Abstract
Genetic bioaugmentation is an in situ bioremediation method that stimulates horizontal transfer of catabolic plasmids between exogenous donor cells and indigenous bacteria to increase the biodegradation potential of contaminants. A critical outcome of genetic bioaugmentation is the expression of an active catabolic phenotype upon plasmid conjugation. Using a pWW0-derivative TOL plasmid, we showed that certain genetic characteristics of the recipient bacteria, including genomic guanine-cytosine (G + C) content and phylogeny, may limit the expression of the transferred catabolic pathway. However, such genetic limitations observed in transconjugants could be overcome by the presence of an additional carbon source. Glucose and Luria-Bertani broth were shown to enhance the toluene degradation rates of transconjugants; these enhancement effects were dependent on transconjugant genomic G + C contents. Based on these observations, thorough genetic characterization of the indigenous microbial community in the contaminated environment of interest may provide a predictive tool for assessing the success of genetic bioaugmentation.
Keywords: TOL plasmid, bioremediation, carbon amendment, genetic bioaugmentation, guanine-cytosine content, plasmid conjugation
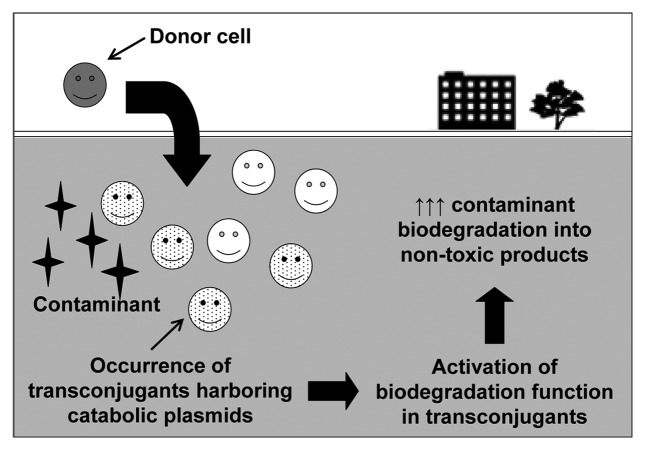
Horizontal gene transfer (HGT) is a widespread phenomenon in the prokaryotic kingdom that occurs readily under harsh environments where genetic adaptation is required for the survival of microorganisms.1-3 As previously suggested by Top et al. (2002),4 HGT could be useful for bioremediation to shift microbial communities in favor of degrading contaminants of concern. In particular, plasmid conjugal transfer is one of the dominant and well-studied mechanisms of HGT in bacteria, by which resistance to toxicants such as metals and antibiotics as well as the ability to degrade complex organic compounds may be spread.1 In genetic bioaugmentation, introduction of environmentally-relevant bacteria harboring self-transmissible catabolic plasmids that encode for the degradation of target contaminants stimulates in situ HGT of the plasmids to indigenous microorganisms, thus resulting in an enhanced contaminant degradation potential at the polluted site (Fig. 1). Genetic bioaugmentation aims to transfer relevant genes into indigenous microorganisms that have greater fitness for survival in the contaminated environments as opposed to conventional bioaugmentation which relies on the survival of exogenous microorganisms. In fact, HGT has been reported to repeatedly occur naturally in contaminated sites to aid in bacterial adaptation to organic pollutants;5 therefore, genetic bioaugmentation aims to enhance the naturally-occurring phenomenon to further improve the biodegradation of target contaminants. This approach is likely to receive much more favorable adoption than the use of genetically engineering microorganisms (GEMs) as GEMs are heavily regulated by the US Environmental Protection Agency for in situ bioremediation. To date, GEMs have only been approved for a limited number of cases.6 However, prior to designing genetic bioaugmentation treatment schemes, the mechanisms and effects of HGT must be extensively characterized.
Figure 1.
Genetic bioaugmentation schematic.
The success of genetic bioaugmentation depends on two critical factors: the successful transfer of the catabolic plasmid to as many indigenous bacteria as possible, and an active contaminant-degrading phenotype in all transconjugants. While there have been previous reports that studied the conjugal transfer of plasmids in various environments, the phenotypic outcome of these conjugation events have been largely ignored. In some studies, the catabolic activity encoded on the transferred plasmid was shown to be nonexistent or greatly limited.7-9 Furthermore, many studies on plasmid conjugal transfer were limited to only one or two recipient bacterial strains and do not provide representative observations for environmental applications. As successful implementation of genetic bioaugmentation heavily relies on an overall increase in catabolic activity of the in situ bacterial community following conjugal transfer, there is a strong need for a thorough investigation on the necessary environmental conditions that trigger efficient expression of transferred catabolic genes.
The effects of various environmental and biological conditions on the TOL plasmid functions of eight transconjugant bacterial strains and the donor strain harboring the TOLgfpmut3b plasmid10 were tested. Recipient genetic characteristics including the genomic guanine-cytosine (G + C) content and phylogenetic relationships were varied as the biological conditions tested. Six of the transconjugants including Escherichia sp TOL, Escherichia coli RP-1,11 Pantoea agglomerans TOL, Enterobacter cloacae TOL, E. coli TOL, and Serratia marcescens TOL belonged to the Enterobacteriaceae family. Pseudomonas fluorescens TOL and Pseudomonas putida TOL were Pseudomonadaceae strains similar to the donor bacterium, P. putida BBC443. These strains had genomic G + C contents ranging from 50 to 60% and were chosen based on their ability to take up the TOLgfpmut3b plasmid. The Enterobacteriaceae transconjugant strains exhibited very limited TOL plasmid functions when toluene was added as the sole carbon source. Such limitations were shown to be due, at least in part, to differences in genomic G + C contents compared with the TOL plasmid average G + C content of 59% as well as the relatively large phylogenetic distances from the donor strain. Following these observations, environmental conditions including differences in pH and the availability of carbon and nitrogen were altered to determine whether the observed genetic limitations on the expression of the catabolic phenotype could be relieved by external changes. Of the environmental factors tested, varying pH (6–8) and nitrogen sources (ammonium sulfate and sodium nitrate) had small and variable effects on the toluene degradation rates of transconjugant strains. On the other hand, when cells were exposed simultaneously to toluene and 1 g/L glucose, a large enhancement of specific toluene degradation rates as well as enzymatic activities and expression of genes encoded by the TOL plasmid were observed in all transconjugant strains tested. These results suggest that the addition of an alternative easily-biodegradable carbon source such as glucose may increase the diversity of transconjugants that have active catabolic phenotypes following conjugal plasmid transfer, and thereby, improve the effectiveness of genetic bioaugmentation.
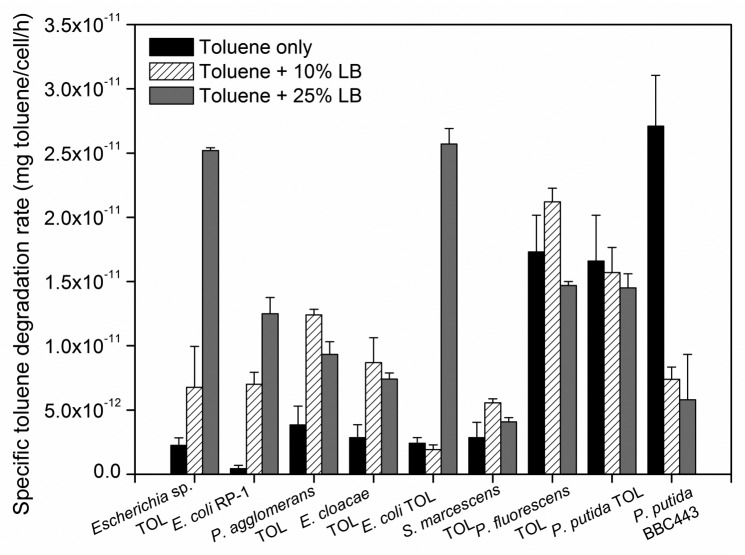
In addition to glucose, the effect of Luria-Bertani (LB) broth as the alternative easily degradable carbon source was investigated. The simultaneous exposure of cells to 10 or 25% LB broth resulted in enhanced specific toluene degradation rates in all Enterobacteriaceae transconjugants (Fig. 2). The enhancement effect of LB broth was as high as 16.0 (± 2.11) and 46.5 (± 10.8) fold for 10 and 25%, respectively. These increases were similar to or higher than the effects of 1 g/L glucose for these strains. However, unlike glucose addition, LB broth exposure resulted in no significant change in specific toluene degradation rates in Pseudomonadaceae transconjugants (Fig. 2), suggesting that the enhancement effects of glucose and LB broth work through different mechanisms. In fact, LB broth is composed of mainly amino acids instead of simple sugars12 and differences in physiology of E. coli cells growing in the presence of LB broth and minimal medium with glucose have been reported in reference 13. In our previous paper, we speculated that LB broth altered the translation or post-translational control of the production of proteins encoded by the TOL plasmid whereas glucose likely affected the transcription of TOL plasmid genes.7 Such mechanistic differences may account for the discrepancy observed in the responses between Enterobacteriaceae and Pseudomonadaceae strains. Furthermore, as both simple sugars and amino acids appear to have an enhancing effect on toluene degradation rates of Enterobacteriaceae transconjugants, it may be unnecessary for a large-scale addition of organic carbon substrates in a field implementation of genetic bioaugmentation for the cleanup of contaminated soils. Soil organic matter (SOM) contains both amino acids and simple sugars; in some soils, amino acids are the dominant form of organic carbon14 while in others, simple sugars account for the majority.15 Thus, the presence of SOM may be sufficient to improve the catabolic activities of the newly-formed transconjugants in situ. However, the addition of glucose may be favorable in some cases to ensure that the enhancement effect occurs widely across different groups of bacteria.
Figure 2.
Specific toluene degradation rates of TOL plasmid-bearing strains exposed to toluene in the presence of 0, 10 and 25% (v/v) LB broth. Error bars represent the standard deviation of at least triplicate samples.
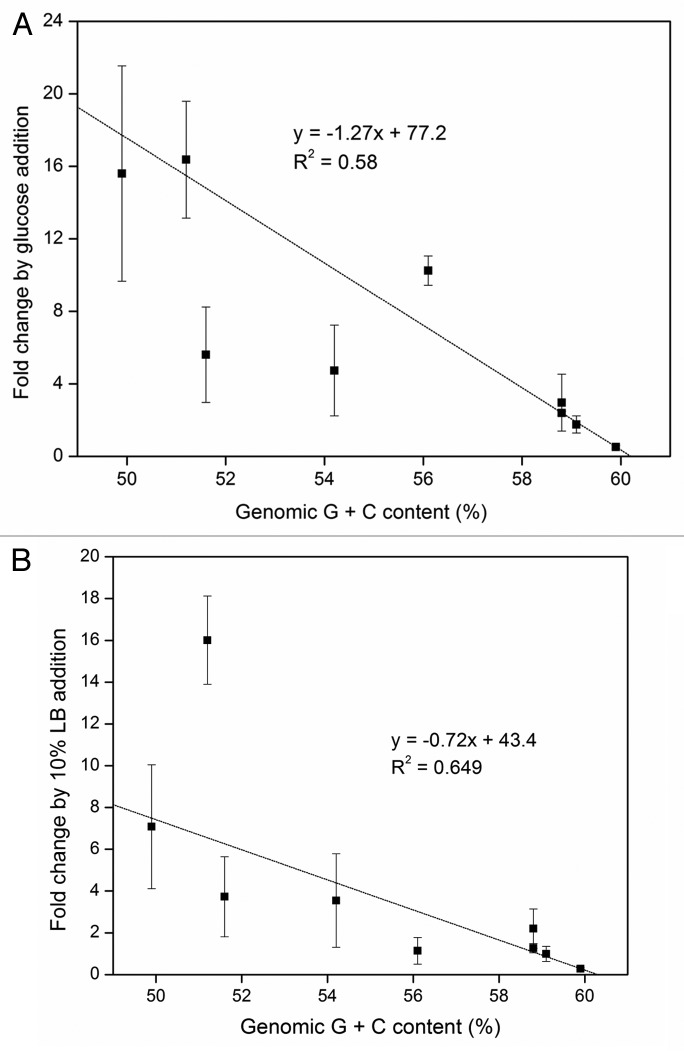
A closer examination of the specific toluene degradation rates revealed that the fold changes induced by glucose addition had a negative linear correlation with genomic G + C content with an R-square value of 0.58 (p < 0.0001) (Fig. 3A). Similarly, the presence of 10% LB broth induced fold changes in specific toluene degradation rates that were negatively correlated with genomic G + C content (R-square value of 0.65; p < 0.01) (Fig. 3B). Glucose appeared to have a stronger correlation with genomic G + C content compared to LB broth. These observations indicate that glucose and LB broth, albeit to different degrees, had larger positive effects on strains with lower genomic G + C contents. We showed that the toluene degradation rates of transconjugant strains in the absence of additional carbon sources were dependent on genomic G + C content, although lacking a clear correlation function.16 However, the limited toluene degradation rates of the tested strains were significantly enhanced by the simultaneous presence of an easily biodegradable carbon source.16 Our results in Figure 2 further imply that the enhancement effects of glucose and LB broth may be larger in transconjugant strains with larger deviation in genomic G + C content from the TOL plasmid average of 59%. On the other hand, the fold changes by glucose or LB broth addition had no correlation with the phylogenetic distance of the transconjugants from the donor strains (data not shown). These findings along with observations discussed above suggest that while phylogenetic relatedness may have stronger impacts on the TOL plasmid phenotype functionality compared with differences in genomic G + C contents in the presence of toluene alone,16 the enhancement by carbon source addition may only directly relieve the phenotypic limitations brought upon by G + C content differences. Furthermore, glucose appeared to have a larger effect on transconjugants with lower genomic G + C contents compared with LB broth, suggesting that glucose would be a more favorable carbon source to add to stimulate genetic bioaugmentation of the TOL plasmid in microbial communities that have lower G + C contents. Additional experiments should be performed under environmentally relevant conditions to further test this observation.
Figure 3.
Fold changes in specific toluene degradation rates induced by the addition of 1 g/L glucose (A) and 10% LB broth (B) in strains with varying genomic G + C contents. Error bars represent the standard deviation of at least triplicate samples.
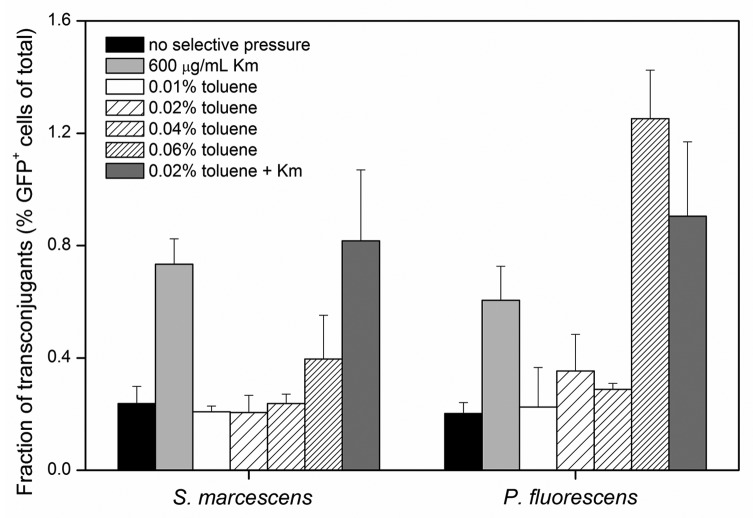
The choice of an appropriate selective pressure that promotes HGT is an important parameter to consider for the successful design of genetic bioaugmentation treatment schemes. In the present study, two recipient bacterial strains, S. marcescens and P. fluorescens, were mated in soil slurry batch reactors with donor P. putida BBC443 bacteria under varying additions of toluene and the antibiotic, kanamycin (Km), as the selective pressures (Fig. 4). The occurrence of transconjugants was monitored by screening for cells expressing green fluorescent protein as encoded by the TOLgfpmut3b plasmid. At least 50% of recipient S. marcescens and P. fluorescens cells were verified to be culturable under these conditions (data not shown). Exposure to toluene as the sole selective pressure resulted in no increase in the percentage of transconjugants except at extremely high concentrations, while the presence of Km alone resulted in transconjugant occurrence that was similar to samples exposed to both Km and 0.02% (v/v) toluene. Toluene degradation occurred in most batches within 24 h (data not shown). These results suggest that at environmentally relevant concentrations, toluene alone may not act as a sufficient selective pressure for the conjugal transfer of the TOL plasmid. The effectiveness of contaminants as a selective pressure for the conjugation of catabolic plasmids may differ significantly depending on the compound and plasmid. As toluene is a contaminant that can be rapidly degraded through various mechanisms, it may exert insufficient stress on cells to act as selective pressure for plasmid uptake. While certain organic contaminants, such as biphenyl17 and 2, 4-D,18,19 have been shown to exert selective pressure for conjugal transfer of catabolic plasmids, the necessary properties that allow a contaminant to act as sufficient selective pressure are unclear. Additional experiments to investigate the chemical and toxicological characteristics of organic contaminants that effectively exert selective pressure may be helpful to determine the triggers necessary for successful genetic bioaugmentation.
Figure 4.
Transconjugant occurrence under varying selective pressures in soil slurry batch mating experiments. S. marcescens and P. fluorescens were used as recipient strains and mated with donor P. putida BBC443 cells in soil slurry under the presence or absence of Km and toluene. Error bars represent standard deviations of triplicate samples.
Future Outlook
Our results indicated that differences in certain genetic characteristics between the recipient bacterial strain and the donor strain or the catabolic plasmid itself may severely limit the expression of the catabolic phenotype following conjugal plasmid transfer. While we could not determine the individual effects of genomic G + C content and phylogenetic relationships, these genetic characteristics are considered interdependent20,21 and therefore, may need to be considered as a combinatorial effect. However, the genetic limitations on phenotype expression were alleviated by the simultaneous addition of an alternative easily biodegradable carbon source. Both simple sugars (glucose) and amino acid-based rich medium (LB broth) showed an enhancement effect on the toluene degradation rates of most transconjugant strains tested in this study. These effects were observed to be negatively correlated with the genomic G + C content of transconjugants, indicating that genetic characteristics may be important not only in the initial expression but also in the stimulation of the transferred catabolic phenotype functions. More information is necessary to determine whether such impacts of transconjugant genetic characteristics are observable in other catabolic plasmid systems. In particular, catabolic plasmids that have broader host ranges than the TOL plasmid should be studied in depth to provide a thorough assessment of the effectiveness of genetic bioaugmentation. Finally, the choice of appropriate in situ selective pressure is critical in ensuring the successful conjugal transfer of the target catabolic plasmids to indigenous bacteria. Overall, together with the growing body of bacterial genome sequences and ever-improving phylogenetic characterization techniques, it may become possible to predict the necessary operational parameters and the overall success of genetic bioaugmentation in a given contaminated environment through the genetic information gathered from the indigenous microbial community.
Acknowledgments
This work is based upon work supported by the National Science Foundation under Grant No. CBET-0846437. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. The authors would like to thank Dr. Søren Molin for the generous donation of P. putida strain BBC443 and the Duke University Biology Department for the donotion of S. marcescens. Special thanks also go to Kevin He and Christina Arnaout for their assistance in the laboratory.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/20551
References
- 1.Sørensen SJ, Bailey M, Hansen LH, Kroer N, Wuertz S. Studying plasmid horizontal transfer in situ: a critical review. Nat Rev Microbiol. 2005;3:700–10. doi: 10.1038/nrmicro1232. [DOI] [PubMed] [Google Scholar]
- 2.Gogarten JP, Townsend JP. Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol. 2005;3:679–87. doi: 10.1038/nrmicro1204. [DOI] [PubMed] [Google Scholar]
- 3.Kurland CG, Canback B, Berg OG. Horizontal gene transfer: a critical view. Proc Natl Acad Sci U S A. 2003;100:9658–62. doi: 10.1073/pnas.1632870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Top EM, Springael D, Boon N. Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol Ecol. 2002;42:199–208. doi: 10.1111/j.1574-6941.2002.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 5.Top EM, Springael D. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr Opin Biotechnol. 2003;14:262–9. doi: 10.1016/S0958-1669(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 6.Sayler GS, Ripp S. Field applications of genetically engineered microorganisms for bioremediation processes. Curr Opin Biotechnol. 2000;11:286–9. doi: 10.1016/S0958-1669(00)00097-5. [DOI] [PubMed] [Google Scholar]
- 7.Ikuma K, Gunsch CK. Effect of carbon source addition on toluene biodegradation by an Escherichia coli DH5alpha transconjugant harboring the TOL plasmid. Biotechnol Bioeng. 2010;107:269–77. doi: 10.1002/bit.22808. [DOI] [PubMed] [Google Scholar]
- 8.Sayler GS, Hooper SW, Layton AC, King JMH. Catabolic plasmids of environmental and ecological significance. Microb Ecol. 1990;19:1–20. doi: 10.1007/BF02015050. [DOI] [PubMed] [Google Scholar]
- 9.Benson S, Shapiro J. TOL is a broad-host-range plasmid. J Bacteriol. 1978;135:278–80. doi: 10.1128/jb.135.1.278-280.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen BB, Sternberg C, Andersen JB, Eberl L, Moller S, Givskov M, et al. Establishment of new genetic traits in a microbial biofilm community. Appl Environ Microbiol. 1998;64:2247–55. doi: 10.1128/aem.64.6.2247-2255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei R, Gunsch CK. Plasmid conjugation in an activated sludge microbial community. Environ Eng Sci. 2009;26:825–31. doi: 10.1089/ees.2008.0236. [DOI] [Google Scholar]
- 12.Sezonov G, Joseleau-Petit D, D’Ari R. Escherichia coli physiology in Luria-Bertani broth. J Bacteriol. 2007;189:8746–9. doi: 10.1128/JB.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao H, Bausch C, Richmond C, Blattner FR, Conway T. Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J Bacteriol. 1999;181:6425–40. doi: 10.1128/jb.181.20.6425-6440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer H, Meyer A, Fischer K, Kuzyakov Y. Carbohydrate and amino acid composition of dissolved organic matter leached from soil. Soil Biol Biochem. 2007;39:2926–35. doi: 10.1016/j.soilbio.2007.06.014. [DOI] [Google Scholar]
- 15.Hertenberger G, Zampach P, Bachmann G. Plant species affect the concentration of free sugars and free amino acids in different types of soil. J Plant Nutr Soil Sci. 2002;165:557–65. doi: 10.1002/1522-2624(200210)165:5<557::AID-JPLN1111557>3.0.CO;2-G. [DOI] [Google Scholar]
- 16.Ikuma K, Gunsch CK. Functionality of the TOL plasmid under varying environmental conditions following conjugal transfer. Appl Microbiol Biotechnol. 2012 doi: 10.1007/s00253-012-3949-8. In press. [DOI] [PubMed] [Google Scholar]
- 17.De Rore H, Demolder K, De Wilde K, Top EM, Houwen F, Verstraete W. Transfer of the catabolic plasmid RP4:Tn4371 to indigenous soil bacteria and its effect on respiration and biphenyl breakdown. FEMS Microbiol Ecol. 1994;15:71–7. doi: 10.1016/0168-6496(94)90027-2. [DOI] [Google Scholar]
- 18.DiGiovanni GD, Neilson JW, Pepper IL, Sinclair NA. Gene transfer of Alcaligenes eutrophus JMP134 plasmid pJP4 to indigenous soil recipients. Appl Environ Microbiol. 1996;62:2521–6. doi: 10.1128/aem.62.7.2521-2526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neilson JW, Josephson KL, Pepper IL, Arnold RB, Di Giovanni GD, Sinclair NA. Frequency of horizontal gene transfer of a large catabolic plasmid (pJP4) in soil. Appl Environ Microbiol. 1994;60:4053–8. doi: 10.1128/aem.60.11.4053-4058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muto A, Osawa S. The guanine and cytosine content of genomic DNA and bacterial evolution. Proc Natl Acad Sci U S A. 1987;84:166–9. doi: 10.1073/pnas.84.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi M, Kryukov K, Saitou N. Estimation of bacterial species phylogeny through oligonucleotide frequency distances. Genomics. 2009;93:525–33. doi: 10.1016/j.ygeno.2009.01.009. [DOI] [PubMed] [Google Scholar]