succinic acid produced; (C) cell mass; (D) residual xylose.35
Martin et al.34 reported that the maximum ethanol concentration achievable in continuous cultures is frequently limited by the ethanol tolerance of the recombinant bacterium. Qureshi et al.15 reported that the maximum ethanol tolerance of the strain FBR5 was 50 gL-1. Asghari et al.36 reported that recombinant E. coli strain K011 produced about 45 gL-1 ethanol from hemicellulose hydrolyzates of agricultural residues (bagasse, corn stover and corn hulls). The ethanol productivity by the recombinant E. coli FBR5 was decreased by 36% when the additional ethanol concentration was increased from 1.5 to 2.5%.35
Lawford and Rousseau7 investigated the factors contributing to the loss of ethanologenicity of E. coli B recombinants pLOI297 and K011 employing glucose- or xylose limited chemostat cultures. Both recombinant strains carry markers (cat gene) for antibiotic resistance. Strain KO11 expressed high levels of cat and was a spontaneous mutant that was selected to high levels (600 ∝gmL-1) of chloroamphenicol. However, both recombinant strains exhibited rapid loss of ethanologenicity in chemostat cultures with glucose even when the selection pressure was imposed by the inclusion of antibiotics in the feed medium. Under xylose limitation, the plasmid-bearing recombinant E. coli strains appeared to be stabilized by antibiotics. These authors concluded that based on an average cost for large bulk quantities of antibiotics at $55 kg-1 and an inclusion level of 40 mgL-1 of fermentation medium, the estimated economic impact regarding the potential stabilization by antibiotics in a plant operating in batch mode, the antibiotics cost can be $0.29 gal-1 of ethanol for antibiotic addition in all fermentation media. Thus, the stable nature of E. coli strain FBR5 without antibiotics is advantageous for industrial production of ethanol from a lignocellulosic hydrolyzate as the antibiotic use will not only add extra cost but also create environmental problems.
Martin et al.34 suggests that if the E. coli FBR5 would have lost the plasmid, the revertant strain would simply be the host strain NZN111. This host strain does not produce ethanol.11,12 In conclusion, the recombinant E. coli FBR5 performed very well over the periods studied with respect to stability and viability without any antibiotics. The information is very important for the use of the recombinant bacterium for continuous production of ethanol from lignocellulosic hydrolyzates. Based on the results of the continuous culture experiments, we have studied the production of ethanol by the recombinant strain E. coli FBR5 at the pilot scale (100 L) level using dilute acid pretreated wheat straw as a feedstock in order to demonstrate the performance of the strain for large scale ethanol production from lignocellulosic hydrolyzates. The results have not yet published.
Concluding Remarks and Future Directions for Research
The recombinant E. coli strain FBR5 performs well in the absence of antibiotics. Under proper conditions, it can ferment all sugars including xylose and arabinose to ethanol quantitatively and is also somewhat tolerant to common fermentation inhibitors such as furfural, HMF and acetic acid. It works better at neutral or near neutral pH range which makes the SSF process using the recombinant bacterium to run at a compromising pH 6.0. In comparison, the conventional yeast performs optimally at pH 4.5–5.0 which is also the optimum pH for action of cellulase and hemicellulase enzymes. The cost of enzymes remains a concern. Approaches can be taken to clone and express cellulase enzymes in the ethanologenic recombinant E. coli strain. The maximum ethanol tolerance of recombinant E. coli strain FBR5 is 50 gL-1. Research needs to be performed to increase the ethanol tolerance of the bacterium. Even though considerable research has been done on the production of ethanol by the recombinant bacterial strain, no pilot plant demonstration of the fermentation process has been performed. To our knowledge, no studies have been done on the optimization of fermentation medium components which is also an essential part to lower the ethanol production cost. Moreover, there is no data available on the utilization of the post fermentation left-over materials including the E. coli cells for use as animal feed or for generating electricity after the product ethanol has been removed from the fermentation broth by distillation. These issues need to be addressed not only for ethanologenic recombinant E. coli strains but also for recombinant S. cerevisiae before their use industrially.
Note
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/19874
References
- 1.Dinneen B. Ethanol Industry Outlook, Renewable Fuels Association, Washington DC 2011. [Google Scholar]
- 2.Saha BC. Hemicellulose bioconversion. J Ind Microbiol Biotechnol. 2003;30:279–91. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- 3.Saha BC. Lignocellulose biodegradation and applications in biotechnology. In: Saha BC, Hayashi K, (Eds). Lignocellulose biodegradation. American Chemical Society, Washington DC 2004; 2-34. [Google Scholar]
- 4.Bothast RJ, Nichols NN, Dien BS. Fermentations with new recombinant organisms. Biotechnol Prog. 1999;15:867–75. doi: 10.1021/bp990087w. [DOI] [PubMed] [Google Scholar]
- 5.Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF. Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol. 1987;53:2420–5. doi: 10.1128/aem.53.10.2420-2425.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta K, Beall DS, Mejia JP, Shanmugam KT, Ingram LO. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase II. Appl Environ Microbiol. 1991;57:893–900. doi: 10.1128/aem.57.4.893-900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawford HG, Rousseau JD. Factors contributing to the loss of ethanologenicity of Escherichia coli B recombinants pL0I297 and KO11. Appl Biochem Biotechnol. 1996;57-58:293–305. doi: 10.1007/BF02941709. [DOI] [PubMed] [Google Scholar]
- 8.Hespell RB, Wyckoff H, Dien BS, Bothast RJ. Stabilization of pet operon plasmids and ethanol production in Escherichia coli strains lacking lactate dehydrogenase and pyruvate formate-lyase activities. Appl Environ Microbiol. 1996;62:4594–7. doi: 10.1128/aem.62.12.4594-4597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mat-Jan F, Alam KY, Clark DP. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol. 1989;171:342–8. doi: 10.1128/jb.171.1.342-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dien BS, Hespell RB, Wyckoff HA, Bothast RJ. Fermentation of hexose and pentose sugars using a novel ethanologenic Escherichia coli strain. Enzyme Microb Technol. 1998;23:366–71. doi: 10.1016/S0141-0229(98)00064-7. [DOI] [Google Scholar]
- 11.Dien BS, Nichols NN, O’Bryan PJ, Bothast RJ. Development of new ethanologenic Escherichia coli strains for fermentation of lignocellulosic biomass. Appl Biochem Biotechnol. 2000;84-86:181–96. doi: 10.1385/ABAB:84-86:1-9:181. [DOI] [PubMed] [Google Scholar]
- 12.Bunch PK, Mat-Jan F, Lee N, Clark DP. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology. 1997;143:187–95. doi: 10.1099/00221287-143-1-187. [DOI] [PubMed] [Google Scholar]
- 13.Nichols NN, Dien BS, Bothast RJ. Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Appl Microbiol Biotechnol. 2001;56:120–5. doi: 10.1007/s002530100628. [DOI] [PubMed] [Google Scholar]
- 14.Dien BS, Cotta MA, Jeffries TW. Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol. 2003;63:258–66. doi: 10.1007/s00253-003-1444-y. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi N, Dien BS, Nichols NN, Saha BC, Cotta MA. Genetically engineered Escherichia coli for ethanol production from xylose substrate and product inhibition and kinetic parameters. Trans IChemE. Part C Food and Bioproducts Processing. 2006;84:114–22. doi: 10.1205/fbp.05038. [DOI] [Google Scholar]
- 16.Saha BC, Iten LB, Cotta MA, Wu YV. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 2005;40:3693–700. doi: 10.1016/j.procbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Saha BC, Cotta MA. Enzymatic hydrolysis and fermentation of lime pretreated wheat straw to ethanol. J Chem Technol Biotechnol. 2007;82:913–9. doi: 10.1002/jctb.1760. [DOI] [Google Scholar]
- 18.Saha BC, Cotta MA. Ethanol production from alkaline peroxide pretreated enzymatically saccharified wheat straw. Biotechnol Prog. 2006;22:449–53. doi: 10.1021/bp050310r. [DOI] [PubMed] [Google Scholar]
- 19.Saha BC, Biswas A, Cotta MA. Microwave pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. J Biobased Materials Bioenergy. 2008;2:210–7. doi: 10.1166/jbmb.2008.412. [DOI] [Google Scholar]
- 20.Wingren A, Galbe M, Zacchi G. Techno-economic evaluation of producing ethanol from softwood: comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog. 2003;19:1109–17. doi: 10.1021/bp0340180. [DOI] [PubMed] [Google Scholar]
- 21.Saha BC, Cotta MA. Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy. 2009;32:971–7. doi: 10.1016/j.biombioe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Saha BC, Iten LB, Cotta MA, Wu YV. Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol Prog. 2005;21:816–22. doi: 10.1021/bp049564n. [DOI] [PubMed] [Google Scholar]
- 23.Saha BC, Cotta MA. Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzyme Microb Technol. 2007;41:528–32. doi: 10.1016/j.enzmictec.2007.04.006. [DOI] [Google Scholar]
- 24.Saha BC, Cotta MA. Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. N Biotechnol. 2010;27:10–6. doi: 10.1016/j.nbt.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi DF, Carvalhal ML, Alterthum F. Ethanol production from pentose and hexoses by recombinant Escherichia coli. Biotechnol Lett. 1994;16:747–50. doi: 10.1007/BF00136484. [DOI] [Google Scholar]
- 26.Saha BC, Nichols NN, Qureshi N, Cotta MA. Comparison of separate hydrolysis and fermentation and simultaneous saccharification and fermentation processes for ethanol production from wheat straw by recombinant Escherichia coli strain FBR5. Appl Microbiol Biotechnol. 2011;92:865–74. doi: 10.1007/s00253-011-3600-0. [DOI] [PubMed] [Google Scholar]
- 27.Nichols NN, Dien BS, Guisado GM, López MJ. Bioabatement to remove inhibitors from biomass-derived sugar hydrolysates. Appl Biochem Biotechnol. 2005;121-124:379–90. doi: 10.1385/ABAB:121:1-3:0379. [DOI] [PubMed] [Google Scholar]
- 28.Galbe M, Sassner P, Wingren A, Zacchi G. Process engineering economics of bioethanol production. Adv Biochem Eng Biotechnol. 2007;108:303–27. doi: 10.1007/10_2007_063. [DOI] [PubMed] [Google Scholar]
- 29.Zacchi G, Axelsson A. Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol Bioeng. 1989;34:223–33. doi: 10.1002/bit.260340211. [DOI] [PubMed] [Google Scholar]
- 30.Saha BC, Nichols NN, Cotta MA. Ethanol production from wheat straw by recombinant Escherichia coli strain FBR5 at high solid loading. Bioresour Technol. 2011;102:10892–7. doi: 10.1016/j.biortech.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 31.Liu T, Lin L, Hu R, Liu S. A study on strain adaptation of recombinant E. coli FBR5 challenged by concentrated wood hydrolyzate. J Biobased Materials Bioenergy. 2009;3:386–92. doi: 10.1166/jbmb.2009.1046. [DOI] [Google Scholar]
- 32.Liu T, Lin L, Sun Z, Hu R, Liu S. Bioethanol fermentation by recombinant E. coli FBR5 and its robust mutant FBRH using hot-water wood extract hydrolyzate as substrate. Biotechnol Adv. 2010;28:601–8. doi: 10.1016/j.biotechadv.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Sanny T, Arnaldos M, Kunkel SA, Pagilla KR, Stark BC. Engineering of ethanolic E. coli with the Vitreoscilla hemoglobin gene enhances ethanol production from both glucose and xylose. Appl Microbiol Biotechnol. 2010;88:1103–12. doi: 10.1007/s00253-010-2817-7. [DOI] [PubMed] [Google Scholar]
- 34.Martin GJO, Knepper A, Zhou B, Pamment NB. Performance and stability of ethanologenic Escherichia coli strain FBR5 during continuous culture on xylose and glucose. J Ind Microbiol Biotechnol. 2006;33:834–44. doi: 10.1007/s10295-006-0129-9. [DOI] [PubMed] [Google Scholar]
- 35.Saha BC, Cotta MA. Continuous ethanol production from wheat straw hydrolysate by recombinant ethanologenic Escherichia coli strain FBR5. Appl Microbiol Biotechnol. 2011;90:477–87. doi: 10.1007/s00253-010-3082-5. [DOI] [PubMed] [Google Scholar]
- 36.Asghari A, Bothast RJ, Doran JB, Ingram LO. Ethanol production from hemicellulose hydrolysates of agricultural residues using genetically engineered Escherichia coli strain K011. J Ind Microbiol. 1996;16:42–7. doi: 10.1007/BF01569920. [DOI] [Google Scholar]


