Abstract
OBJECTIVE
Sleep disorders and subjective sleep complaints have been associated with increased risk of type 2 diabetes. The evidence with respect to insulin resistance (IR) and insulin secretion in individuals without type 2 diabetes has been scarce and elusive. We examined if subjective sleep complaints and their co-occurrence were associated with IR and insulin secretion in adult women and men without diabetes.
RESEARCH DESIGN AND METHODS
Women (n = 442) and men (n = 354) 18–75 years of age without type 2 diabetes underwent an oral glucose tolerance test (OGTT), with insulin and glucose measured at fasting and at 30 and 120 min. Complaints related to sleep apnea, insomnia, and daytime sleepiness were self-rated with the Basic Nordic Sleep Questionnaire.
RESULTS
In comparison with individuals with no or minor sleep complaints, those with more frequent complaints of sleep apnea, insomnia, and daytime sleepiness were more insulin resistant, as evidenced by higher fasting insulin concentrations and insulin and glucose responses to OGTT, and more frequently had high homeostasis model assessment of IR and low insulin sensitivity index values. The likelihood of being insulin resistant increased significantly and linearly according to the accumulation of co-occurring sleep complaints. These associations changed only a little when adjusted for mediating and confounding factors and for depressive symptoms. Sleep complaints were not associated with indices of deficiency in insulin secretion.
CONCLUSIONS
Subjective sleep complaints were associated with IR. The likelihood of being insulin resistant increased according to accumulation of co-occurring sleep complaints. Sleep complaints were not associated with deficiency in insulin secretion.
In several cross-sectional and prospective studies, sleep apnea, sleep disordered breathing, habitual snoring, insomnia, difficulties in initiating and maintaining sleep, and daytime sleepiness have been associated with the prevalence and the incidence of type 2 diabetes (1–6). Although these findings suggest that sleep disorders and subjective sleep complaints may carry an increased risk for type 2 diabetes, the evidence with respect to insulin resistance (IR) and insulin secretion, two major features of type 2 diabetes, in individuals without type 2 diabetes has been scarce and elusive.
In individuals without a history of or concurrently diagnosed type 2 diabetes, polysomnography-based sleep disordered breathing was associated with impaired fasting glucose (IFG), impaired glucose tolerance (IGT) (2), a higher degree of IR (7,8), and a decreased degree of insulin sensitivity and pancreatic β-cell function during a frequently sampled intravenous glucose tolerance test (9). However, subjective complaints of sleep apnea, habitual snoring, and daytime sleepiness were not associated with IFG, IGT, and IR (10). Finally, a recent study has reported that subjective complaints of frequent snoring were not associated with fasting insulin, glucose, and IR, whereas insomnia, a combined measure of actigraphy-based sleep latency and fragmentation, and subjective complaints were associated with lower fasting insulin values and a lower likelihood of being insulin resistant (4).
We studied whether subjective complaints of sleep apnea (habitual snoring and sleep disordered breathing), insomnia (difficulties in initiating and/or maintaining sleep), and daytime sleepiness were associated with IR and insulin secretion in a population-based sample of 18–75-year-old Finnish women and men without a history of or concurrently diagnosed type 2 diabetes. Our study contributes to previous studies in two ways. First, we tested if the degree of glycemia and IR increased according to accumulation of subjective sleep complaints that often co-occur together (11,12). Second, we tested if symptoms of depression accounted for the associations. The latter was seen as relevant since sleep complaints may indicate the presence of depression (13), and symptoms of depression have been linked with the prevalence and the incidence of type 2 diabetes (14,15), and with IR in populations without type 2 diabetes (16).
RESEARCH DESIGN AND METHODS
Participants
The population-based Prevalence, Prediction, and Prevention of Diabetes (PPP)–Botnia Study has been described in detail elsewhere (16–19). Of the 9,518 invited individuals, 5,208 (2,443 men and 2,765 women, 55%) participated. A psychological survey including questions on sleep complaints was added later to the study protocol and administered to 1,335 consecutive individuals (59.8% of 2,232) recruited from the Vasa area. A total of 1,066 (79.9%) individuals returned the questionnaire, with 949 (535 women and 414 men) providing complete data on subjective sleep complaints. Of these, we excluded 47 with previously and 24 with newly diagnosed diabetes. An additional 82 individuals had missing oral glucose tolerance test (OGTT) values of insulin/glucose. In total, 796 participants (442 women and 354 men) without type 2 diabetes had complete data available on all study variables. The included participants differed from the entire PPP-Botnia sample without type 2 diabetes (n = 3,885 after excluding 257 with history of/new diabetes) by being older, more frequently retired, consuming more alcohol, reporting regular exercise more frequently, and having higher fasting and 120-min glucose. All participants gave their written informed consent, and the study protocol was approved by the Ethics Committee of the Helsinki University Central Hospital, Finland.
Insulin resistance and insulin secretion
The subjects participated in an OGTT by ingesting 75 g of glucose after a 12-h overnight fast. During the OGTT, venous samples for plasma glucose and serum insulin were drawn at 0, 30, and 120 min. The homeostasis model assessment of IR method (HOMA-IR) (20), insulin sensitivity index (ISI) (21), corrected insulin response (CIR) (22), and disposition index (DI) (23) were used as indices of IR and insulin secretion. The following formulas were used to calculate these variables: HOMA-IR = (fasting plasma insulin [mU/L] × fasting plasma glucose level [mmol/L])/22.5; ISI = 10,000/√(fasting plasma glucose level [mmol/L] × fasting insulin [mU/L]) × (mean OGTT glucose [mmol/L] × mean OGTT insulin [mU/L]); CIR = (100 × insulin [mU/L] at 30 min)/glucose (mmol/L) at 30 min × (glucose [mmol/L] at 30 min – 3.89 mmol/L); and DI = CIR × ISI. Area under the curve (AUC) of insulin and AUC glucose were calculated as follows: AUC insulin = 15 × fasting plasma insulin (mU/L) + 15 × insulin (mU/L) at 30 min + 45 × insulin (mU/L) at 30 min + 45 × insulin (mU/L) at 120 min; AUC glucose = 15 × fasting plasma glucose (mmol/L) + 15 × glucose (mmol/L) at 30 min + 45 × glucose (mmol/L) at 30 min + 45 × glucose (mmol/L) at 120 min.
Assays
Plasma glucose was measured with a glucose dehydrogenase method (HemoCue, Ängelholm, Sweden) and serum insulin by a fluoroimmunoassay (Delphia; Perkin-Elmer Finland, Turku, Finland).
Sleep complaints
Self-reported complaints of sleep apnea, insomnia, and daytime sleepiness the previous 3 months were assessed with the Basic Nordic Sleep Questionnaire (24). Complaints of sleep apnea (frequency and quality of snoring and frequency of breathing pauses), insomnia (frequency of difficulties in falling asleep and maintaining sleep and frequency of awakenings per night), and daytime sleepiness (frequency of feeling excessively sleepy in the morning after awakening, during daytime, and napping) were rated on a scale ranging from never or less than once per month (1) to every day/night or almost every day/night per week (5); the quality of snoring was assessed using a scale ranging from “I don’t snore” (1) to “I snore very loud and intermittently (there are silent breathing pauses when snoring is not heard and at times very loud snorts with gasping)” (5), and frequency of awakenings during one night was assessed using a scale ranging from “I do not wake up at night” (1) to at least five times per night (5).
Answers to questions on sleep apnea, insomnia, and daytime sleepiness were summed, and the top quartile was used as a cutoff for identifying individuals with more frequent/severe complaints. The group whose complaints of sleep apnea, insomnia, and daytime sleepiness fell below the top quartile and who also reported using no sleeping pills (9.4%, n = 75, reported using sleeping pills) was used as the reference group (from here on referred to as “no or minor sleep complaints”).
Mediating and confounding factors
The subjects self-reported their weekly alcohol consumption (g/week), current smoking status (yes vs. no or former smoker), occupational status (categorized according to the classification system of Statistics Finland: manual workers, junior clericals, senior clericals, students, and retirees), and family history of known diabetes (yes vs. no) in at least one first-degree relative (father, mother, sibling, or child). In addition, frequency and intensity of current physical activity and physical activity during the past 12 months were assessed using the validated Kuopio Ischemic Heart Disease Questionnaire (25). This questionnaire provides detailed information on common lifestyle, commuting, and leisure-time physical activity and enables assessment of total physical activity as metabolic equivalent (MET) hours per week (MET × hours/week). Based upon leisure-time activity, the participants were assigned into two groups: the regularly exercising group performed >30 min physical activity three or more times per week with intensity resulting in breathlessness and/or sweating and the less/no exercising group performed less or no physical activity. Body weight and height were measured, BMI was calculated, and depressive symptoms were self-rated using the Beck Depression Inventory II (26).
Statistical analyses
Multiple linear regression analyses, unstandardized regression coefficients, and 95% CIs were computed to examine associations between sleep complaints and fasting, 120-min, and AUC glucose and insulin. Logistic regression analyses, odds ratios (ORs), and 95% CIs were computed to examine if sleep complaints were associated with HOMA-IR, ISI, CIR, and DI indices of IR and insulin secretion dichotomized such that the top quartile in the HOMA-IR was contrasted with the lower three quartiles, and the bottom quartiles in the ISI, CIR, and DI were contrasted with the upper three quartiles. Variables were log transformed where appropriate, and the associations were adjusted for mediating and confounding factors.
RESULTS
Of the individuals who during the previous 3 months reported more frequent/severe complaints of sleep apnea (n = 162), 143 (88%) reported snoring at least three nights per week, 47 (29%) reported at least very loud and intermittent snoring, and 34 (21%) reported breathing pauses at least three nights per week. Of those with more frequent/severe insomnia (n = 163), 39 (24%) reported having problems in initiating sleep at least three nights per week, 120 (74%) reported waking up every night or almost every night per week, and 52 (32%) reported waking up at least three times per night. Of those with more frequent/severe daytime sleepiness (n = 202), 95 (47%) reported feeling sleepy in the morning at least three days per week, 103 (51%) reported feeling sleepy during daytime at least three days per week, and 94 (47%) reported having had naps at least three days per week.
Of the sleep complaints, daytime sleepiness occurred most frequently alone (n = 104, 40%), followed by sleep apnea (n = 81, 31%) and insomnia (n = 77, 29%). The most common combination of two co-occurring complaints was insomnia–daytime sleepiness (n = 38, 41%), followed by sleep apnea–daytime sleepiness (n = 33, 36%) and sleep apnea–insomnia (n = 21, 23%). All three sleep complaints were present in only 27 individuals and thus were not separately analyzed.
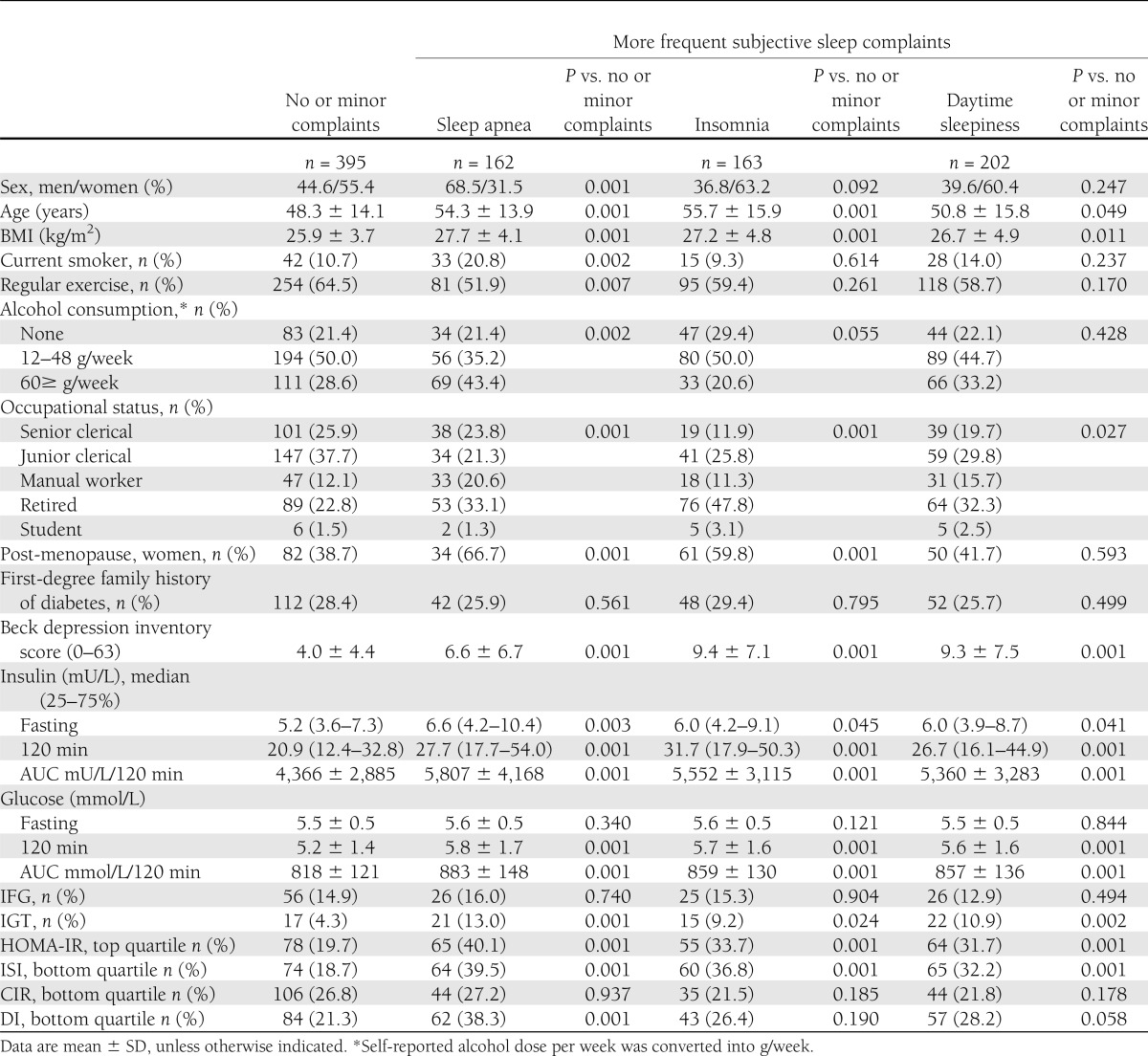
Table 1 shows that individuals with more frequent complaints of sleep apnea, insomnia, and daytime sleepiness were older, heavier, more frequently manual workers/retired, and more depressed, and those with sleep apnea were additionally more frequently men, current smokers, more frequent alcohol users, and those who exercised regularly less frequently. Further, complaints of sleep apnea and insomnia were more frequent in post- than premenopausal women.
Table 1.
Characteristics of the subjects with no or minor complaints and more frequent subjective sleep complaints
Sleep complaints and IR and insulin secretion
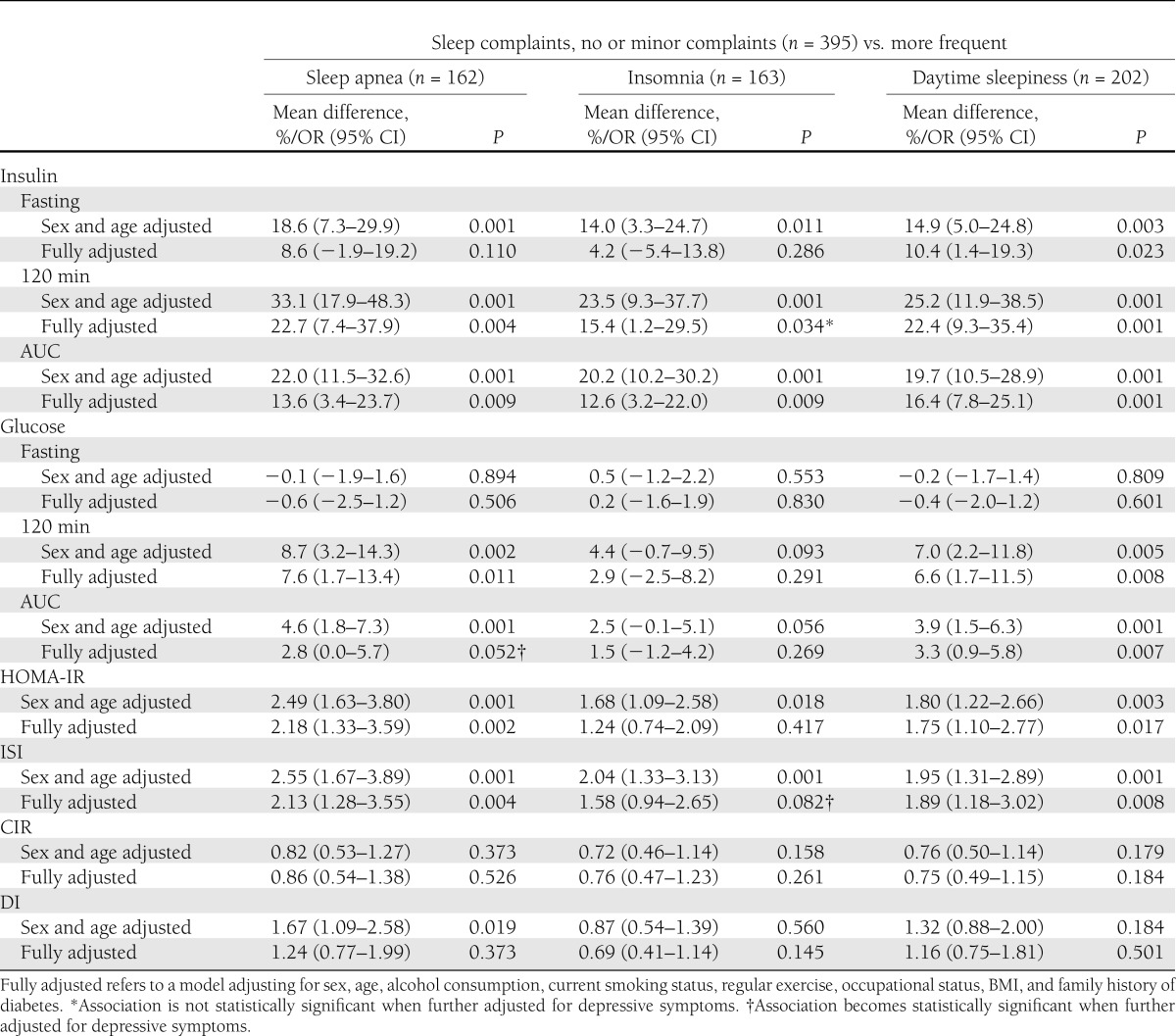
Those with more frequent sleep complaints (sleep apnea, insomnia, or daytime sleepiness) were significantly more insulin resistant than individuals with no or minor subjective sleep complaints, as evidenced by higher fasting and 120-min insulin, higher 120-min glucose concentrations, and higher AUC for insulin and glucose, as well as a higher likelihood to have high HOMA-IR and low ISI (Table 1). In addition, sleep apnea was associated with lower DI (Table 1). When adjustments were made for sex and age, and further for all mediating and confounding factors, the associations of sleep apnea with fasting insulin and DI, and of insomnia with fasting insulin, 120-min glucose, AUC for glucose, and HOMA-IR and ISI were rendered nonsignificant (Table 2).
Table 2.
Associations between subjective sleep complaints and insulin and glucose values during an OGTT, and indices of IR and insulin secretion
Co-occurrence of sleep complaints and IR and insulin secretion
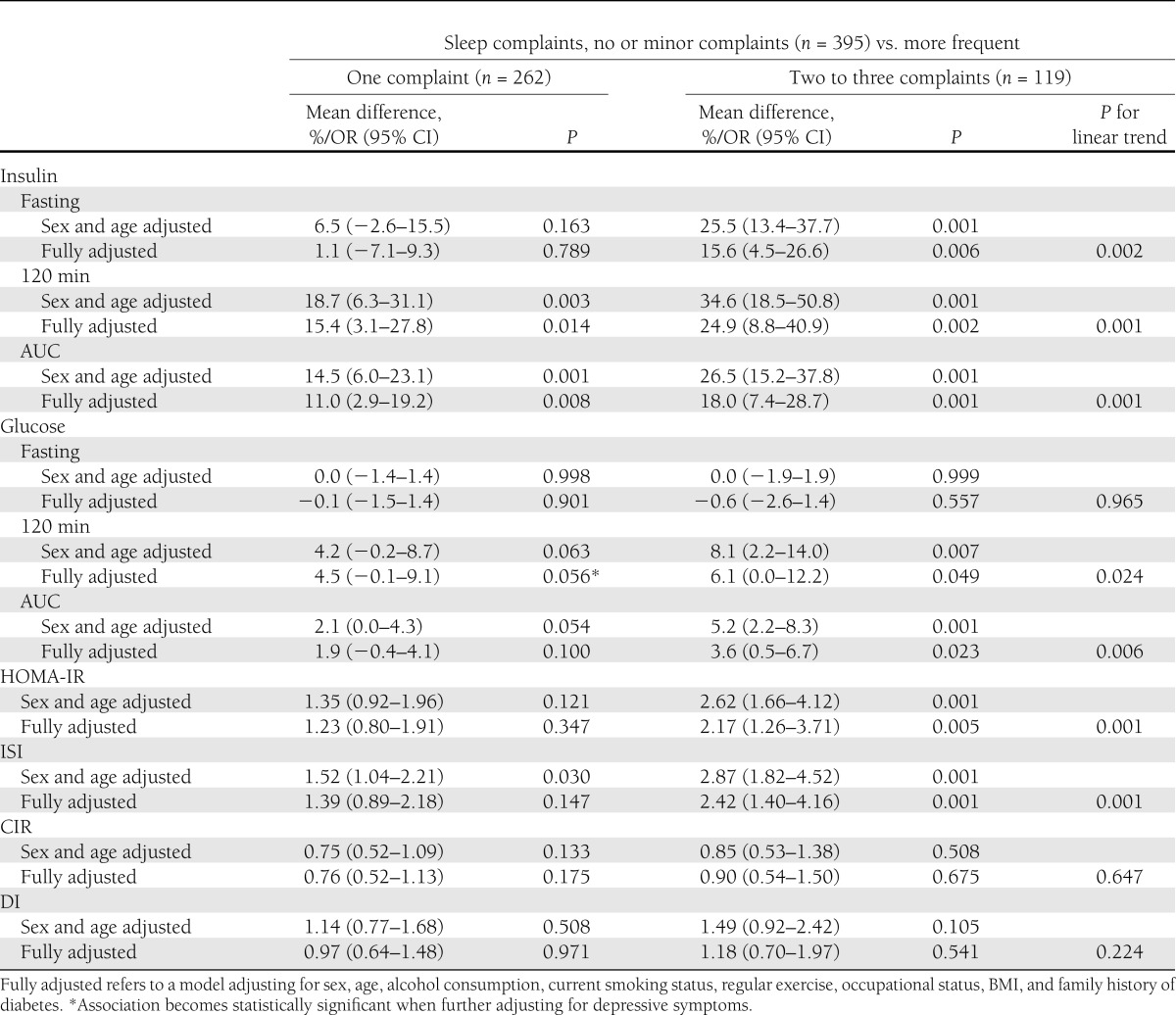
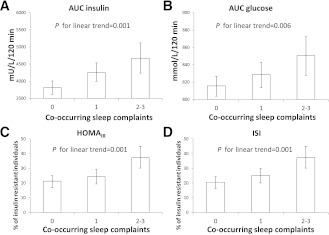
The likelihood of being insulin resistant, as evidenced by higher fasting and 120-min insulin and 120-min glucose concentrations and higher AUC for insulin and glucose, and higher likelihood of having high HOMA-IR and low ISI, increased according to the accumulation of co-occurring sleep complaints (Table 3). Figure 1 displays graphically the significant linearly increasing trends of AUC insulin and AUC glucose, and the percentage of individuals with high HOMA-IR and low ISI according to the accumulation of co-occurring sleep complaints. These associations remained significant in the fully adjusted models (Table 3). These associations changed only a little when we made further adjustments for depressive symptoms (see footnotes in Tables 2 and 3).
Table 3.
Associations between accumulation of co-occurring subjective sleep complaints and insulin and glucose values during an OGTT, and indices of IR and insulin secretion
Figure 1.
Geometric means of AUC of insulin (A) and glucose (B) and adjusted percentage of insulin-resistant individuals (percentage of individuals in the top quartile of HOMA-IR [C] and in the bottom quartile of ISI [D], adjusted for sex, age, alcohol consumption, current smoking status, regular exercise, occupational status, BMI, and family history of diabetes) and 95% CIs (error bars) according to accumulation of co-occurring sleep complaints.
Finally, we tested if the associations were driven by IFG- and/or IGT-associated abnormalities in insulin and glucose concentrations. We reran all the analyses, first after excluding individuals with IGT (n = 55) and then after excluding individuals with IFG or IGT (n = 166). In the first set of analyses, the following associations were rendered nonsignificant: sleep apnea and daytime sleepiness with 120-min glucose, insomnia with 120-min insulin, and accumulation of co-occurring sleep complaints with 120-min and AUC glucose. In the second set of analyses, the following additional associations were rendered nonsignificant: sleep apnea with 120-min and AUC insulin and ISI. The other associations remained significant (all P values <0.05; data not shown).
CONCLUSIONS
We examined associations between subjective sleep complaints and their co-occurrence with IR and insulin secretion in individuals without a history of or concurrently diagnosed type 2 diabetes. Our findings showed that in comparison with individuals with no or minor sleep complaints, those with more frequent complaints of sleep apnea, insomnia, and daytime sleepiness were significantly more insulin resistant. Further, our findings showed that the likelihood of being insulin resistant increased significantly and linearly according to co-occurring sleep complaints. Although the associations were somewhat attenuated when we excluded individuals with IFG and/or IGT, sleep complaints and their co-occurrence significantly increased the likelihood of being insulin resistant. These associations changed only a little when we made adjustments for sex, age, BMI, lifestyle, occupation, and family history of diabetes. Nor did the associations change when adjustments were made for depressive symptoms. Complaints of sleep apnea were associated with deficiency in insulin secretion (DI), but this association was rendered nonsignificant when adjusted for mediating and confounding factors. Our findings thus suggest that IR, rather than deficiency in insulin secretion, is a characteristic of individuals reporting more frequent sleep complaints. These associations do not merely characterize individuals without diabetes who display abnormalities in insulin and glucose concentrations as a consequence of IFG and/or IGT but also appear to characterize individuals whose OGTT values fall within the normoglycemic range.
Our findings agree with the previous research suggesting that those who suffer from sleep disordered breathing by displaying a more severe apnea-hypopnea index in polysomnography were more likely to be insulin resistant (2,7,9). Our findings, however, disagree with a report (9) showing that sleep apnea was associated with impairments in pancreatic β-cell function (DI) (9). In the current study, sleep apnea and DI were associated in an unadjusted model, but when we made adjustments for mediating and confounding factors, this association was rendered nonsignificant. Our findings also disagree with the null associations found between subjective complaints of sleep apnea/snoring and IR (4,10). Nor do our findings confirm either the null associations of complaints of daytime sleepiness with IR (10) or the association of actigraphy- and complaint-based measures of insomnia with a lower likelihood of being insulin resistant (4).
The discrepant findings may rise from methodological differences. Our participants underwent an OGTT, providing us with the opportunity to exclude individuals with new type 2 diabetes based on fasting and 2-h values for type 2 diabetes. Except for Seicean et al. (2) and Renko et al. (10), individuals with new type 2 diabetes have, in the previous studies, been excluded based on fasting values for type 2 diabetes only (4,8), even if an OGTT (7) or an intravenous glucose tolerance test (9) has identified additional responses characteristic of type 2 diabetes. It should also be kept in mind that we measured subjective sleep complaints, although with a validated questionnaire (24), which, as a methodology, is different from polysomnography and actigraphy. Nevertheless, epidemiological evidence points to an increased risk of type 2 diabetes also for individuals with subjective sleep complaints (1–6). Further, different self-reported questionnaires have been used in different studies, and therefore the findings are not directly comparable. The questions to capture sleep apnea, insomnia, and/or daytime sleepiness in questionnaires, such as the Basic Nordic Sleep Questionnaire (24), the Pittsburgh Sleep Quality Index (27), and the Berlin Sleep Apnea Questionnaire (28), are, however, similar, although the time frame and quantitative and qualitative measurement scales vary slightly. The questionnaires also vary in the degree to which input from a roommate/bed partner is requested in questions capturing sleep apnea. Finally, a majority of the existing studies has focused on sleep disordered breathing, and in only two of the studies has more than one sleep disorder/complaint been measured. Even though sleep disorders and complaints co-occur (11,12), no previous study has tested if the likelihood of being insulin resistant increases according to co-occurring sleep complaints.
Due to the cross-sectional study design, we cannot draw causal inferences from the associations, and hence, we cannot rule out that the associations are reciprocal. A recent longitudinal study suggests that higher insulin, but not glucose, values may increase the risk of sleep apnea (29), and insomnia and daytime sleepiness may result from hyperglycemia. Nor can we unravel the mechanisms underlying these associations. We did adjust the association for BMI, lifestyle, occupation, family history of diabetes, and depression, suggesting that these may not explain the associations. Yet, we found that individuals with sleep complaints were heavier, older, more frequently manual workers or retired, and more depressed, and those with sleep apnea were additionally more frequently men, current smokers, more frequent alcohol users, and those who exercised regularly less frequently, a finding in general agreement with earlier reports (2–5,10,12,29). Therefore, the role of these factors cannot be entirely ruled out when interpreting the findings, as they all contribute to a higher likelihood of being insulin resistant. The mechanisms may relate to physical and psychological stresses that accompany complaints of sleep apnea, insomnia, and daytime sleepiness, such as hypoxia, sleep fragmentation, nonrestorative sleep, irritable mood, and problems in cognitive performance. These stresses may result in sympathetic arousal (12,30–34), hypothalamic-pituitary-adrenal axis dysfunction (35,36), and inflammatory response (37). Finally, a common genetic basis may underlie these findings. This basis may arise from genetic variants that participate in the regulation of the human sleep-wake cycle and are implicated in diabetes, such as MTNR1B (melatonin receptor 1B) on chromosome 11 (38). A further study limitation relates to the generalizability of our findings beyond Caucasians. Our findings suggest that strategies aimed at improving sleep quality in individuals without type 2 diabetes may be an additional tool in diabetes prevention.
Acknowledgments
The PPP-Botnia Study was financially supported by grants from the Finnish Academy, Sigrid Juselius Foundation, Folkhälsan Research Foundation, Nordic Center of Excellence in Disease Genetics, Signe and Ane Gyllenberg Foundation, Swedish Cultural Foundation in Finland, Finnish Diabetes Research Foundation, Foundation for Life and Health in Finland, Finnish Medical Society, Finnish Ministry of Education and Culture, Paavo Nurmi Foundation, Perklén Foundation, Ollqvist Foundation, and Närpes Health Care Foundation. This study was also supported by the Municipal Health Care Center and Hospital in Jakobstad and Health Care Centers in Vasa, Närpes, and Korsholm.
L.G. was a consultant for and has served on advisory boards for sanofi-aventis, GSK, Novartis, Merck, Tethys Bioscience, and Xoma and has received lecture fees from Eli Lilly and Novartis. J.G.E. was a consultant for and has served on advisory boards for MSD, BMS, AstraZeneca, Roche, Eli Lilly, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
A.-J.P. and K.R. researched data, contributed to discussion, and wrote the manuscript. B.I. and L.G. researched data, contributed to discussion, and reviewed and edited the manuscript. A.-K.P. reviewed and edited the manuscript. J.G.E. contributed to discussion and reviewed and edited the manuscript. T.T. researched data and reviewed and edited the manuscript. A.-J.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors gratefully acknowledge the skillful assistance of the Botnia Study Group.
References
- 1.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010;33:414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care 2008;31:1001–1006 [DOI] [PubMed] [Google Scholar]
- 3.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol 2005;99:2008–2019 [DOI] [PubMed] [Google Scholar]
- 4.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care 2011;34:1171–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson PM, Rööst M, Engström G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care 2004;27:2464–2469 [DOI] [PubMed] [Google Scholar]
- 6.Meisinger C, Heier M, Loewel H, MONICA/KORA Augsburg Cohort Study Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia 2005;48:235–241 [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 2002;165:677–682 [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE, Sleep Heart Health Study Investigators Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530 [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med 2009;179:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renko AK, Hiltunen L, Laakso M, Rajala U, Keinänen-Kiukaanniemi S. The relationship of glucose tolerance to sleep disorders and daytime sleepiness. Diabetes Res Clin Pract 2005;67:84–91 [DOI] [PubMed] [Google Scholar]
- 11.Ohayon MM, Caulet M, Lemoine P. Comorbidity of mental and insomnia disorders in the general population. Compr Psychiatry 1998;39:185–197 [DOI] [PubMed] [Google Scholar]
- 12.Barceló A, Barbé F, de la Peña M, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax 2008;63:946–950 [DOI] [PubMed] [Google Scholar]
- 13.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA 1989;262:1479–1484 [DOI] [PubMed] [Google Scholar]
- 14.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006;49:837–845 [DOI] [PubMed] [Google Scholar]
- 16.Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, Isomaa B. Depressive symptoms, antidepressant medication use, and insulin resistance: the PPP-Botnia Study. Diabetes Care 2011;34:2545–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isomaa B, Forsén B, Lahti K, et al. A family history of diabetes is associated with reduced physical fitness in the Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia Study. Diabetologia 2010;53:1709–1713 [DOI] [PubMed] [Google Scholar]
- 18.Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, Isomaa B. Stressful life events and the metabolic syndrome: the Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia Study. Diabetes Care 2010;33:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, Isomaa B. Association between depressive symptoms and metabolic syndrome is not explained by antidepressant medication: results from the PPP-Botnia Study. Ann Med 2012;44:279–288 [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 21.Bergman RN, Prager R, Volund A, Olefsky JM. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 1987;79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 2000;151:190–198 [DOI] [PubMed] [Google Scholar]
- 23.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 24.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res 1995;4(Suppl. 1):150–155 [DOI] [PubMed] [Google Scholar]
- 25.Salonen JT, Lakka TA. Assessment of physical activity in population studies–validity and consistency of the methods in the Kuopio Ischemic Heart Disease Risk Factor Study. Scand J Sports Sci 1987;9:89–95 [Google Scholar]
- 26.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories IA and II in psychiatric outpatients. J Pers Assess 1996;67:588–597 [DOI] [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213 [DOI] [PubMed] [Google Scholar]
- 28.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491 [DOI] [PubMed] [Google Scholar]
- 29.Balkau B, Vol S, Loko S, et al. Epidemiologic Study on the Insulin Resistance Syndrome Study Group High baseline insulin levels associated with 6-year incident observed sleep apnea. Diabetes Care 2010;33:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martikainen S, Pesonen AK, Feldt K, et al. Poor sleep and cardiovascular function in children. Hypertension 2011;58:16–21 [DOI] [PubMed] [Google Scholar]
- 31.Keckeis M, Lattova Z, Maurovich-Horvat E, et al. Impaired glucose tolerance in sleep disorders. PLoS ONE 2010;5:e9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 2008;105:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birketvedt GS, Florholmen J, Sundsfjord J, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA 1999;282:657–663 [DOI] [PubMed] [Google Scholar]
- 34.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens 2001;14:304S–309S [DOI] [PubMed] [Google Scholar]
- 35.Pesonen AK, Kajantie E, Heinonen K, et al. Sex-specific associations between sleep problems and hypothalamic-pituitary-adrenocortical axis activity in children. Psychoneuroendocrinology 2012;37:238–248 [DOI] [PubMed] [Google Scholar]
- 36.Räikkönen K, Matthews KA, Pesonen AK, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab 2010;95:2254–2261 [DOI] [PubMed] [Google Scholar]
- 37.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 2000;85:1151–1158 [DOI] [PubMed] [Google Scholar]
- 38.Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009;41:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]