Abstract
OBJECTIVE
To test whether safe and effective glycemic control could be achieved in type 1 diabetes using a bihormonal bionic endocrine pancreas driven by a continuous glucose monitor in experiments lasting more than two days and including six high-carbohydrate meals and exercise as challenges to glycemic control.
RESEARCH DESIGN AND METHODS
Six subjects with type 1 diabetes and no endogenous insulin secretion participated in two 51-h experiments. Blood glucose was managed with a bionic endocrine pancreas controlling subcutaneous delivery of insulin and glucagon with insulin pumps. A partial meal-priming bolus of insulin (0.035 units/kg/meal, then 0.05 units/kg/meal in repeat experiments) was administered at the beginning of each meal (on average 78 ± 12 g of carbohydrates per meal were consumed). Plasma glucose (PG) control was evaluated with a reference quality measurement on venous blood every 15 min.
RESULTS
The overall mean PG was 158 mg/dL, with 68% of PG values in the range of 70–180 mg/dL. There were no significant differences in mean PG between larger and smaller meal-priming bolus experiments. Hypoglycemia (PG <70 mg/dL) was rare, with eight incidents during 576 h of closed-loop control (0.7% of total time). During 192 h of nighttime control, mean PG was 123 mg/dL, with 93% of PG values in the range of 70–180 mg/dL and only one episode of mild hypoglycemia (minimum PG 62 mg/dL).
CONCLUSIONS
A bihormonal bionic endocrine pancreas achieved excellent glycemic control with minimal hypoglycemia over the course of two days of continuous use despite high-carbohydrate meals and exercise. A trial testing a wearable version of the system under free-living conditions is justified.
Development of a fully or semiautomated device that achieves glycemic levels demonstrated to reduce long-term complications (1–4) while lowering the risk for hypoglycemia (5) and reducing patient burden has long been a goal in the treatment of type 1 diabetes and would improve quality of life for people with type 1 diabetes. We previously demonstrated the feasibility of safe and effective bihormonal therapy with subcutaneous insulin and glucagon directed by a computer algorithm using frequently sampled venous plasma glucose (PG) in sedentary subjects over the course of 27 h (6). In the same study we also compared the accuracy and reliability of three commercially available continuous glucose monitors (CGMs) in each subject. Based on these results and preclinical studies in diabetic pigs, we hypothesized that glycemic control could be achieved in humans with type 1 diabetes using glucose values from one of these CGMs as the sole input to the controller. Here, we report the results of a study testing this hypothesis in experiments more than 2 days in length that included six high-carbohydrate meals and a period of exercise as challenges to glycemic control. Subcutaneous dosing of glucagon and insulin was controlled by an algorithm requiring only the subject weight for initialization.
RESEARCH DESIGN AND METHODS
Subjects
The protocol was approved by the Massachusetts General Hospital (MGH) and Boston University Human Research Committees, and all participants gave written informed consent. At baseline, subjects were required to be 18 years of age or older, have had type 1 diabetes for at least 1 year, and be treated with an insulin pump. In addition, glycated hemoglobin A1c (HbA1c) had to be ≤9.0%, BMI had to be 20–35 kg/m2, daily insulin requirement was ≤1 units/kg/day, and a peak stimulated C-peptide level had to be ≤0.1 nmol/L after a mixed meal tolerance test. Other criteria are detailed in the Supplementary Data.
Closed-loop glucose control system
Insulin and glucagon were administered under closed-loop control (Supplementary Fig. 1) using control algorithms similar to those previously described (6). The only input signal was data from the Freestyle Navigator (Abbott Diabetes Care), an interstitial fluid CGM approved by the U.S. Food and Drug Administration (FDA). Insulin dosing was controlled by a customized model predictive control algorithm incorporating a pharmacokinetic model for insulin lispro that assumed a tmax of 65 min. Glucagon dosing was controlled by a customized proportional derivative algorithm. Insulin lispro and glucagon (Eli Lilly) were administered subcutaneously by OmniPod patch pumps (Insulet). With the exception of a weight-based partial meal-priming bolus, delivered at the beginning of each meal, the system was fully automated, and all of the components were worn by the subject except for the CGM receiver and a computer, which were mounted on an intravenous line pole to allow freedom of movement.
The control system came online with the first CGM glucose (CGMG) measurement. There was no device learning period, nor was there any information about the subject’s usual insulin regimen provided to the algorithm. The control system received CGMG readings and commanded dosing of insulin or glucagon (or both) every 5 min. Because the lag between PG and CGMG handicaps the system in controlling postmeal glycemic excursions, a meal-priming insulin bolus based only on subject weight (0.035 units/kg in the initial experiments and 0.05 units/kg in repeat experiments) was administered at the beginning of each meal. These meal-priming boluses were intended to provide less than half the insulin required for each meal. Although the priming bolus was automatically delivered, this was possible only because the timing of the six meals was known in advance. In normal use, this would not be the case, and the user would need to indicate meal timing manually.
Closed-loop glucose control experiments
Subjects were admitted to the MGH Clinical Research Center and received basal insulin from their own pumps until initiation of closed-loop control. The Navigator CGM, inserted the day before, was linked wirelessly to the system and calibrated strictly according to the manufacturer’s instructions, except that venous PG values were used for calibration. Venous PG levels, from which the primary outcomes were derived, were measured every 15 min with the GlucoScout (International Biomedical) and were confirmed hourly with a YSI 2300 STAT Plus Analyzer (YSI Life Sciences). Only CGMG values were available to the control system.
Two OmniPod pumps, one filled with U-100 (100 units per mL) insulin lispro and the other filled with 1 mg/mL glucagon, were attached to the subject, activated, and linked wirelessly to the system. The glucagon pump was replaced approximately halfway through the 2-day experiment so that administered glucagon was never in solution for more than 27 h (Supplementary Data). At 3:00 p.m., closed-loop glucose control was initiated. Six meals (each consumed in 30 min) were provided over each of the 51-h experiments, with ≥50% of calories from carbohydrate. The mean carbohydrate consumption was 78 ± 12 g per meal (60–117 g per meal). Exercise on a stationary bicycle began at 4:00 p.m. on the second day, regardless of the PG at the time, and lasted ∼30 min with a target heart rate of 120–140 bpm, until a total of 4,000 heartbeats were reached. The experiment ended at 6:00 p.m. on the third day, after 51 h of closed-loop control.
Hypoglycemia was defined as venous PG <70 mg/dL. It was treated with fruit juice if PG sampled every 15 min remained <70 mg/dL for three consecutive measurements, <60 mg/dL for two consecutive measurements, <50 mg/dL once, or if subjects had symptoms of hypoglycemia concurrent with PG <70 mg/dL (Supplementary Data).
Laboratory and pharmacokinetic analyses
Samples for insulin and glucagon measurements were obtained at 30-min intervals from 7:00 p.m. to 4:00 a.m. and at 60-min intervals otherwise. Immunoassays were used to measure insulin (Architect insulin assay, Abbott Laboratories) and glucagon (glucagon radioimmunoassay, Millipore). Blood obtained during screening for HbA1c measurement was assayed by high-performance liquid chromatography (7). C-peptide was measured by a two-site immunometric assay using electrochemiluminescence detection (Roche). Plasma tmax for lispro was derived as previously described (6).
Statistical analyses
The prespecified primary outcomes were: mean PG; percent of PG values <70 mg/dL, 70–120 mg/dL, 70–180 mg/dL, and >180 mg/dL; and number of total and carbohydrate-treated hypoglycemic events. Outcomes were calculated for the last 48 h of each experiment to reduce the influence of pre-experimental conditions. The time from 11:00 p.m. to 7:00 a.m. was defined as night.
Three experiments were affected by technical failures associated with insulin delivery, but the results of these experiments were included in the analyses. Other than pump replacements when pump or infusion catheter malfunction was clinically suspected, no additional interventions were made; the control system was allowed to recover and manage the glycemic consequences autonomously.
Statistical analyses were performed in Excel (Microsoft). Comparisons between groups were performed with the paired sample heteroskedastic Students t test. Because there were no significant differences in the primary outcome measures between the experiments using the two different meal-priming boluses, the two groups of experiments were combined for these analyses. Calculations of mean intrasubject and intersubject differences are described in the Supplementary Data.
RESULTS
Subjects
Six subjects (three male, three female) each participated in two 51-h closed-loop blood glucose control experiments. Subjects were 52 ± 14 (33–72) years of age, had type 1 diabetes for 32 ± 14 (17– 50) years, had a peak stimulated C-peptide after a mixed meal tolerance test that was below the assay limit of detection (C-peptide <0.033 nmol/L) and had HbA1c at screening of 7.4 ± 0.7% (6.4–8.3%). Their average body mass was 72 ± 10 kg (54–85 kg); they had a BMI of 25 ± 3 kg/m2 (22–30 kg/m2) and a total daily dose (TDD) of insulin of 0.45 ± 0.09 units/kg (0.31–0.56 units/kg) with their usual insulin regimen (Supplementary Table 1).
Glycemic control
The aggregate results of all experiments are shown in Fig. 1. The bionic endocrine pancreas achieved an aggregate mean PG of 158 ± 44 mg/dL (range 36–563 mg/dL) over 576 h of control (Table 1). Sixty-eight percent of PG values were in the target range of 70–180 mg/dL (8) (Fig. 2). The mean of the peak postprandial PG levels of all 72 meals across the 12 subjects was 257 ± 69 mg/dL (the means of the peak postprandial PG levels after the breakfast, lunch, and dinner meals were 269 ± 64, 230 ± 46, and 270 ± 85 mg/dL, respectively). The mean PG during night hours was 123 ± 13 mg/dL (range 62–406 mg/dL) over 192 h of closed-loop control, with 93% of PG values in the range of 70–180 mg/dL.
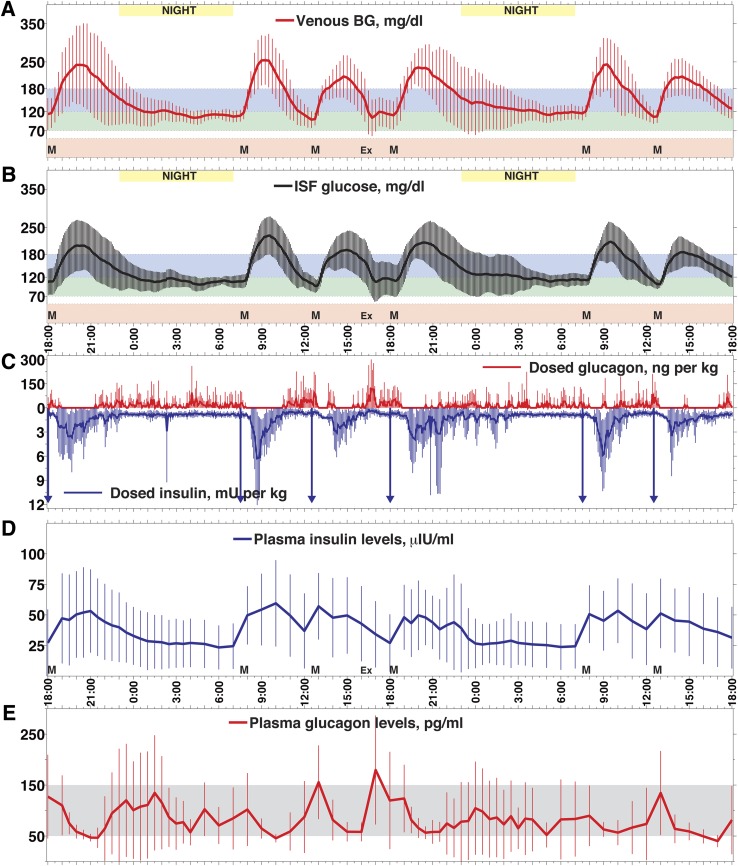
Figure 1.
Mean (SD) of venous PG (A), CGMG (B), insulin and glucagon doses (C), plasma insulin levels (D), and glucagon levels (E) for all experiments. A: The mean (SD) of venous PG levels with 15-min sampling is shown from all (N = 12) 48-h experiments in six subjects. The maximum in the mean PG was 254 mg/dL at 9:15 a.m. after the first breakfast, and the mean nadir was 99 mg/dL at 12:30 p.m. at the start of the first lunch. The overall mean of all 48-h PG results (N = 193 measurements per experiment) was 158 ± 44 mg/dL. The overall mean PG during nighttime (11 p.m.–7 a.m.) was 123 ± 13 mg/dL (N = 66 measurements per experiment). The six meals are indicated by the letter M. The exercise period is indicated by Ex. B: The mean (SD) of CGMG levels, measured every 5 min, are shown. The mean peak CGMG was 229 mg/dL, recorded at 9:30 a.m. after the first breakfast, and the mean nadir was 97 mg/dL at 12:30 p.m. at the start of the first lunch. The overall mean of all CGMG measurements was 145 ± 35 mg/dL (N = 577 measurements per experiment). The overall mean CGMG overnight was 116 ± 9 mg/dL (N = 194 measurements per experiment). C: The means of all subcutaneous insulin doses (vertical lines below), including meal-priming insulin doses (indicated by downward arrows), and glucagon doses (vertical lines above), administered by the bionic endocrine pancreas, are indicated. The mean daily doses of insulin and glucagon administered by the program were 0.5 units/kg/day and 3.64 μg/kg/day, respectively. D: The mean (SD) plasma insulin levels, measured every 30–60 min, with mean over 48 h of 38 ± 10 μIU/mL. The tmax for insulin absorption ranged from 24–166 min in all subjects and was 70 min on average. E: The mean (SD) plasma glucagon levels, measured every 30–60 min. The mean glucagon level over 48 h was 83 ± 28 pg/mL, with peak mean levels increasing only transiently over the normal fasting range (indicated by shaded area) to 180 pg/mL at 5:00 p.m. after exercise. The peak level is consistent with the increased glucagon dosing at the time of exercise seen in (C).
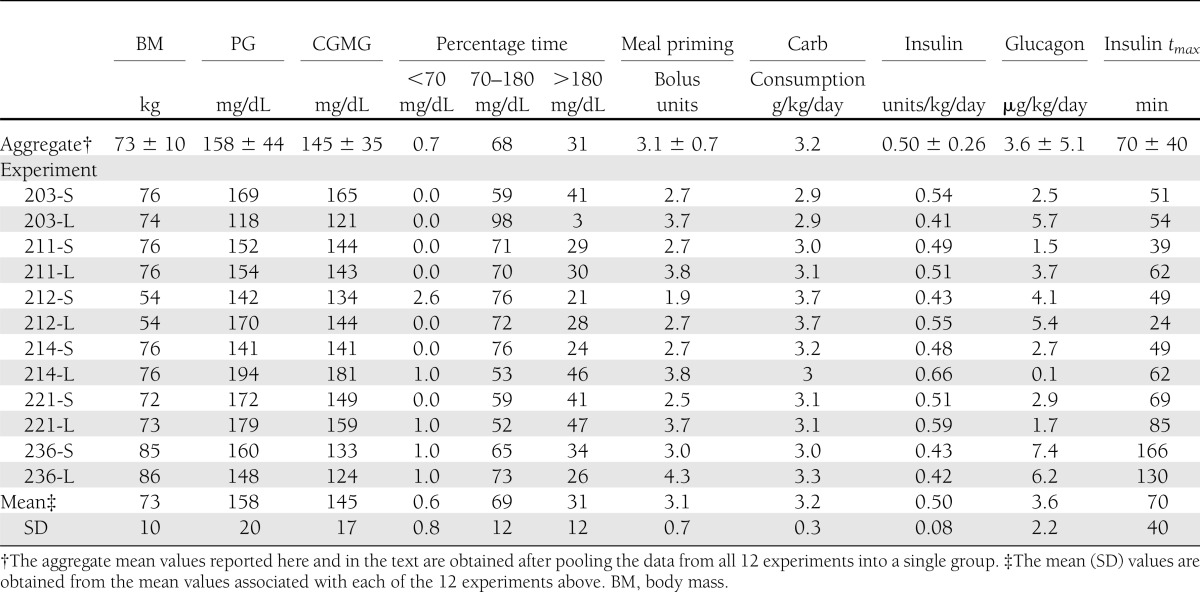
Table 1.
Summary results of all 48-h closed-loop experiments
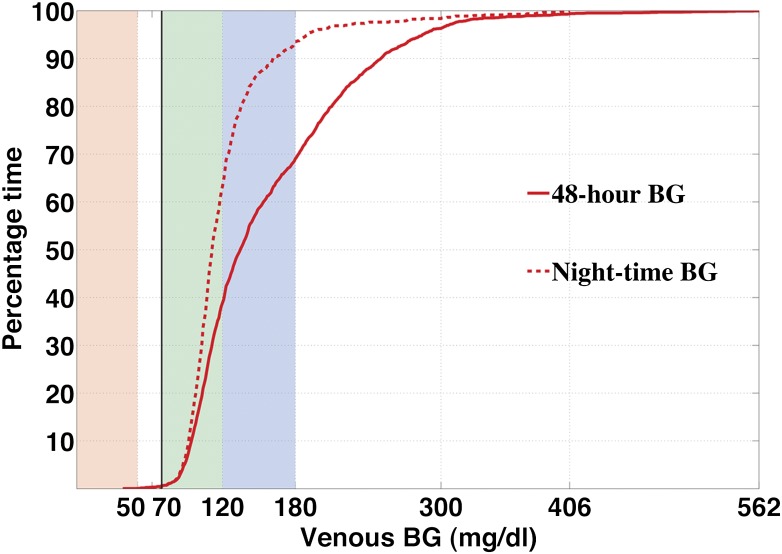
Figure 2.
Cumulative distributions for 48-h and night PG profiles. The solid curve shows the distribution of all recorded venous PG levels (N = 2078) in the twelve 48-h closed-loop experiments. Venous PG was <70 mg/dL 0.7% of total study time, within 70–120 mg/dL 38% of time, and within the American Diabetes Association target range of 70–180 mg/dL 68% of the time. The dashed curve shows the distribution of all night (11:00 p.m.–7:00 a.m.) venous PG levels (676 measurements over 2 nights for each experiment). Venous PG was <70 mg/dL 0.5% of total study time, within 70– 120 mg/dL 62% of the time, and within 70–180 mg/dL 93% of the time. A previous meta-analysis of closed-loop control studies limited to the night hours reported 76% of PG values in adults with type 1 diabetes were within their specified target range of 70–144 mg/dL (11). In this study, 84% of venous PG values were within this range despite one of the insulin delivery failures occurring during night hours (Supplementary Fig. 7).
During structured exercise, which occurred in the late postprandial period after lunch, the mean rate of decrease in PG was more than four-fold greater than in the same period on the day without exercise (1.8 ± 1.2 vs. 0.4 ± 0.4 mg/dL/min, P = 0.002) (Fig. 1A). Furthermore, the mean PG was markedly lower at the PG check after exercise than it was 24 h later (104 ± 48 mg/dL vs. 159 ± 32 mg/dL, P = 0.003). This nadir was followed by a rebound in mean PG to a maximum of 120 ± 38 mg/dL 45 min after exercise, and was associated with a peak period in glucagon dosing. This pattern was absent on the day without exercise. However, there was no trend for reduced mean PG (156 ± 27 vs. 161 ± 22 mg/dL) between the first and second 24-h periods or between the first and second nights (119 ± 22 vs. 128 ± 38 mg/dL). In fact, the mean PG tended to be higher during the night after exercise. However, with 12 experiments, our study lacks sufficient power to exclude a difference in glucose control between the first and second 24-h periods or between the nights before and after exercise.
Hypoglycemia
There were eight episodes of hypoglycemia with no more than mild symptoms in five subjects, with 0.7% of all PG values <70 mg/dL. Six episodes occurred during the first 24-h period and two occurred during the second 24-h period (Fig. 3). During the night hours there was a single episode of hypoglycemia (lowest PG 62 mg/dL) with 0.5% of night PG values <70 mg/dL. The longest episode of PG <70 mg/dL was 32 min, although the duration of episodes treated with carbohydrates likely would have been longer had carbohydrates not been administered. Two of the episodes resolved without oral carbohydrate treatment (Fig. 3A, B), and one of these appeared to be a measurement artifact (Fig. 3A). The six remaining hypoglycemic episodes, five in the late postprandial period and one nocturnal (1:15 a.m.) (Fig. 3C–F), were treated with juice. Two of the episodes immediately followed the period of exercise (Fig. 3C, D). One episode occurred when the meal-priming bolus was administered according to schedule, but the meal was presented to the subject 20 min late (Fig. 3D, Supplementary Fig. 6).
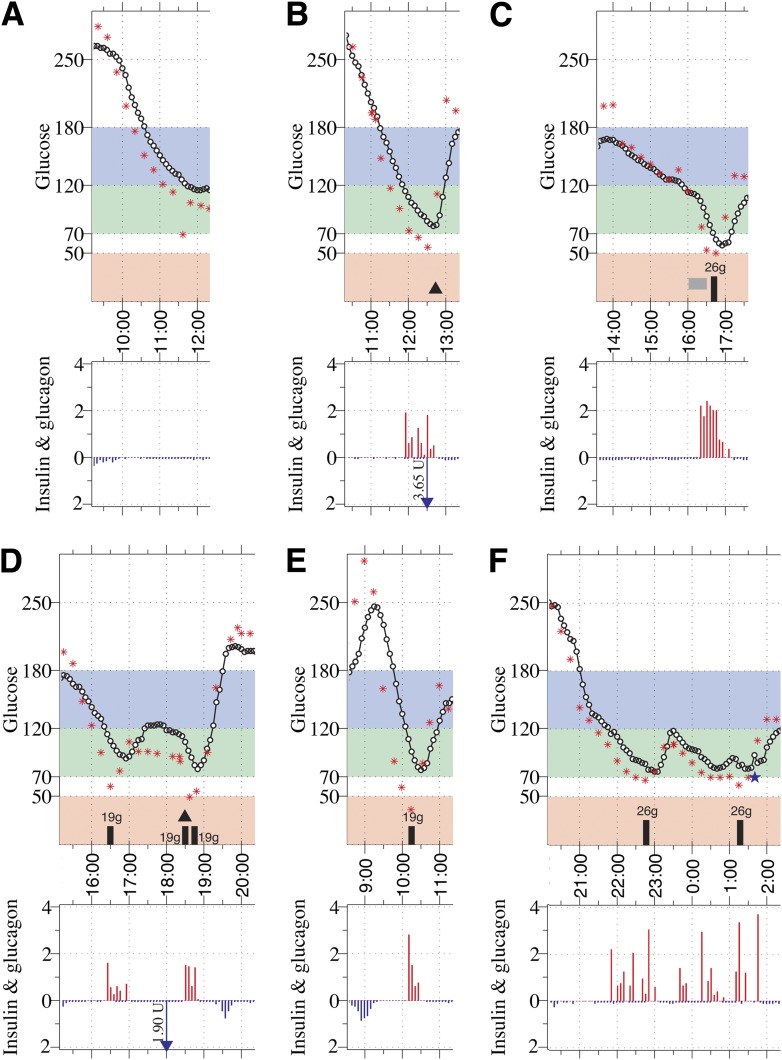
Figure 3.
Glucose profiles and the corresponding insulin–glucagon doses that were administered by the control algorithm are shown for all eight hypoglycemic episodes. A–C: Hypoglycemic episodes (defined as events with PG <70 mg/dL) that occurred during experiments using 0.05 units/kg meal-priming boluses (venous PG shown as stars and CGMG as circles). D–F: Hypoglycemic episodes that occurred during experiments that used 0.035 units/kg meal-priming boluses. The isolated PG value of 69 mg/dL in (A) appears to be a measurement artifact. The first PG value <70 mg/dL in each of the five hypoglycemic episodes shown in (B–E) were 28, 35, 47, 48, and 89 mg/dL lower than their corresponding CGMG values, which were 94, 88, 107, 97, and 148 mg/dL, respectively. Both interventions in (F) were at the request of the subject and, in both cases, PG was >60 mg/dL and CGMG was >80 mg/dL. Note that in (D) the meal was presented to the subject 20 min late, at 18:20, although the meal-priming bolus was administered on time, at 18:00. (tmax for that experiment was 49 min.)
Insulin and glucagon administration and pharmacokinetics
Mean insulin usage was 0.5 ± 0.1 units/kg/day (Table 1), with 0.49 ± 0.1 and 0.51 ± 0.08 units/kg/day in the first and second 24-h periods, respectively (P = 0.62), similar to the daily dose in the home setting (Supplementary Table 1). On average, less than half of the insulin for each meal was provided by the meal-priming bolus, with the remainder delivered by the algorithm. In particular, for the 0.035 units/kg group, the priming bolus doses, controller bolus doses, and automated basal doses accounted, on average, for 22%, 27%, and 51% of the TDD of insulin, respectively. In the case of the 0.05 units/kg group, this distribution was 29%, 23%, and 48%. More of the prandial insulin (delivered in the 4 h after presentation of the meal) came from the meal-priming bolus in the 0.05 units/kg group than in the 0.035 units/kg group (42 ± 8% vs. 30 ± 3%, P < 0.001). However, the mean intrasubject difference in insulin lispro TDD was not significantly different from the mean intersubject difference derived by comparing each of the smaller priming bolus experiments with all of the larger priming bolus experiments, and vice versa (0.09 vs. 0.086 units/kg/day, P = 0.90).
As we found previously (6), there were large intersubject and intrasubject variations in insulin lispro pharmacokinetic parameters (Table 1) with tmax from 24 to 166 min (mean 70 ± 40 min). The mean intersubject difference in insulin lispro tmax was significantly larger than the mean intrasubject difference (44.3 vs. 19.3 min, P < 0.001). Three of the hypoglycemic events treated with carbohydrate occurred in a single subject (subject 236) who had markedly slow insulin lispro absorption (Table 1); lispro tmax for this subject was 133 min in one experiment (one carbohydrate treatment; Fig. 3C) and 166 min in another (two carbohydrate treatments; Fig. 3F). The mean glucagon usage was 3.8 μg/kg/day (0.01–0.63 mg/day), with the largest single dose being 50 μg. Mean plasma glucagon levels were in the normal range for the fasted state (50–150 pg/mL), except during the period immediately after exercise when the mean level was 180 ± 107 pg/mL (compared with 40 ± 12 pg/mL at the same time on the day without exercise). The subject with the highest glucagon usage (7.4 and 6.2 μg/kg/day vs. 3.6 ± 2.2 μg/kg/day for mean glucagon usage) was the subject with the slowest insulin lispro absorption (subject 236), consistent with our previous finding that slow insulin absorption is associated with increased glucagon usage (9).
Performance of the CGM
A single Navigator sensor was used throughout each experiment and no extra calibrations were performed, so that the final 41–42 h of each experiment were performed without any Navigator calibrations. The Navigator CGM performed well relative to PG measurements, with a mean absolute relative difference of 11.8% (10). However, the mean CGMG (145 ± 35 mg/dL) was 13 mg/dL lower than the mean PG, and CGMG tracked PG into the hypoglycemic range during only one of the eight hypoglycemic episodes (Fig. 3). In six of the eight cases of hypoglycemia, the rate of decline in PG was >1 mg/dL/min (Fig. 3A–E and Supplementary Figs. 6, 9, 11, and 13). In these cases, the physiologic lag between PG and interstitial fluid glucose may have contributed to the failure to prevent hypoglycemia.
Technical failures
Two experiments were affected by a failure to deliver insulin. Both clinically suspected failures that prompted pump replacement were later confirmed with insulin measurements (Supplementary Figs. 7 and 9). Another experiment (Supplementary Fig. 2) was affected by a spurious report from the pump that a large dose of insulin (∼61 units) had been delivered. Because the algorithm considers insulin-on-board when calculating dosing, it refrained from dosing insulin for several hours, and hyperglycemia ensued. This same experiment also was affected by a 2-h period during which the system was offline and had to be restarted.
All data from each experiment are included in the outcome measures, including those affected by technical failures. None of the failures resulted in hypoglycemia. The mean PG for the nine experiments without technical failures was 151 ± 45 mg/dL, with 71% of PG measurements in the range of 70–180 mg/dL; the mean PG at night for these nine experiments was 115 ± 7 mg/dL.
Adverse events
The subject with the slowest insulin absorption and who received the most glucagon reported nausea on several occasions. However, the timing of these symptoms did not correspond to periods of high plasma glucagon levels (Supplementary Figs. 12 and 13). There were no other adverse events other than the nonsevere hypoglycemic episodes described.
CONCLUSIONS
These results demonstrate the feasibility of closed-loop blood glucose control with a bihormonal bionic endocrine pancreas utilizing CGMG measurements. The CGM and drug delivery components of the system are all off-the-shelf and approved by the FDA for diabetes management. The computational requirements of the algorithm are modest and easily could be met by a mobile device with less processing power than a smart phone.
These studies differ from previous work in closed-loop glucose control in several important respects. First, the control algorithm requires no information about the subject’s usual insulin regimen or any other data other than the body mass for initialization. The control algorithm and control parameters were identical for all 12 experiments. The system does not have to be customized for each subject as it does in other closed-loop insulin delivery systems (12–15); it automatically adapts to the subject’s needs in real time. Second, as in our previous study (6), we enrolled only subjects without stimulated C-peptide secretion, ensuring endogenous insulin secretion was not assisting closed-loop control. Third, we report data for closed-loop control over 48 continuous hours, which is longer than has been reported previously, during which subjects consumed six high-carbohydrate meals. Fourth, this is the first published study to evaluate closed-loop control during exercise, a common cause of hypoglycemia in patients with type 1 diabetes (16). Fifth, we monitored PG every 15 min, in contrast to studies with up to 60-min intervals between measurements that may have missed episodes of hypoglycemia (12,13,15). Sixth, we used only a single CGM sensor throughout the entire duration of each experiment with no calibrations for the last 41–42 h (the longest period of closed-loop control without CGM calibration yet reported), allowing us to realistically assess the potential of the system to operate fully autonomously. Seventh, only our previous study and one other study lasting more than 24 h (14) have tested systems using both insulin and glucagon. Finally, we have reported all subject-level data for every experiment performed with this control system, and we included all data in calculating outcome measures, including experiments with technical failures.
In addition to the delay in the absorption of subcutaneously administered insulin, the lag in CGMG relative to PG further compounds the challenge of regulating hyperglycemic excursions after meals. Therefore, we used a meal announcement to trigger automated delivery of a partial weight-based meal-priming insulin bolus. Interestingly, the glycemic control achieved with two different meal-priming insulin doses did not differ; the control algorithm appeared to compensate fully for the smaller meal-priming bolus with more reactive dosing of insulin. Additional study is required to determine if further increasing the meal-priming bolus can decrease mean PG without increasing postprandial hypoglycemia.
The period of exercise markedly increased glucose clearance and was associated with increased glucagon dosing and levels during and immediately after the exercise period. However, no difference was observed in overall glycemic control between the nights before and after the period of exercise, in contrast to a previous report that found a lower mean glucose and more hypoglycemia during nights under closed-loop control after a period of structured exercise that took place prior to the start of closed-loop therapy (17). This apparent difference from our findings may be explained by the lack of a counter-regulatory capability in the insulin-only system used in the previous report. However, although our results show a trend toward higher mean PG during the night after exercise, the study lacks sufficient power to exclude a reduced mean PG on the nights after exercise.
Limitations of our study include technical problems, primarily failures associated with insulin delivery. Because we report each complete study in the data analyses, including those with technical failures, the failures may have led us to underestimate the potential of the control approach. However, the failures allowed us to observe that the control system succeeded in returning PG to target range after each failure, thereby demonstrating the robustness of the system. The occurrence of such failures suggests that more reliable insulin delivery systems will improve system performance in the outpatient setting.
Another limitation is the lack of an open-loop control group in which subjects manage their blood glucose levels in the Clinical Research Center setting. Without data from such a study, we cannot assess how open-loop blood glucose control achieved by the subjects would have compared with the performance of the bionic pancreas in the Clinical Research Center with similar meals and a similar level of activity. In pilot open-loop control experiments, we found that subjects tested their blood glucose much more frequently than in usual care (as determined by self-reported testing frequency and meter downloads), achieved a much lower mean blood glucose than in usual care (determined by comparing mean blood glucose with estimated mean blood glucose based on HbA1c), and had more frequent hypoglycemia requiring carbohydrate treatment than they reported during usual care (unpublished observations). This problem could be dealt with by a protocol that regulates the number and timing of blood glucose checks, but that approach also would alter subject behavior, limiting the utility of the control group. We anticipate that control groups using sensor-augmented pump therapy will be less vulnerable to confounding when trials can be performed in less supervised environments with subjects involved in their normal daily activities.
Another limitation is the small number of subjects in this trial. However, the total time under closed-loop control in our study (576 h) was comparable with the largest of previously published studies comprising experiments lasting ≥24 h (578 h) (13) and was greater than in other recent studies (6,12,14,15). Furthermore, within our cohort there was a wide variation in age (33–72 years), body mass (54–86 kg), insulin lispro absorption characteristics (tmax 24–166 min), and TDD administered under closed-loop control (0.41–0.66 units/kg/day). Interestingly, the mean difference in insulin lispro TDD between experiments in the same subject was just as large as the mean difference in TDD between subjects. Therefore, in terms of interaction between the algorithm and the subject via insulin dosing, the six subjects participating in two experiments each were indistinguishable from 12 subjects each participating in a single experiment.
A limitation of the bionic endocrine pancreas is the accuracy of the CGM. Although overall accuracy was good, the Navigator underestimated PG during hyperglycemia, resulting in a lower overall mean CGMG than PG (145 vs. 158 mg/dL). We also observed that CGMG failed to detect most of the hypoglycemic events that were documented with PG measurements every 15 min. The errors in CGMG we observed occurred despite our use of reference-quality PG measurements for calibrations. Calibration protocols that do not rely on reference-quality PG measurements must be explored for use in the outpatient environment and sensors that perform better in tracking PG would be expected to improve mean PG and reduce hypoglycemia.
Despite technical limitations of the pump and CGM components, we have shown that a bihormonal bionic endocrine pancreas is capable of achieving good PG control with minimal hypoglycemia during two continuous days in the face of high-carbohydrate meals and exercise. Control was particularly good at night, achieving mean PG values in the normal range with no clinically significant hypoglycemia. The current study opens the way for longer-term and more definitive studies of a wearable version of this system incorporating more robust pump technology. We anticipate that these studies will be at least five days in length, with subjects following their normal routines, eating when and what they choose, and exercising at will on our hospital campus. Such studies will lead the way to testing a bionic endocrine pancreas in the outpatient setting.
Acknowledgments
This study was supported by grant R01-DK-085633 to E.R.D., from the National Institutes of Health; grants M01-RR-01066 and UL1-RR-025758, through the General Clinical Research Center and Clinical and Translational Science Center programs from the National Institutes of Health National Center for Research Resources; Clinical Investigations research grant 22-2009-798 to E.R.D., from the Juvenile Diabetes Research Foundation; and a grant to D.M.N. from the Charlton Fund for Innovative Research in Diabetes.
F.H.K. and E.R.D. have one patent and one pending patent on the closed-loop algorithm. No other potential conflicts of interest relevant to this article was reported.
S.J.R., E.R.D., F.H.K., and D.M.N. designed the study, performed the analysis, and interpreted the data. F.H.K. and E.R.D. designed and built the closed-loop control algorithm. F.H.K., J.J., and E.R.D. built the device. S.J.R. supervised the human studies. J.J. assisted in the design of several study procedures. K.L.M. assisted with the design of the human studies, coordinated subject enrollment, and was the primary liaison to the Clinical Research Center nursing staff. S.J.R. wrote the first draft of the manuscript. S.J.R., E.R.D., D.M.N., F.H.K., and K.L.M. participated in revision of the manuscript for important intellectual content. E.R.D. had full access to the data and takes full responsibility for this work as a whole, including the study design and the decision to submit and publish the manuscript.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank the volunteers for their time and enthusiasm; the diabetes care providers who referred potential subjects for the study; the nurses and laboratory staff of the MGH Clinical Research Center, especially Kathy Hall and Kathy Grinke, and the study staff at the Diabetes Research Center, including Kerry Grennan, Richard Pompei, Cathy Beauharnais, and Laurel Macey, for their dedicated effort and careful execution of the experimental protocol; Mary Larkin, Camille Collings, and Nancy Kingori, Diabetes Research Center, MGH, for organizational and logistical support; Patrick Sluss, Reproductive Endocrine Laboratory, MGH, for performing insulin and glucagon assays; Timothy Goodnow, Marc Taub, and Erwin Budiman, Abbott Diabetes Care, for providing Navigator hardware and software support and technical advice; Robert Campbell and Steve Gemmell, Insulet Corporation, for providing Insulet OmniPod hardware and software support and technical advice; John Segars and Jennifer Isenberg, International Biomedical, for providing GlucoScout monitors and technical assistance in their use; John Godine, Deborah Wexler, and Carl Rosow for serving on the data safety and monitoring board for the study, the members of the Partners Human Research Committee and Boston University Medical Campus Institutional Review Board for their oversight of the study; Charles Zimliki, Keith Marin, and Patricia Beaston, Office of Device Evaluation, FDA, for their helpful suggestions during the process of obtaining the investigational device exemption for this study; and Toby Milgrome, Reliant Medical Group, for her valuable input and advice.
Footnotes
See accompanying commentary, p. 2111.
Clinical trial reg. no. NCT01161862, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0071/-/DC1.
S.J.R. and F.H.K. share equal responsibility for this work.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the diabetes control and complications trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 5.Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care 2003;26:1176–1180 [DOI] [PubMed] [Google Scholar]
- 6.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Trans Med 2010;2:27ra27 [DOI] [PMC free article] [PubMed]
- 7.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 1984;310:341–346 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell SJ, El-Khatib FH, Nathan DM, Damiano ER. Efficacy determinants of subcutaneous microdose glucagon during closed-loop control. J Diabetes Sci Tech 2010;4:1288–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damiano ER, El-Khatib FH, Magyar KL, Nathan DM, Russell SJ. A comparative analysis of three continuous glucose monitors: not all are created equal (Abstract). Diabetes 2012;61(Suppl. 1): A2
- 11.Kumareswaran K, Elleri D, Allen JM, Harris J, Xing D, Kollman C, et al. Meta-analysis of overnight closed-loop randomized studies in children and adults with type 1 diabetes: The Cambridge Cohort. J Diabetes Sci Technol 2011;5:1352–1362 [DOI] [PMC free article] [PubMed]
- 12.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 13.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 14.Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003;26:1902–1912 [DOI] [PubMed] [Google Scholar]
- 17.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]