Abstract
OBJECTIVE
Diabetes is associated with increased brachial and central blood pressure and aortic stiffness. We examined the effect of intensive multifactorial treatment in general practice on indices of peripheral and central hemodynamics among patients with screen-detected diabetes.
RESEARCH DESIGN AND METHODS
As part of a population-based screening and intervention study in general practice, 1,533 Danes aged 40–69 years were clinically diagnosed with screen-detected diabetes. General practitioners were randomized to provide intensive multifactorial treatment or routine care. After a mean follow-up of 6.2 years, an unselected subsample of 456 patients underwent central hemodynamic assessments by applanation tonometry. Central pressure was derived from the radial pulse wave. Aortic stiffness was assessed as carotid-femoral pulse wave velocity (aPWV). The intervention effect on each index of central hemodynamics was analyzed by mixed-effects models adjusted for heart rate, cluster randomization, age, and sex.
RESULTS
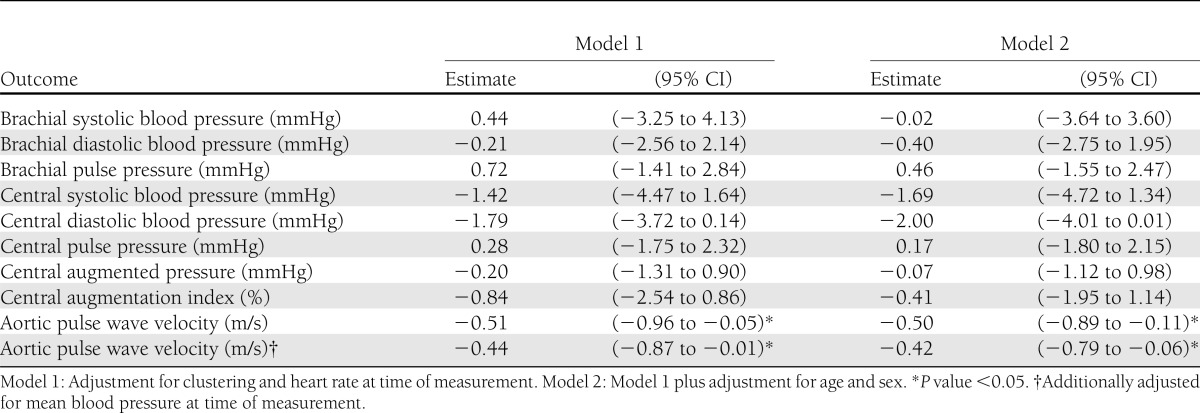
At screening, median age was 59.2 years (interquartile range 55.2–64.6); 289 patients (63%) were in the intensive treatment group, and 278 patients (61%) were men. Patients in the intensive treatment group had a 0.51 m/s (95% CI −0.96 to −0.05, P = 0.03) lower aPWV compared with routine care. Respective differences for central augmentation index (−0.84% [−2.54 to 0.86]), pulse pressure (0.28 mmHg [−1.75 to 2.32]), and systolic (−1.42 mmHg [−4.47 to 1.64]) and diastolic (−1.79 mmHg [−3.72 to 0.14]) blood pressure were not statistically significant.
CONCLUSIONS
Intensive multifactorial treatment of screen-detected diabetes during 6 years in general practice has a significant impact on aortic stiffness, whereas the effects on other hemodynamic measures are smaller and not statistically significant.
Patients with clinically diagnosed type 2 diabetes have a raised cardiovascular risk profile and often have undiagnosed diabetes complications at the time of diabetes diagnosis (1,2). The clear benefit of close individual cardiovascular risk factor management in patients with a new clinical diagnosis of type 2 diabetes (3–5) has long driven calls for screening for undiagnosed diabetes. However, until recently the direct evidence needed to extend these benefits to those with screen-detected diabetes was unavailable. The Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION-Europe trial) evaluated the effect of intensive multifactorial treatment compared with routine care of individuals with screen-detected diabetes and found a nonstatistically significant 17% risk reduction of a composite cardiovascular end point (6).
Intervention studies with cardiovascular events as outcome require very large study samples or a long follow-up. Intermediate end points that lie in the causal pathway toward the clinically relevant outcomes are consequently a useful tool for assessing the effect of treatment in clinical trials short of cardiovascular events beyond a calculated cardiovascular risk estimate. As reported in a recent review and meta-analysis (7), several studies in the general population and in high-risk populations have shown that aortic stiffness, measured by carotid-femoral pulse wave velocity (aPWV), is a strong predictor of cardiovascular disease (CVD) and mortality independent of brachial blood pressure and other cardiovascular risk factors. The meta-analysis included studies with various measurement methods of aPWV and showed that each additional 1 m/s in aPWV is associated with an increased CVD risk in the range of 5–35% (7). Accordingly, aortic stiffness is increasingly used as an intermediate end point in clinical trials (8,9).
In short- and long-term clinical trials, several classes of antihypertensive agents have been shown to lower aortic stiffness (8,10,11). Lipid-lowering treatment with statins has shown to reduce aortic stiffness in high-risk patients (9,12), and small clinical trials have indicated a beneficial effect of weight loss as well as exercise training on aortic stiffness (13,14). Only few studies have examined the association between glucose-lowering treatment and aortic stiffness, and their results are inconclusive (15,16). To our knowledge, no studies have examined the effect of multifactorial treatment of cardiovascular risk factors on aortic stiffness.
In clinical practice, cardiovascular risk factors are usually treated simultaneously in diabetic patients, and before implementing a screening program for diabetes it is important to know whether intensive multifactorial treatment of screen-detected diabetes lowers the risk of CVD. We examined the effect of intensive multifactorial treatment compared with routine care on the following central hemodynamics: aortic stiffness, central systolic and diastolic blood pressure, pulse pressure, augmented pressure, and augmentation index as intermediate cardiovascular end points among individuals with screen-detected diabetes in the Danish arm of the ADDITION-Europe trial.
RESEARCH DESIGN AND METHODS
The design and the rationale for the ADDITION-Europe study have previously been reported (17,18). In brief, the Danish arm of the ADDITION-Europe study (ADDITION-Denmark) consisted of two phases: 1) a screening phase and 2) a pragmatic cluster-randomized parallel-group trial. In five Danish regions, 744 general practices were invited to participate. Of these, 190 agreed to participate and were allocated to screening and randomized to provide either routine care or intensive multifactorial treatment of patients with diabetes detected by screening.
Population
The screening phase took place in 2001–2006 and consisted of a population-based stepwise screening program to identify individuals with screen-detected diabetes. It was conducted among the participating general practitioners’ patients aged 40–69 years without known diabetes. The recruitment and selection methods have previously been described in detail (19,20). In total, 1,533 individuals were diagnosed with diabetes according to the 1999 World Health Organization criteria (21) and were enrolled in the trial. In 2009, a follow-up examination was performed. In two of five study centers (Holstebro Hospital and Steno Diabetes Center), measurements of central hemodynamics were carried out and successfully obtained in 456 of 486 attending patients. These patients constitute our study sample. Of the 456 patients, 420 had measurements of both aPWV and central blood pressure, 9 had measurements of aPWV only, and 27 had measurements of central blood pressure only (Supplementary Fig. 1). The reasons for missing measurements were as follows: station closure owing to insufficient staff (n = 27), irregular pulse (aPWV, n = 4; central blood pressure and augmentation index, n = 12), and for aPWV, that the carotid or femoral artery pulse could not be found (n = 26).
The study was approved by the local ethics committee (Region Midt, Denmark) and was conducted in accordance with the 1996 Helsinki Declaration. Written informed consent was obtained from each patient at baseline and at follow-up.
Intervention
The specific characteristics of the interventions to promote intensive treatment have previously been described in detail (17). We aimed to educate and support general practitioners and practice nurses in target-driven management (using medication and promotion of healthy lifestyle) of hyperglycemia, hypertension, and hypercholesterolemia based on the stepwise regimen used in the Steno-2 study (22,23). Treatment thresholds, targets, and algorithms are provided in Supplementary Table 1. Intensive treatment was promoted through the addition of several features to existing diabetes care. Practice personnel were provided with educational material for patients, and patients were sent reminders if annual check-up appointments were overdue. Practices received additional funding to support the delivery of extra care added to the usual care and consultations. Although targets for treatment were specified and classes of medication recommended, decisions on prescriptions, including choice of individual drugs, were made by general practitioners and patients in cooperation.
The general practitioners in the routine care group were provided with diagnostic test results only; their patients with screen-detected diabetes received standard care of diabetes according to the Danish national guidelines (24).
Outcomes
In this secondary analysis, the outcomes were central and peripheral hemodynamics: aPWV, central systolic and diastolic blood pressure, pulse pressure, augmented pressure, augmentation index, brachial systolic and diastolic blood pressure, and brachial pulse pressure. The staff obtaining the outcome measures was blinded for randomization group.
Aortic pulse wave velocity.
With the patient in a supine position, brachial blood pressure was measured after 10 min of rest (Omron M6 comfort; Omron Healthcare, Milton Keynes, U.K.). From the supine systolic and diastolic blood pressure, mean blood pressure was calculated (diastolic blood pressure + 0.4 × pulse pressure) (25). The velocity of the pulse wave was then assessed between the right carotid and femoral sites by applanation tonometry using the SphygmoCor device (version 8; Atcor Medical) and a high-fidelity tonometer, which is a validated method of measuring aPWV (26). The tonometer was used to capture the wave forms at the carotid and subsequently at the femoral artery simultaneously with an electrocardiogram recording using the intersecting tangent (27). The transit time was based on the mean of 10 pulse waves. The distance from the suprasternal notch to the carotid artery was measured with a tape measure and from the suprasternal notch to the femoral artery with an anthropometer (seca; Medical Scales and Measuring Systems, Hamburg, Germany) to avoid overestimation of the distance and subsequently the velocity in obese individuals. The path length was determined by subtracting the carotid-sternal notch distance from the femoral-sternal notch distance. In each patient, aPWV was measured twice. If the difference of aPWV between the two measurements was larger than 0.5 m/s, a third measurement was taken. In the statistical analysis, the average of the two closest measurements in each patient was used.
Central blood pressure and augmentation index.
With the patient in a supine position, peripheral pressure waveforms were recorded at the radial artery using applanation tonometry (version 8, SphygmoCor system; Atcor Medical). Based on a built-in generalized transfer function using the supine brachial systolic and diastolic blood pressure for calibration, central waveforms were calculated from the radial arterial waveforms. From the central waveforms, central systolic and diastolic blood pressure, central pulse pressure, central augmented pressure, and central augmentation index were estimated. Central augmented pressure was calculated as the contribution of the pulse wave reflection to central systolic pressure, and central augmentation index was calculated as the ratio between central augmented pressure and central pulse pressure.
Peripheral blood pressure.
Brachial systolic and diastolic blood pressures were measured three times after a 10-min rest with an automated oscillometric blood pressure recorder (Omron M6 comfort) with the patient in a sitting position. Brachial pulse pressure was calculated as the difference between brachial systolic and diastolic blood pressure. The average of all three measurements for each parameter was used in the analysis.
Measurements at baseline and at follow-up
Health assessments at baseline and at follow-up were performed at the Danish ADDITION study centers by trained staff blinded for randomization group following standard operating procedures (6). Briefly, height, weight, waist circumference, and brachial blood pressure were measured. In venous blood samples, HbA1c, serum total cholesterol, serum HDL cholesterol, serum triglycerides, and plasma creatinine were measured. Serum LDL cholesterol was calculated using Friedewald equation (28). Urinary albumin and urinary creatinine were measured on spot urine. At baseline and follow-up, self-report questionnaires were used to collect information on ethnicity, smoking status, alcohol consumption, medication, and CVD (previous myocardial infarction and stroke). Furthermore, at follow-up we collected information on self-reported episodes with angina pectoris or arrhythmia and self-reported cardiovascular revascularization.
Statistical analysis
Patient characteristics at baseline and at follow-up are presented as means (SD) or, in cases of skewed distributions, as median (interquartile range [IQR]). For proportions, exact CIs were calculated (29).
The effect of intensive multifactorial treatment on central hemodynamics was analyzed by mixed-effects models with adjustment for clustering (randomization by general practice) and heart rate at time of measurement, taking its direct functional effect on the hemodynamic markers into account. The analysis of aPWV was also adjusted for mean blood pressure at time of measurement. In addition to regression coefficients, standardized regression coefficients (SD change in the outcome) are presented.
Aortic stiffness is associated with the intrasubject variation of the central hemodynamic measurements (the higher the level of stiffness, the larger the intrasubject variation). To assure a full spectrum of aortic stiffness, no measurements were excluded in the primary analysis. For comparison, we subsequently performed a subanalysis, excluding measurements with large intrasubject variation according to the manufacturer’s guidelines (30,31). In the subanalysis of aPWV, measurements with a ratio >0.2 between aPWV SEM and aPWV were excluded. For the measurements of central blood pressure and augmentation index, the software provides an operator index based on the height of the pulse waves, variations in the height of the pulse waves, diastolic variation, and deviation in the shape of the pulse waves. In the subanalysis of central blood pressure and augmentation index, measurements with an operator index <75 were excluded. A significance level of 5% was used. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
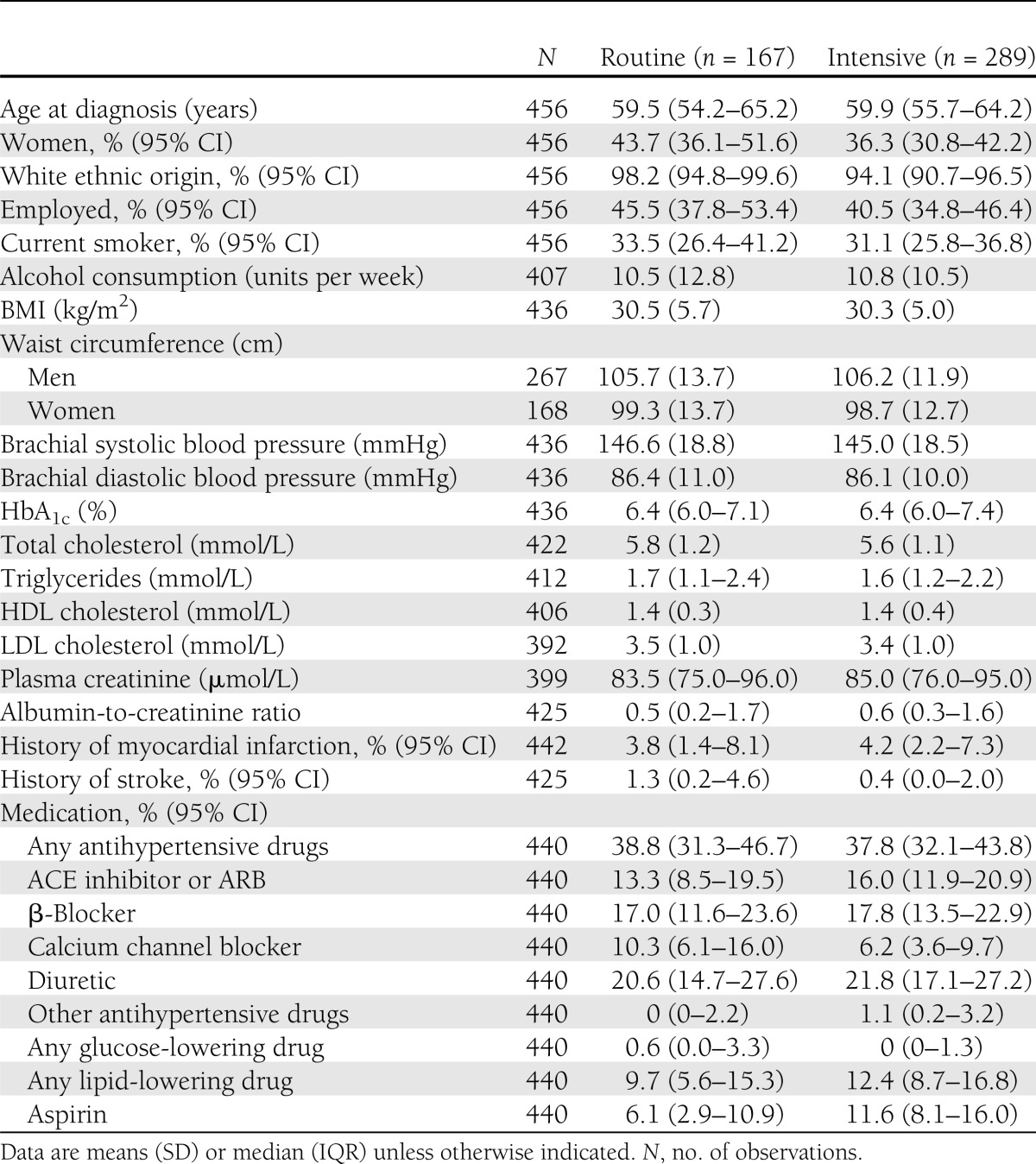
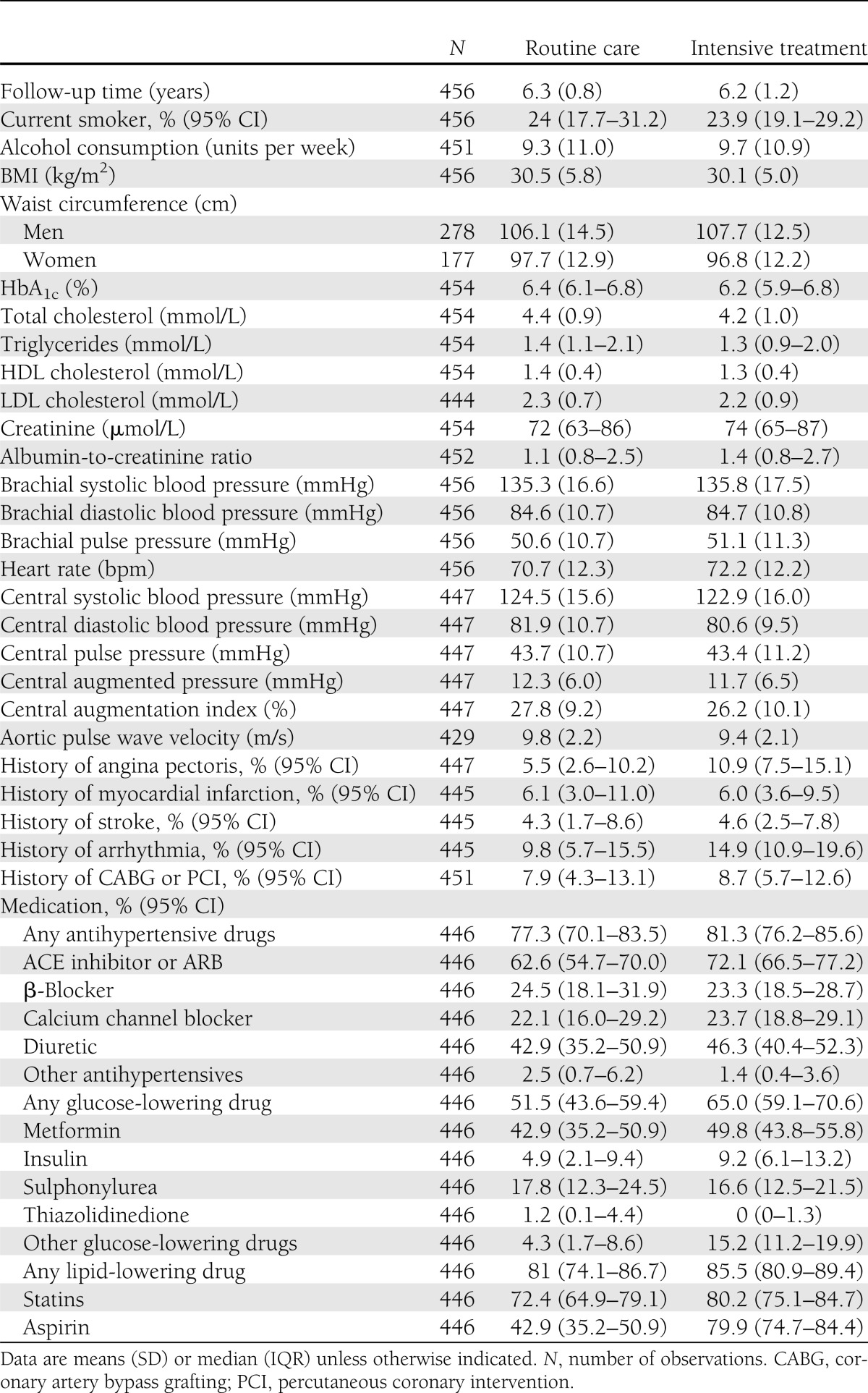
In our study sample, 61% of the patients were men, mean age was 59 years at screening, and mean follow-up time was 6.2 years. At screening, modifiable cardiovascular risk factors were elevated (Table 1). Furthermore, 38% of the patients were treated with antihypertensive drugs, and lipid-lowering treatment was used by only 10% in the routine care group and by 12% in the intensive treatment group. During follow-up, HbA1c, total cholesterol, LDL cholesterol, triglycerides, creatinine, and the proportion of smokers decreased in both treatment arms, whereas waist circumference, BMI, and HDL cholesterol did not change (Table 2). At follow-up, the medication use was more or less similar between treatment groups. Only ACE inhibitor or angiotensin receptor blocker (ARB), aspirin, overall glucose-lowering drugs, and other glucose-lowering drugs, which mainly accounted for repaglinide, were more frequently used by the intensive treatment group.
Table 1.
Baseline characteristics of the study sample
Table 2.
Follow-up characteristics of the study sample
At follow-up, mean aPWV was 9.8 (SD 2.2) m/s in the routine care group and 9.4 (2.1) m/s in the intensive treatment group (Table 2). With the cluster randomization and heart rate at time of measurement taken into account, aPWV was 0.51 m/s lower (95% CI −0.96 to −0.05; P = 0.03) in the intensive treatment group compared with routine care (Table 3). We found no statistically significant differences between treatment arms in brachial systolic blood pressure, diastolic blood pressure, or pulse pressure or central systolic blood pressure, diastolic blood pressure, pulse pressure, augmented pressure, or augmentation index. When we excluded aPWV measurements with large intrasubject variation (72 out of a total of 1,140 measurements), 15 patients were excluded from the analysis (2 from routine care and 13 from intensive treatment group), and the difference in aPWV between treatment arms was −0.39 m/s (−0.82 to 0.05). For the analysis of central blood pressure and augmentation index, excluding measurements with an operator index <75 (178 measurements of 1,046; 27 patients, 6 from routine care and 21 from intensive treatment) did not change the results substantially.
Table 3.
Effect of intensive treatment compared with routine care on peripheral and central hemodynamics
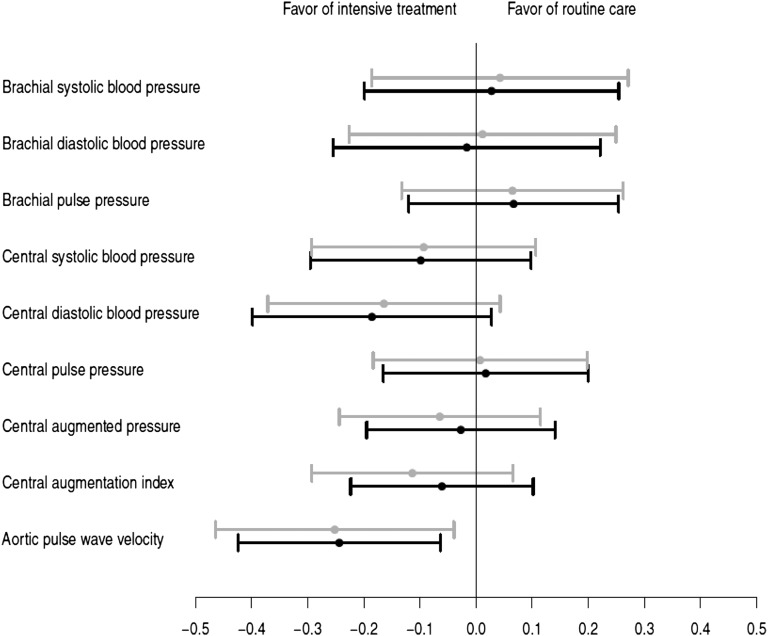
Comparison of the intervention effect on peripheral and central hemodynamics in standardized analyses showed that the largest intervention effect was on aPWV (Fig. 1) and, subsequently, central diastolic and systolic blood pressure. The effect on the peripheral hemodynamics was further down the line or in the opposite direction.
Figure 1.
The effect of the intervention on the population SD change in the hemodynamic marker. Gray, model 1: adjusted for clustering (general practice) and heart rate at time of measurement; black, model 2: model 1 plus adjustment for age and sex.
CONCLUSIONS
In this secondary analysis of a subset of 456 individuals with screen-detected type 2 diabetes who participated in the ADDITION-Denmark trial, we found that individuals attending practices randomized to provide intensive treatment had lower aortic stiffness based on aPWV after 6 years of follow-up compared with those attending practices randomized to provide routine care. Furthermore, we found a statistically nonsignificant indication of lower central systolic and diastolic blood pressure, augmented pressure, and augmentation index in the intensive treatment group.
Our results should be seen in the light of the findings from the ADDITION-Europe trial, which found a statistically significant difference in the cardiovascular risk factors between routine care and intensive treatment: HbA1c, brachial blood pressure, total cholesterol, LDL cholesterol, and triglycerides and a statistically nonsignificant 17% risk reduction of a composite cardiovascular end point after 5.3 years (6). In the ADDITION-Europe trial, there was an indication that the rate of cardiovascular events started to separate after 4 years of treatment. Similar findings have been observed in other trials of intensive treatment in patients with established type 2 diabetes (5,22,32), indicating that in the presence of a relatively low absolute event rate, the effect of cardiovascular risk reduction takes some time to translate into a lower rate of hard events. Our findings lend support to the notion that during the early phases of cardiovascular risk lowering, subclinical functional and/or structural cardiovascular changes occur that underlie the later effect of treatment on hard cardiovascular events. However, the occurrence of hard cardiovascular events in this early time window may still depend on the state of subclinical cardiovascular function and structure before the start of treatment. Based on this effect-lag concept and our results, it is possible that the incidence curves in the ADDITION-Europe trial will continue to separate as cardiovascular events continue to accrue in the posttrial follow-up. Results from the Danish Steno-2 study (22) further support this concept.
The level of cardiovascular risk factors at follow-up was similar between treatment arms. Nonetheless, we found a difference in aortic stiffness. Similarly, the Steno-2 study also found a small or nonexistent difference in several of the cardiovascular risk factors after a mean follow-up of 7.8 years, and the cardiovascular events were still reduced by 53% in the intensive treatment group (22). Addressing several cardiovascular risk factors simultaneously, which is usually the approach in general practice, therefore seems to lower cardiovascular risk. Because of the pragmatic study design and the treatment strategy, which covered several treatment components and features to provide intensive treatment, we cannot conclude on the impact of each single treatment component; we can speculate on the mechanisms involved only.
In our study, brachial systolic blood pressure, central systolic blood pressure, central pulse pressure, central augmented pressure, and augmentation index were not significantly lower in the intensive treatment group compared with the routine care group at follow-up. This is in contrast to what we had expected, given that the brachial blood pressure decreased significantly in the intensive treatment group in the ADDITION-Europe trial. Further, central aortic pressures and augmentation index do not necessarily reflect the same arterial wall properties as measured by aPWV. The velocity of the pulse wave assessed at the carotid and femoral artery is a direct measure of the elasticity of the aorta, whereas augmented pressure, augmentation index, central systolic blood pressure, and pulse pressure are determined by the reflective properties of the entire arterial tree, distance to the reflection sites, the velocity of the forward and reflected wave, and stroke volume (33). The reflective properties of the arterial wall can be modulated independently of aortic stiffening (34,35) and vice versa (11,36). In line with our study, Karalliedde et al. (36) found a reduction only in aPWV in diabetic patients treated with an ARB over 24 weeks compared with a calcium channel blocker, despite similar reductions in augmentation index and brachial and central pulse pressure. Moreover, Mitchell et al. (11) found a reduced aPWV in individuals with stable coronary artery disease treated with an ACE inhibitor for 4.5 years compared with placebo and no difference in augmentation index and brachial and central pulse pressure between the two groups. In our study, the first antihypertensive drug of choice in the intensive treatment group was ACE inhibitor, and at follow-up, 72% of the intensive treatment arm patients were treated with either an ACE inhibitor or ARB compared with 62% in the routine care arm. This could be the main reason for lower aPWV in the intensive treatment group, as previous studies have shown that blockade of the renin-angiotensin system has a beneficial effect on the elastic properties of the arterial wall (37).
In the analysis of aPWV, we also adjusted for mean blood pressure at time of measurement to see whether the effect on aPWV was driven by the effect on mean blood pressure, and the effect was attenuated only modestly. Hence, the effect of intensive multifactorial treatment is independent of the direct effect on blood pressure, suggesting that the intervention had a direct effect on the vessel wall.
The treatment algorithm in the intensive treatment group included statins to all patients and aspirin to patients receiving antihypertensive medication. At follow-up, 80% of patients in the intensive treatment group were treated with statins compared with 72% in the routine care group, and aspirin was more frequently used in the intensive treatment group. The difference in statin treatment could also explain part of the difference in aortic stiffness at follow-up, as a few studies have shown that statin treatment lowers aortic stiffness (9,12), whereas the destiffening effect of aspirin remains speculative.
The most frequently used glucose-lowering drug in our study was metformin, which has a beneficial effect on cardiovascular risk (4). Nevertheless, a meta-analysis has shown that metformin does not reduce brachial systolic and diastolic blood pressure (38). Few studies have examined the effect of glucose-lowering treatment on aortic stiffness (15,39), and studies conducted among individuals with diabetes are sparse (15).
There was no difference in BMI or waist circumference at follow-up between the treatment arms, and the proportion of smokers decreased equally in the treatment arms during follow-up. It is therefore unlikely that the lifestyle component of the intensive treatment intervention led to the observed aortic stiffness effects.
This study is the first examining the effect of intensive multifactorial treatment on central hemodynamics among individuals with screen-detected diabetes in general practice. All patients were screened and treated by their general practitioner, showing that the screening strategy and intervention are feasible in general practice. During follow-up, the evidence-based guidelines of diabetes care in Denmark (24,40) changed toward the treatment targets in the intensive treatment arm, which could have evened out the effect of intensive treatment. Nevertheless, we found a lower aortic stiffness in patients treated intensively. This suggests either that the cardiovascular risk factor levels differed more markedly in the earliest years of the trial and had an effect on aortic stiffness that carried forward or that even relatively small differences in cardiovascular risk factor levels can impact aortic stiffness if consistent over 6 years.
The main limitation of this secondary trial analysis is that our central hemodynamic outcomes were not prespecified before initiation of the study. Statistically, the addition of nine outcomes increases the possibility of getting statistically significant results simply by chance. However, the nine outcomes should not be regarded as independent, as they are all expressions of the functional and structural processes underlying the prespecified outcome: CVD. The number of patients in the intensive treatment group was larger than in the routine care group, even though there were no differences in the number and types of practices in the two groups. The practices were randomized before screening and inclusion of patients, but it seems that the intervention allocation enhanced the focus on screening and thereby inclusion of patients in the intensive treatment practices. Still, the patients did not differ between groups regarding cardiovascular risk factors at baseline. Another limitation is the lack of measurements of central hemodynamics at baseline, but based on the study design and similar levels of cardiovascular risk factors, we believe it is reasonable to assume that the level of aortic stiffness and central blood pressure were similar in both groups at baseline and, consequently, that the observed differences occurred during the study. Because of the study design, the general practices were not blinded to treatment allocation, but the research personnel conducting the measurements at follow-up were unaware of the treatment allocation.
Several prospective observational studies have shown that a lower level of aortic stiffness is associated with a lower cardiovascular risk, as reported in a recent meta-analysis (7). With our results extrapolated based on estimates from this meta-analysis, which included studies with various measures of aortic stiffness, the difference of 0.51 m/s would correspond with a relative CVD risk reduction of 7% by intensive multifactorial treatment compared with routine care. However, we cannot conclude that reducing aortic stiffness also reduces the incidence of cardiovascular events, even though this hypothesis might be plausible. To confirm this theory, we need to follow our population for future cardiovascular events.
In this secondary analysis of 456 patients with screen-detected diabetes in a cluster randomized trial, we found that patients treated intensively by general practitioners have a lower level of aPWV than individuals receiving routine care after 6 years of treatment. This means that screening for type 2 diabetes followed by intensive multifactorial treatment in general practice leads to lower aortic stiffness, a key intermediate cardiovascular outcome, during the time window when treatment effects on hard cardiovascular outcomes are not yet observable.
Acknowledgments
ADDITION-Denmark was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and South Jutland in Denmark; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; the Novo Nordisk Foundation; the Danish Center for Evaluation and Health Technology Assessment; the Diabetes Fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. Parts of the grants from Novo Nordisk Foundation and Danish Council for Strategic Research were transferred to other European centers.
ADDITION-Denmark has been given unrestricted grants from Novo Nordisk AS, Novo Nordisk Scandinavia AB, Novo Nordisk UK, ASTRA Denmark, Pfizer Denmark, GlaxoSmithKline Pharma Denmark, Servier Denmark A/S, and HemoCue Denmark A/S. Parts of the grants from Novo Nordisk AS were transferred to the other European centers. N.B.J. is funded by an unrestricted grant from the European Foundation for the Study of Diabetes/Pfizer for research into CVD risk reduction in patients with diabetes (74550801). N.B.J., D.V., and D.R.W. are employed by Steno Diabetes Center A/S, which is a research and teaching hospital collaborating with the Danish National Health Service and owned by Novo Nordisk A/S. N.B.J., D.V., N.W., K.B.-J., T.L., and D.R.W. hold shares in Novo Nordisk A/S. K.B.-J. has been an invited speaker with honorarium paid by Eli Lilly, Novo Nordisk, Takeda, and sanofi-aventis within the past 5 years. T.L. has held five lectures for the medical industry within the past 2 years. No other potential conflicts of interest relevant to this article were reported.
N.B.J. analyzed the data and wrote the manuscript. M.C. collected data and reviewed and edited the manuscript. D.V. analyzed data and reviewed and edited the manuscript. S.S.R. and N.W. reviewed and edited the manuscript. K.B.-J. and T.L. designed the study and reviewed and edited the manuscript. A.S. designed the study, collected data, and reviewed and edited the manuscript. D.R.W. analyzed data and reviewed and edited the manuscript. N.B.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in poster form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
Clinical trial reg. no. NCT00237549, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0176/-/DC1.
References
- 1.Glümer C, Jørgensen T, Borch-Johnsen K, Inter99 study Prevalences of diabetes and impaired glucose regulation in a Danish population: the Inter99 study. Diabetes Care 2003;26:2335–2340 [DOI] [PubMed] [Google Scholar]
- 2.Sandbaek A, Griffin SJ, Rutten G, et al. Stepwise screening for diabetes identifies people with high but modifiable coronary heart disease risk. The ADDITION study. Diabetologia 2008;51:1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016 [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–713 [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 2011;378:156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–1327 [DOI] [PubMed] [Google Scholar]
- 8.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol 2009;54:505–512 [DOI] [PubMed] [Google Scholar]
- 9.Mäki-Petäjä KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007;50:852–858 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell GF, Izzo JL, Jr, Lacourcière Y, et al. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation 2002;105:2955–2961 [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF, Dunlap ME, Warnica W, et al. Prevention of Events With Angiotensin-Converting Enzyme Inhibition Investigators Long-term trandolapril treatment is associated with reduced aortic stiffness: the prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension 2007;49:1271–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension 2009;54:763–768 [DOI] [PubMed] [Google Scholar]
- 13.Dengo AL, Dennis EA, Orr JS, et al. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010;55:855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care 2009;32:1531–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman S, Ismail AA, Ismail SB, Naing NN, Abdul Rahman AR. Effect of rosiglitazone/ramipril on preclinical vasculopathy in newly diagnosed, untreated diabetes and IGT patients: 1-year randomised, double-blind, placebo-controlled study. Eur J Clin Pharmacol 2007;63:733–741 [DOI] [PubMed] [Google Scholar]
- 16.Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M. Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism 2011;60:1278–1284 [DOI] [PubMed] [Google Scholar]
- 17.Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BHR, Rutten G, Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord 2000;24(Suppl. 3):S6–S11 [DOI] [PubMed] [Google Scholar]
- 18.The ADDITION study. Anglo-Danish-Dutch Study of Intensive Treatment in People with Screen-Detected Diabetes in Primary Care [article online], 2009. Available from http://www.clinicaltrials.gov/ct2/show/NCT00237549?term=addition&rank=21 Accessed 10 October 2005
- 19.Christensen JO, Sandbaek A, Lauritzen T, Borch-Johnsen K. Population-based stepwise screening for unrecognised Type 2 diabetes is ineffective in general practice despite reliable algorithms. Diabetologia 2004;47:1566–1573 [DOI] [PubMed] [Google Scholar]
- 20.van den Donk M, Sandbaek A, Borch-Johnsen K, et al. Screening for type 2 diabetes. Lessons from the ADDITION-Europe study. Diabet Med 2011;28:1416–1424 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classificaiton of Diabetes Mellitus. Geneva, World Health Org., 1999 [Google Scholar]
- 22.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 23.Rubak S, Sandbæk A, Lauritzen T, Borch-Johnsen K, Christensen B. Effect of “motivational interviewing” on quality of care measures in screen detected type 2 diabetes patients: a one-year follow-up of an RCT, ADDITION Denmark. Scand J Prim Health Care 2011;29:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royal College of General Practitioners in Denmark Type 2 Diabetes in General Practice: an Evidence Based Guideline. Copenhagen, Denmark, Royal College of General Practioners, 2004:1–58 [Google Scholar]
- 25.Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens 2007;25:751–755 [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 1998;16:2079–2084 [DOI] [PubMed] [Google Scholar]
- 27.Hermeling E, Reesink KD, Reneman RS, Hoeks AP. Measurement of local pulse wave velocity: effects of signal processing on precision. Ultrasound Med Biol 2007;33:774–781 [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 29.Harte D. Non Asymtotic Binomial Confidence Intervals. Statistics Research Associates, Wellington, New Zealand: 2002, p. 1–3 [Google Scholar]
- 30.Software Operator's Guide: Pulse Wave Velocity Assessment System. Sydney, Australia, AtCor Medical Pty. Ltd., 2008;9.1:13–14 [Google Scholar]
- 31.Software Operator's Guide: Central Blood Pressure Assessment System. Sydney, Australia, AtCor Medical Pty. Ltd., 2008;9.1:27–31 [Google Scholar]
- 32.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 33.Nichols WW, Denardo SJ, Wilkinson IB, McEniery CM, Cockcroft J, O’Rourke MF. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens (Greenwich) 2008;10:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutouyrie P, Achouba A, Trunet P, Laurent S, EXPLOR Trialist Group Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension 2010;55:1314–1322 [DOI] [PubMed] [Google Scholar]
- 35.Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension 2001;37:1429–1433 [DOI] [PubMed] [Google Scholar]
- 36.Karalliedde J, Smith A, DeAngelis L, et al. Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension 2008;51:1617–1623 [DOI] [PubMed] [Google Scholar]
- 37.Boutouyrie P, Lacolley P, Briet M, et al. Pharmacological modulation of arterial stiffness. Drugs 2011;71:1689–1701 [DOI] [PubMed] [Google Scholar]
- 38.Wulffelé MG, Kooy A, de Zeeuw D, Stehouwer CD, Gansevoort RT. The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med 2004;256:1–14 [DOI] [PubMed] [Google Scholar]
- 39.Stakos DA, Schuster DP, Sparks EA, Wooley CF, Osei K, Boudoulas H. Long term cardiovascular effects of oral antidiabetic agents in non-diabetic patients with insulin resistance: double blind, prospective, randomised study. Heart 2005;91:589–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Royal College of General Practitioners in Denmark. Type 2-Diabetes in General Practice: Diagnosis and Treatment. Copenhagen, Denmark, Royal College of General Practioners, 1999, p. 1–29 [Google Scholar]