Abstract
OBJECTIVE
To determine the effect of adding cilostazol (100 mg b.i.d.) to standard-dose clopidogrel (75 mg/d) (TRIPLE) compared with double-dose clopidogrel (150 mg/d) (DOUBLE) and the influence of the cytochrome P450 (CYP2C19*2/*3, CYP3A5*3)and ATP-binding cassette subfamily B1(ABCB1 C3435T) genetic polymorphisms in type 2 diabetes (T2DM) patients.
RESEARCH DESIGN AND METHODS
T2DM patients were treated with TRIPLE (n = 41) or DOUBLE (n = 39) after percutaneous coronary intervention. Conventional aggregometry and VerifyNow were performed at baseline and at 30 days. The primary end point was absolute change in 20-μM ADP-induced maximal platelet aggregation (ΔMPA20) between baseline and switching values.
RESULTS
TRIPLE versus DOUBLE showed greater ΔMPA20 (22.9 ± 11.6 vs.12.7 ± 15.5%; difference, 10.2% [95% CI 4.2–16.3]; P < 0.001). Carriage of one (β coefficient, −5.4%; P = 0.162) and two CYP2C19 loss-of-function allele(s) (−8.3%; P = 0.007) were associated with lower ΔMPA20 in DOUBLE–treated patients, but not in TRIPLE-treated patients.
CONCLUSIONS
Among T2DM patients, adding cilostazol achieves greater platelet inhibition compared with clopidogrel (150 mg/d), which is not influenced by genetic polymorphisms.
Patients with type 2 diabetes (T2DM) have greater morbidity and mortality from cardiovascular disease than patients without T2DM. Moreover, increased short- and long-term ischemic event occurrences have been observed in diabetes patients treated with percutaneous coronary intervention (PCI) (1). Dual-antiplatelet therapy with aspirin and a P2Y12 inhibitor has been the mainstay to prevent ischemic events among T2DM patients undergoing PCI. However, the antiplatelet and clinical responses to clopidogrel in PCI-treated patients can be influenced by several single nucleotide polymorphisms of the gene encoding cytochrome P450 (CYP2C19*2/*3, CYP3A5*3) and the ATP-binding cassette gene B1 (ABCB1) (2,3).
Cilostazol is a dual-inhibitor of phosphodiesterase type 3 (PDE3) and adenosine reuptake in endothelium, vascular smooth muscle cells, inflammatory cells, and platelets (4). These pleiotropic effects, in addition to platelet inhibition, may affect the occurrence of atherothrombosis in cilostazol-treated patients. The pharmacodynamic and clinical benefit of cilostazol is more prominent in high-risk settings, particularly in diabetes patients (4,5). Cilostazol maintains intraplatelet cyclic AMP levels, which are markedly abnormal in diabetes patients, making them more susceptible to cilostazol effects (5). However, cilostazol metabolism also shows the substantial interindividual variability via CYP3A5 and CYP2C19 polymorphisms (6).
The current analysis evaluated the antiplatelet effect of adding cilostazol (TRIPLE) or double-dose clopidogrel (150 mg/d) (DOUBLE) compared with standard-dose clopidogrel in high-risk T2DM patients undergoing PCI. We also assessed the influence of single nucleotide polymorphisms on the effect of these regimens.
RESEARCH DESIGN AND METHODS
This ACCEL-DM (Adjunctive Cilostazol versus double-dose ClopidogrEL in Diabetes Mellitus) study was a subanalysis of T2DM Korean patients who were recruited from prospective, randomized, platelet-function studies. T2DM was defined according to the criteria of American Diabetes Association (7). The local ethics committee approved the study protocol, and signed informed consent was obtained from all patients. Patients receiving elective PCI received a 300-mg clopidogrel loading dose at least 12 h before PCI or were receiving chronic clopidogrel therapy (75 mg/d ≥5 days). Patients with acute myocardial infarction (AMI) patients being treated with emergency PCI received a 600-mg clopidogrel loading dose, followed by clopidogrel (75 mg/d) for at least 5 days before randomization. After randomization, the TRIPLE group received cilostazol (100 mg b.i.d.), clopidogrel (75 mg/d), and aspirin (200 mg/d) for 30 days. The DOUBLE group received clopidogrel (150 mg/d) and aspirin (200 mg/d) for 30 days.
Blood samples for platelet function were collected immediately before elective PCI or at least 5 days later after emergency PCI, and 2–6 h after the last dose at the 30-day follow-up. Light transmittance aggregometry (LTA) and VerifyNow (Accumetrics, San Diego, CA) were used as previously described (8). Platelet aggregation (PA) values (maximal and 5-min final) induced by ADP (5 and 20 μmol/L) or collagen (6 μg/mL) were determined using an AggRAM aggregometer (Helena Laboratories Corp., Beaumont, Texas). Absolute changes in PA (ΔPA) were defined as changes of values between baseline and 30-day follow-up: ΔPA = baseline PA − 30-day PA.
CYP2C19 genotyping used the ABI SNaPshot reaction. Genotyping for CYP3A5*3 and ABCB1 C3435T was performed using the TaqMan method (Applied Biosystems, Foster City, CA).
The primary end point was the absolute change in maximal PA induced by 20 μmol/L ADP (ΔMPA20ADP). High on-treatment platelet reactivity (HPR) was defined as 5 μmol/L ADP-induced maximal PA >46% (LTA) or P2Y12 reaction units (PRU) >235 (VerifyNow) (9).
The sample size calculation was based on an earlier observed difference in 20 μmol/L ADP-induced maximal PA after adding cilostazol or doubling of the clopidogrel dose (8). At least 38 patients in each group were needed to detect an absolute difference in 20 μmol/L ADP-induced maximal aggregation of 15% with a power of 90%, a two-sided α = 0.05, and a SD of 0.2.
Continuous variables were compared using the Student t test, Mann-Whitney U test, or one-way ANOVA; categoric variables were compared using χ2 or the Fisher exact test. To evaluate the effect of covariates on ΔMPA20ADP, a multivariate linear regression analysis was performed including variables showing P < 0.2 in univariate analysis. Analyses were performed with SPSS 18.0 software (SPSS, Inc., Chicago, IL), and P < 0.05 was considered significant.
RESULTS
Among 80 T2DM patients with available genotype, 39 were admitted for AMI and 77 were treated with a drug-eluting stent. Baseline characteristics were well matched (Supplementary Table 1). In the TRIPLE group (n = 41), there were five cases of transient headache and three cases of palpitation for 3–5 days after the study was initiated regimen. In the DOUBLE group (n = 39), two patients presented with transient headache and two with gastrointestinal discomfort. These adverse events were well tolerated overall, and no major ischemic or bleeding events occurred during the study period. Baseline platelet reactivity and HPR prevalence before randomization did not differ between the TRIPLE (n = 41) and DOUBLE (n = 39) groups. At the 30-day follow-up, platelet reactivity and the prevalence of HPR in the TRIPLE group was consistently lower than in the DOUBLE group (P ≤ 0.124; Supplementary Table 2).
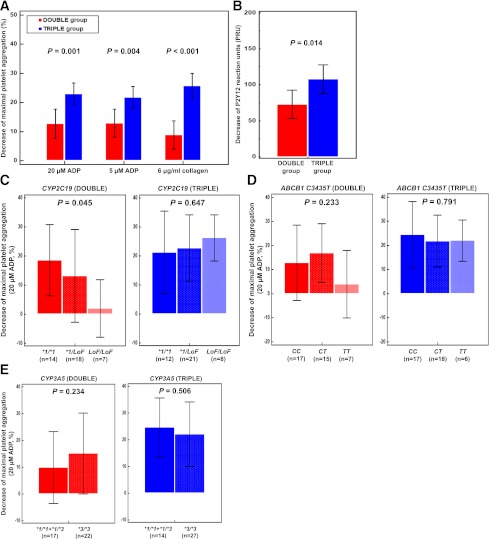
TRIPLE was associated with a greater ΔMPA20ADP of 22.9 ± 11.6% compared with 12.7 ± 15.5% for DOUBLE (difference, 10.2% [95% CI 4.2–16.3%]; P < 0.001; Fig. 1A). Other changes of LTA-based PAs also showed the same results (P ≤ 0.021). TRIPLE achieved a higher ΔPRU of 108 ± 63 compared with 73 ± 61 for DOUBLE (difference, 35 [7–62]; P = 0.014; Fig. 1B). Furthermore, a significant decrease in prevalence of HPR was observed with TRIPLE compared with DOUBLE based on the criteria of LTA (61.0 vs. 20.5%; P < 0.001) and VerifyNow (58.5 vs. 33.3%; P = 0.024).
Figure 1.
Decreases of maximal platelet aggregations (A) and P2Y12 reaction units (B) between baseline and the 30-day follow-up. Decreases of 20 μmol/L ADP-induced maximal platelet aggregation by double-dose clopidogrel (left bars, red) or adding cilostazol (right bars, blue): CYP2C19(C), ABCB1 C3435T(D), and CYP3A5 (E) genotypes. Results are expressed as means with the 95% CIs (error bars).
Carriage of the CYP2C19 loss-of-function (LoF) allele (*2 or *3) was relatively high, with 39 intermediate (48.8%) and 15 poor (18.7%) metabolizers (Supplementary Table 3). In the DOUBLE group, ΔMPA20ADP was associated with only the number of the CYP2C19 LoF alleles (Fig. 1C–E). Compared with noncarriers, carriers of one (β coefficient, −5.4% [SE 4.7%]; P = 0.162) and two CYP2C19 LoF alleles (−8.3% [2.7%]; P = 0.007) showed reduced values of ΔMPA20ADP (Supplementary Table 4). None of the clinical characteristics or genetic polymorphisms significantly influenced the effect of adding cilostazol (Fig. 1C–E, Supplementary Table 5).
CONCLUSIONS
To the best of our knowledge, the ACCEL-DM study is the first to compare the pharmacodynamic effect of TRIPLE versus DOUBLE in high-risk T2DM patients after PCI (10). This study demonstrated that the antiplatelet effect of adding cilostazol is not influenced by genetic variations and demographic characteristics and that the double-dose clopidogrel effect is significantly influenced by the CYP2C19 LoF variant, which is in line with the recent pharmacokinetic and pharmacodynamic studies (11,12). A recent study suggested that tripling the maintenance dose of clopidogrel (225 mg/d) in the CYP2C19*2 heterozygotes achieved levels of platelet reactivity similar to the standard 75-mg dose in noncarriers, but the maintenance dose (300 mg/d) did not result in comparable platelet inhibition among the CYP2C19*2 homozygotes (12).
A recent meta-analysis demonstrated that the addition of cilostazol might reduce long-term mortality by 31% over control in PCI-treated patients, without the increase of bleeding (13). Despite the same HPR during clopidogrel therapy, the linked magnitude of HPR to post-PCI ischemic events appeared greater in the diabetic cohort compared with the nondiabetic cohort (14). Diabetes itself increases the activity of inflammation, oxidative stress, and coagulation activity, which can increase the influence of platelet reactivity on clot formation (15). The inhibitory effect of cilostazol on PDE3, together with its effect on signaling through adenosine, prostaglandin, and nitric oxide on platelets, vascular smooth muscle cells, endothelium, and inflammation cascades are likely to contribute to its overall clinical benefits in diabetes patients (4). In addition, the antiplatelet effect of adding cilostazol appears not to be influenced by the CYP2C19 genotype. Taken together, adding cilostazol may be a safe and commendable antiplatelet regimen to reduce PCI-related clinical events. However, this concept, which is based on several transitional research projects, needs to be validated by large-scale future clinical trials.
This study has several limitations. First, this study was a subgroup analysis with a relatively small number of patients. Because the genetic effect in response to treatments was evaluated with exploratory purposes, this analysis should be conceived as a “proof of concept” investigation. Second, this study was performed using candidate gene analysis, and other unknown genetic variants may be relevant in cilostazol and clopidogrel responses. Finally, this study may have overestimated the antiplatelet effect of each treatment because platelet reactivity after PCI can vary over time. However, the observed change between baseline and the 30-day follow-up was 73 PRU in the DOUBLE group, which was similar with ∼80 PRU result observed from the previous study (11).
Acknowledgments
This study was partly supported by grants from the Research Foundation of Gyeongsang National University Hospital and the Institute of the Health Sciences, Gyeongsang National University.
Y.-H.J. has received honoraria for lectures from sanofi-aventis, Daiichi Sankyo, Inc., and Otsuka. P.A.G. has received research grants, honoraria, and consultant fees from Haemoscope, AstraZeneca, Schering-Plough/Merck, Medtronic, Lilly/Daiichi Sankyo, Inc., sanofi-aventis/Bristol-Myers Squibb, Portola, Boston Scientific, Bayer, Norvatis, Accumetrics, Boehringer Ingelheim, and Johnson & Johnson. No other potential conflicts of interest relevant to this article were reported.
Y.-H.J., U.S.T., and P.A.G., researched the data, reviewed and edited the manuscript, and contributed to discussion. Y.P., T.J.K., J.R.P., S.-J.H., and C.H.K. researched the data. K.P.B., E.-H.K., J.-Y.H., and S.K. contributed to discussion. Y.-H.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented as an abstract for presentation at the 61st Annual Scientific Sessions of the American College of Cardiology, Chicago, Illinois, 24–27 March 2012.
Footnotes
Clinical trial reg. nos. NCT00915733 and NCT01012193, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2351/-/DC1.
References
- 1.Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 2011;123:798–813 [DOI] [PubMed] [Google Scholar]
- 2.Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010;376:1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh JW, Koo BK, Zhang SY, et al. Increased risk of atherothrombotic events associated with cytochrome P450 3A5 polymorphism in patients taking clopidogrel. CMAJ 2006;174:1715–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong YH, Tantry US, Bliden KP, Gurbel PA. Cilostazol to overcome high on-treatment platelet reactivity in korean patients treated with clopidogrel and calcium-channel blocker. Circ J 2011;75:2534–2536 [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Capranzano P, Ferreiro JL, et al. Impact of adjunctive cilostazol therapy on platelet function profiles in patients with and without diabetes mellitus on aspirin and clopidogrel therapy. Thromb Haemost 2011;106:253–262 [DOI] [PubMed] [Google Scholar]
- 6.Yoo HD, Cho HY, Lee YB. Population pharmacokinetic analysis of cilostazol in healthy subjects with genetic polymorphisms of CYP3A5, CYP2C19 and ABCB1. Br J Clin Pharmacol 2010;69:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2012;35(Suppl. 1):S64–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong YH, Lee SW, Choi BR, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With Clopidogrel Resistance) randomized study. J Am Coll Cardiol 2009;53:1101–1109 [DOI] [PubMed] [Google Scholar]
- 9.Bonello L, Tantry US, Marcucci R, et al. Working Group on High On-Treatment Platelet Reactivity Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919–933 [DOI] [PubMed] [Google Scholar]
- 10.Ferreiro JL, Ueno M, Desai B, Capranzano P, Capodanno D, Angiolillo DJ. Impact of adjunctive cilostazol therapy versus high maintenance dose of clopidogrel in suboptimal responders with diabetes mellitus. Rev Esp Cardiol 2012;65:105–106 [DOI] [PubMed] [Google Scholar]
- 11.Price MJ. Murray SS, Angiolillo DJ, et al. Influence of genetic polymorphisms on the effect of high- and standard-dose clopidogrel after percutaneous coronary intervention: the GIFT (Genotype Information and Functional Testing) study. J Am Coll Cardiol 2012;59:1928–1937 [DOI] [PubMed] [Google Scholar]
- 12.Mega JL, Hochholzer W, Frelinger AL, 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 2011;306:2221–2228 [DOI] [PubMed] [Google Scholar]
- 13.Takagi H, Umemoto T. Benefit, rather than safety, of cilostazol for long-term mortality in patients undergoing percutaneous coronary intervention: a meta-analysis of randomized trials. Int J Cardiol 2011;153:74–76 [DOI] [PubMed] [Google Scholar]
- 14.El Ghannudi S, Ohlmann P, Jesel L, et al. Impaired inhibition of P2Y(12) by clopidogrel is a major determinant of cardiac death in diabetes mellitus patients treated by percutaneous coronary intervention. Atherosclerosis 2011;217:465–472 [DOI] [PubMed] [Google Scholar]
- 15.Hess K, Grant PJ. Inflammation and thrombosis in diabetes. Thromb Haemost 2011;105(Suppl. 1):S43–S54 [DOI] [PubMed] [Google Scholar]