Abstract
OBJECTIVE
Skin intrinsic fluorescence (SIF) reflects many factors, including the presence of certain advanced glycation end products. We investigated whether SIF was associated with coronary artery disease (CAD) in type 1 diabetes and whether this relationship was independent of renal disease.
RESEARCH DESIGN AND METHODS
SIF was measured in 112 subjects from the Pittsburgh Epidemiology of Diabetes Complications (EDC) study and 60 from MedStar Health Research Institute when mean age and diabetes duration were 48 and 36 years, respectively. Cumulative glycemic exposure (updated mean A1C) represented a mean of 18 years’ follow-up in EDC and 10.3 in MedStar.
RESULTS
Of the 172 participants, 30 had CAD (15 male and 15 female). SIF levels were higher in those with CAD (P < 0.0001). SIF was strongly associated with CAD (odds ratio [OR] 3.5 [95% CI 2.1–6.1]). After age, duration, and updated mean A1C were controlled for, SIF remained associated with CAD (2.4 [1.3–4.4]), more strongly in men (5.6 [2.1–14.6]) than in women (1.4 [0.61–3.3]). As there was no significant sex interaction, further analyses were conducted combining the sexes. Further accounting for sex and nephropathy status did not improve the model fit, though with nephropathy in the model, the OR for SIF was reduced to 1.7 (95% CI 0.89–3.4).
CONCLUSIONS
SIF has a significant cross-sectional association with CAD. This association is strongly linked to age and duration and, to a lesser degree, to mean A1C and renal disease. SIF therefore may be a useful overall marker of CAD risk in type 1 diabetes.
Coronary artery disease (CAD) is a leading cause of death in type 1 diabetes (1). While risk factors for CAD include those observed in the general population, with a greater role for renal disease, much of the excess CAD in type 1 diabetes remains unexplained. Recently, increased plasma levels of advanced glycation end products (AGEs), specifically N′-(carboxyethyl)lysine, N′-(carboxymethyl)lysine, and pentosidine, were found to predict incident cardiovascular disease and all-cause mortality, independent of markers of renal impairment and other cardiovascular disease risk factors, in a population with type 1 diabetes (2).
AGEs are products formed in later-stage reactions of the glycation of proteins, amino acids, basic lipids, and nucleotides by monosaccharides and related monosaccharide derivatives (3) and are typically characterized by their ability to form cross-links with other proteins. When so linked (e.g., with collagen), AGEs may represent glycoxidation-induced vascular damage and are increased both in renal disease (4) and in diabetes (5). The renal retention likely reflects AGE-free adducts (4). AGE cross-link formation increases the stiffness of the arterial wall, while vascular matrix AGEs interfere with nitric oxide (NO) action (6), providing an important link between renal disease and CAD, and may account for some of the unexplained CAD in type 1 diabetes. Some AGEs, such as pentosidine and cross-lines, fluoresce when excited with near-ultraviolet and blue light (7). These AGEs are found in the dermis. In addition, epidermal fluorescence due to nicotinamide adenine dinucleotide and flavin adenine dinucleotide are indicators of cell metabolism and oxidative stress (8,9). These dermal and epidermal fluorophores both contribute to skin intrinsic fluorescence (SIF). We recently showed, in individuals with type 1 diabetes, that SIF was independently associated with the severity of coronary artery calcification (10), a marker of atherosclerotic burden, and with cardiac autonomic neuropathy (11), a strong predictor of CAD and sudden death. Based on our findings of an association of SIF with coronary artery calcification, we pursued the current investigation to determine the relationship between SIF and clinical CAD in 172 individuals with long duration of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Participants from the Epidemiology of Diabetes Complications (EDC) study and the MedStar Health Research Institute Diabetes Complications (MDC) Study were enrolled in this cross-sectional study. The EDC cohort is a well-defined population (n = 658) with type 1 diabetes diagnosed before the age of 17 years at the Children’s Hospital of Pittsburgh and followed since their baseline examinations between 1986 and 1988, when mean age was 28 years and mean duration of diabetes was 19 years (12). The MDC cohort represents a population of patients with type 1 diabetes followed for their clinical care for a minimum of 4 years and mean of 10 years. A convenience sample of 172 participants with type 1 diabetes was enrolled at both sites. The EDC participants (n = 112) had noninvasive SIF assessment performed during their 20th year of follow-up, ∼2 years after their last clinical exam, and were selected on a first-to-respond basis from those living within 50 miles of Pittsburgh with a goal of 100 participants. Though they were marginally older (P = 0.02) and more likely to have CAD (P = 0.02), no differences in diabetes duration, most recent A1C, or renal disease were seen compared with nonparticipants living within the 50-mile range. MDC participants (n = 60) were offered participation in this study following their routine clinical visit; no MedStar patients declined to participate. All study procedures were approved by the institutional review boards of the University of Pittsburgh and MedStar Health Research Institute.
Blood samples were assayed for lipids, lipoproteins, glycated hemoglobin, and creatinine. For the EDC study population, stable HbA1 was originally measured in saline-incubated samples by microcolumn cation exchange chromatography (Isolab, Akron, Ohio). On 26 October 1987, the method was changed to high-performance liquid chromatography (HPLC) (Diamat; Bio-Rad Laboratories, Hercules, CA). The two methods were highly correlated (r = 0.95; Diamat HbA1 0.18 ± 1.00 Isolab HbA1). Beginning in 1998, A1C was measured using the DCA2000 analyzer. Original HbA1 (1986–1998) and A1C (1998–2004) were converted to Diabetes Control and Complications Trial (DCCT)-aligned A1C values using regression formulas derived from duplicate analyses (DCCT A1C = [0.83 × EDC HbA1] + 0.14; DCCT A1C = [EDC A1C − 1.13]/0.81). For the MedStar study population, A1C was obtained with DCCT-aligned DCA 2000/DCA 2000+ instruments or by local laboratory, and historic A1C values (also DCCT aligned) were obtained through chart review. Cumulative glycemic exposure was determined using updated mean A1C, calculated by taking the sum of an individual’s A1C values over the duration of follow-up and dividing by the number of A1C measurements taken on the individual (biennially in EDC and approximately every 3 months in MDC).
CAD was defined as a history of myocardial infarction, revascularization for symptoms (bypass surgery or percutaneous coronary intervention), or stenosis >50%. The albumin-to-creatinine ratio was determined by spot urine collections in both EDC and MedStar, while albuminuria was determined by timed urine collections in EDC and by spot urine collections in MedStar. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula (13). Overt nephropathy was defined as an albumin excretion rate >200 μg/min or a history of dialysis/kidney transplantation.
SIF was noninvasively measured from the skin of the left volar forearm using the SCOUT DS (an investigational device in the U.S.; VeraLight, Albuquerque, NM) skin fluorescence spectrometer (14,15). SIF was excited with a light-emitting diode (LED) centered at 405 nm and was detected over the emission range of 441–482 nm. The skin reflectance at the excitation wavelength was used to compensate for absorbance owing to melanin and hemoglobin (16). The intrinsic fluorescence correction is expressed in the following equation:
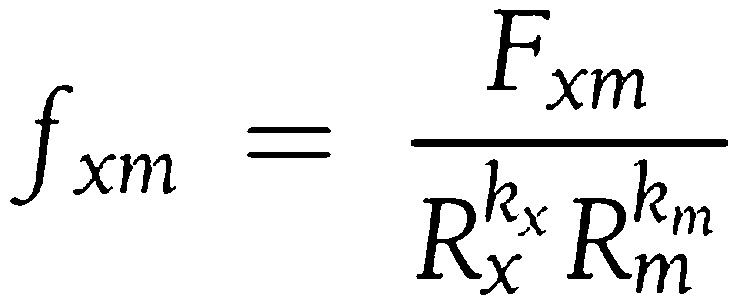 |
where the measured fluorescence (Fxm) is divided by reflectance values at the excitation and emission wavelengths (Rx and Rm), respectively. The reflectance values are adjusted by the dimensionless exponents (kx and km). For these analyses, kx was set to 0.9 and km was set to 0.0. The resulting intrinsic fluorescence (fxm), was integrated over the 441–482 nm spectral region to give the SIF sum. The interday within-subject skin variation in SIF assessed by the SCOUT DS had previously been determined in a large diabetes screening study of 421 subjects at risk for developing type 2 diabetes (17). The interday Hoorn coefficient of variation (fasting versus nonfasting) was 5.1% for SCOUT DS–measured SIF.
Student t and χ2 tests were used to examine univariate correlates of CAD. Logistic regression analysis was used to determine the independent association of SIF with the prevalence of CAD. Odds ratios (ORs) are expressed as per-SD change in continuous variables. Statistical analysis was conducted using SAS version 9.3 (Cary, North Carolina). Model fits were compared using Akaike information criteria (18).
RESULTS
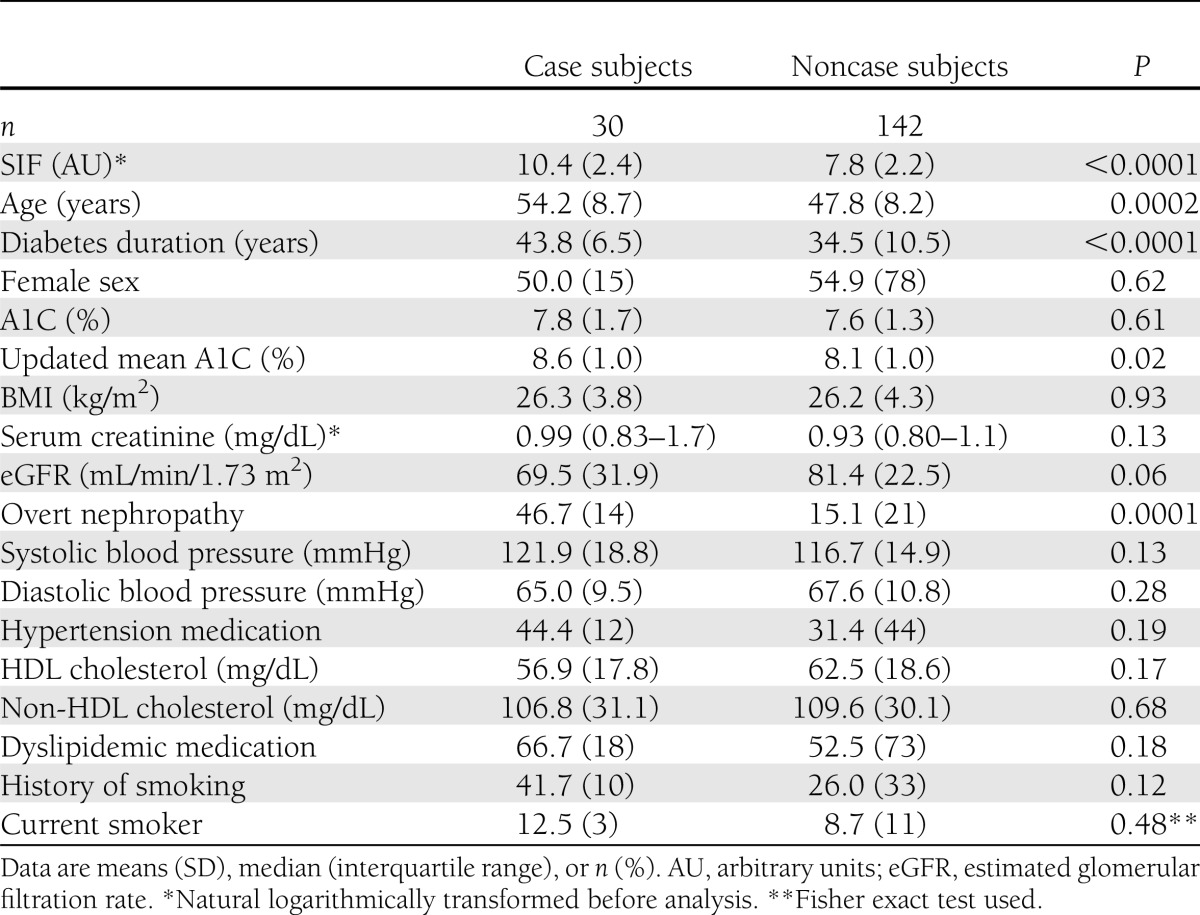
Eighteen percent (n = 30) of the study participants had CAD. Characteristics of the study participants by CAD status are presented in Table 1. Participants with CAD were on average ∼6 years older (54.2 vs. 47.8 years) and had 9 years longer (43.8 vs. 34.5 years) duration of diabetes. Participants with CAD also had higher SIF values and updated mean A1C, but no difference in their most recent A1C levels, and were more likely to have overt nephropathy. Increased SIF in those with CAD was seen in each cohort (OR 2.8 [95% CI 1.6–5.1] in EDC; 8.1 [1.5–45.3] in MDC). SIF correlated modestly with updated mean A1C (R = 0.44, P < 0.0001). No other differences by CAD status were apparent.
Table 1.
Characteristics of study participants by CAD status
Sex-specific analyses revealed a strong association between SIF and CAD in men (OR 5.6 [95% CI 2.1–14.6]) in analyses controlled for age and updated mean A1C. A weaker, nonsignificant, association was observed in women (1.4 [0.61–3.3]). However, as formal analyses revealed no significant effect modification by sex (P = 0.52) and there was not a significant difference in SIF distribution with sex stratification (P = 0.55), the sexes were combined for the remainder of the analyses.
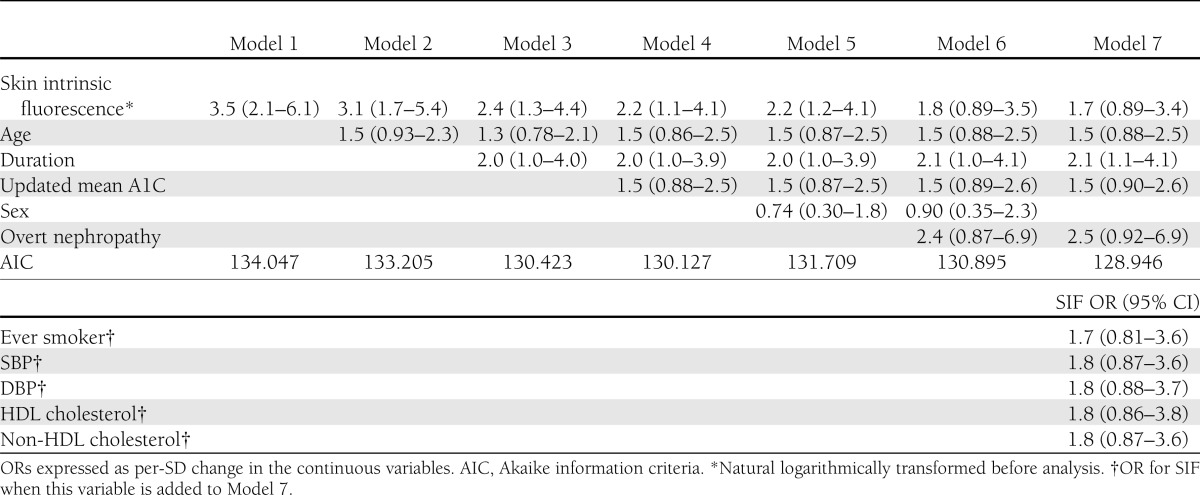
The independent relationship of SIF with CAD is presented in Table 2. Model 1 shows the relationship of SIF with CAD before any adjustment (OR 3.5), while models 2–6 show the effect of progressively accounting for age, duration, updated mean A1C, and sex. As can be seen, the OR for SIF was particularly reduced by the additions of age and duration to 2.4, while further control for updated mean A1C and sex had a smaller impact and the model fit did not improve (models 4 and 5). Model 7 (full adjustment but without sex) provided the best fit, but this was not materially better than without overt nephropathy. Each SD change in the natural log of SIF was therefore univariately associated with a 3.5-greater likelihood of having CAD, and after full adjustment there was still a 1.7-greater risk. Using model 7 (best fit), we further explored other CAD risk factors in type 1 diabetes, namely, blood pressure, lipids, and smoking. Individually adding these variables to model 7 provided no additional information.
Table 2.
Multivariable-adjusted correlates of CAD
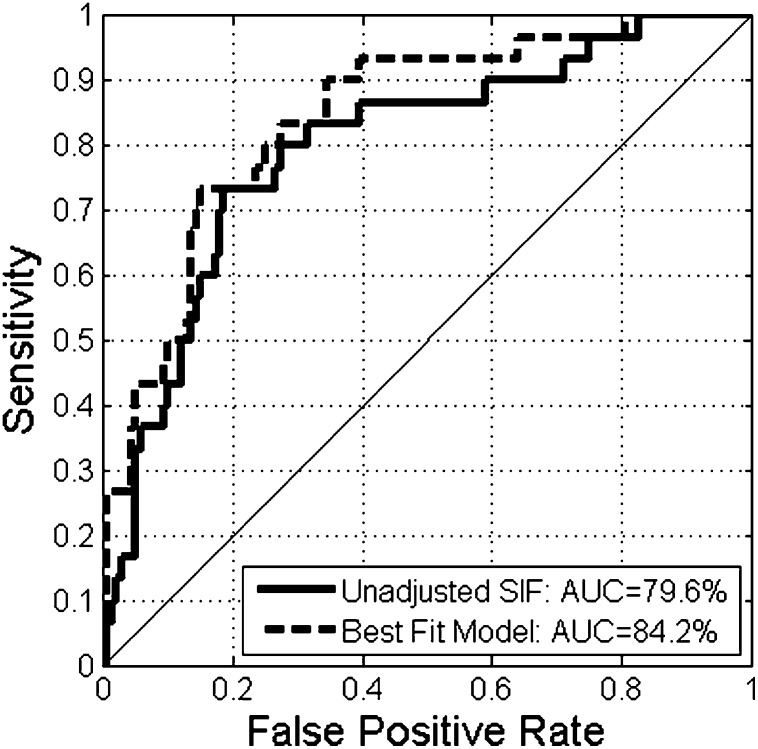
The discriminative ability of SIF to detect CAD is depicted in receiver operator characteristic curves in Fig. 1. SIF alone accounted for 79% of the area under the curve for CAD. Addition of age, diabetes duration, updated mean A1C, and overt nephropathy to the model with SIF increased the area under the curve slightly to 84% with a small gain in the equal error rate (sensitivity = specificity).
Figure 1.
Receiver operator characteristic curves of the discriminative ability of unadjusted and adjusted SIF to detect CAD in individuals with type 1 diabetes. The adjusted receiver operator characteristic curve was derived from the best-fit model and depicts the discriminative ability of SIF, age, diabetes duration, updated mean A1C, and overt nephropathy to detect CAD in individuals with type 1 diabetes.
CONCLUSIONS
In individuals with type 1 diabetes, SIF was strongly associated with the presence of CAD. This association was closely linked to age and duration of diabetes and less so to updated mean A1C and nephropathy. Even though it was statistically nonsignificant in the fully adjusted model, the OR remained increased at 1.7. These results suggest that SIF may therefore be a useful overall marker of CAD risk in type 1 diabetes. It may also represent part of the biological basis for the associations of these factors with CAD and potentially contribute above and beyond these factors. These observations may thus help explain the increased CAD risk in those with type 1 diabetes.
AGEs are products formed in later-stage reactions of the glycation of proteins, amino acids, basic lipids, and nucleotides by monosaccharides and related monosaccharide derivatives (3). AGEs form in tissues and accumulate over time when attached to proteins like collagen (19); however, increased exposure to hyperglycemia and oxidative stress, such as occurs in diabetes, accelerates their rate of formation (19). AGEs are also known to increase with decreasing renal function and increasing renal damage (probably reflecting accumulation of AGE adducts) (4,20), due to decreased renal clearance of AGEs, another mechanism by which AGEs may be elevated in diabetes. Furthermore, derivatives of AGEs not cleared by the kidneys form highly reactive second-generation AGEs that are thought to contribute to the progression of renal damage (21). That our findings were not independent of overt nephropathy when both age and duration were controlled for suggests that AGEs may contribute to the strong risk that renal disease plays in CAD in individuals with long duration of type 1 diabetes.
AGEs accumulate in the heart and large blood vessels, forming cross-links between adjacent proteins in the basement membrane of the extracellular matrix as well as activating the AGE receptor on vessel wall membranes. AGE cross-linking in vessel wall collagen increases the area of the extracellular matrix, resulting in increased stiffness of the vessel wall. AGEs have also been shown to interact with NO, reducing its bioavailability and activity (22), and to increase the adhesion of macromolecules, such as macrophages and LDL, to the vessel wall (23), and the adhesion of leukocytes to coronary fibroblasts (24). AGEs have been observed in atherosclerotic plaques, foam cells, and fatty streaks (25). Glycated LDL particles may be more atherogenic than regular LDL cholesterol (25,26). Finally, activation of the AGE receptor by AGE stimulates secretion of inflammatory proteins via activation of nuclear factor-κB (27). While AGEs and their pathophysiologic effects are likely important components of the SIF/CAD association reported, it should also be noted that SIF reflects much more than AGEs, e.g., the fluorescent enzymes nicotinamide adenine dinucleotide and flavin adenine dinucleotide. To what degree these latter factors add to the association is unclear but merits further investigation.
Our results are in agreement with studies that have shown increased levels of certain AGEs (2,28) in association with cardiovascular disease in individuals with diabetes. Nin and colleagues prospectively followed 339 individuals with type 1 diabetes for incident cardiovascular disease and all-cause mortality and found that baseline levels of two plasma AGEs [N′-(carboxyethyl)lysine and pentosidine] were predictive of outcomes 12 years later, independently of markers of renal function, inflammation, endothelial dysfunction, or arterial stiffness (2). These data suggest the association is not purely cumulative, as serum AGEs are reflective of plasma protein turnover (29) and renal function (4,20). Kilhovd et al. (28) found that higher levels of serum AGEs were predictive of CAD mortality in women, but not men, with type 2 diabetes. This apparent contrast to our findings of a stronger association in men of SIF with CAD morbidity is intriguing. Unfortunately, we do not have mortality follow-up at this time to address SIF and mortality, and it should be noted that risk predictors of CAD mortality (as in Kilhovd et al. [28]) and morbidity (current report) are often different. For example, we have shown that glycemic control is more strongly related to CAD mortality than to morbidity (30), a finding also apparent in the Wisconsin Epidemiologic Study of Diabetic Retinopathy (31,32). Nonetheless, in the EDC study glycemic control was a stronger predictor in men than women (30), consistent with our current findings. These findings therefore raise the possibility that glycemia, serum AGEs, and SIF may differ in their metabolism and/or impact on CAD risk by sex—a finding that may also reflect the non–AGE-related determinants of SIF.
Our results are also in agreement with others showing a relationship between skin autofluorescence and heart disease in European populations (33–36). Skin autofluorescence was cross-sectionally associated with coronary heart disease and predicted coronary heart disease mortality in 48 individuals with type 1 diabetes and in 69 with type 2 diabetes (35), and with stable CAD independent of diabetes status and renal function in 68 participants (36). We have previously shown a strong association between SIF and the severity of coronary artery calcification in the EDC population (10). This association was strongest in those with a coronary artery calcification score of at least 400, a threshold associated with clinical CAD (37). Our current results support this finding.
This study has a number of strengths, including the well-documented risk factor status of participants and the confirmation of strong CAD/SIF associations in two independent cohorts. However, a major limitation of this study is the cross-sectional nature of the study design. SIF measurements were taken on average 2 years after clinical exams in the EDC population. Unfortunately, the SCOUT technology became available during a phase of EDC when only surveys were being collected. Another limitation of these analyses is that our population was made up of middle-aged, predominantly Caucasian, adults of long diabetes duration, and thus these results are not generalizable to children, young adults, other ethnic groups, or individuals with short diabetes duration. Felipe et al. (38) found a significant difference in SIF when stratifying a cohort of 110 pediatric subjects by sex. We hypothesize that the effects of much older age (48.9 ± 8.6 vs. 13.2 ± 3.8 years) and longer duration of diabetes (36.1 ± 10.5 vs. 5.9 ± 3.6 years) in the EDC/MDC cohort overwhelmed the SIF sex difference observed in that pediatric cohort.
Some limitations arise from the measurement of SIF itself. In a study of 421 subjects at risk for type 2 diabetes, the interday, within-subject Hoorn coefficient of variation was 5.1% (15). In addition, in the SCOUT substudy of 1,036 patients with long-duration of type 1 diabetes from the DCCT/Epidemiology of Diabetes Interventions and Complications Study, the SIF within-day, within-subject Hoorn coefficient of variation was 4.3% (39). A factor that contributes to SIF measurement variation is skin heterogeneity from freckles, hair follicles, sweat glands, and wrinkles. Skin pigmentation differences between patients and within a patient over time (more or less tan) are mitigated by measuring the reflectivity of the skin and using the measured reflectance to correct the distortion of the melanin. A further limitation is the potential for selection bias, as not everyone in the 50-mile catchment area of the EDC clinic was studied. As shown, the EDC population with SIF measurements was older, and this may have introduced a bias in terms of general health and CAD prevalence, though it is difficult to conceive a selection bias that would falsely strengthen a SIF-CAD association. Indeed, as age is strongly related to both CAD and SIF, the older age of this population would tend to make it more difficult to show an independent association of SIF with CAD, as the current data demonstrate. We thus feel that selection bias is unlikely to have led to a spurious association of SIF with CAD. Finally, owing to sample size limitations, we were unable to more fully explore potential differences by sex in the relationship of SIF with CAD.
In conclusion, we have demonstrated a strong association between SIF and CAD in middle-aged individuals with type 1 diabetes. SIF partially reflects the influence of skin AGEs, skin markers of oxidative stress and cell metabolism, subject age, diabetes duration, long-term glycemic control, and renal disease, which are associated with increased CAD risk.
Acknowledgments
This work was supported by grant DK 34818 (to T.J.O.) and grant UL1RR031975 (to R.E.R.) from the National Institutes of Health.
This project was also funded by VeraLight, Inc. J.D.M. and N.M. are employees of VeraLight, Inc., the manufacturer of the SCOUT DS used to determine SIF in this study. V.R.A., S.F., and R.E.R. are employees of MedStar Health Research Institute, which received research support from VeraLight, Inc. V.R.A. was also a paid consultant of VeraLight, Inc., in 2009. T.J.O. received research support from VeraLight, Inc. B.N.C. received partial travel support from VeraLight, Inc. to attend the American Heart Association's Epidemiology & Prevention/Nutrition, Physical Activity & Metabolism 2012 Scientific Sessions, San Diego, California, 13–16 March 2012. No other potential conflicts of interest relevant to this article were reported.
B.N.C. wrote the manuscript and collected and analyzed data. V.R.A. collected data, reviewed the manuscript, and contributed to the discussion. J.D.M. reviewed and edited the manuscript and contributed to the methods and discussion. N.M. analyzed data and reviewed and edited the manuscript. S.F. analyzed data. R.E.R. collected data and reviewed the manuscript. T.J.O. conceived the study, collected data, directed the analyses, reviewed and edited the manuscript, and contributed to the discussion. B.N.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009 and at the American Heart Association's Epidemiology & Prevention/Nutrition, Physical Activity & Metabolism 2012 Scientific Sessions, San Diego, California, 13–16 March 2012.
The authors thank the participants of the EDC study and the MedStar Research Institute’s Diabetes Clinic for their dedication and support and for making this work possible.
References
- 1.Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care 2006;29:2528–2538 [DOI] [PubMed] [Google Scholar]
- 2.Nin JW, Jorsal A, Ferreira I, et al. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care 2011;34:442–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabbani N, Thornalley PJ. Glycation research in amino acids: a place to call home. Amino Acids 2012;42:1087–1096 [DOI] [PubMed] [Google Scholar]
- 4.Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol 2005;289:F645–F659 [DOI] [PubMed] [Google Scholar]
- 5.Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JEB. Diabetes and advanced glycoxidation end products. Diabetes Care 2006;29:1420–1432 [DOI] [PubMed] [Google Scholar]
- 6.Vlassara H. Advanced glycation end-products and atherosclerosis. Ann Med 1996;28:419–426 [DOI] [PubMed] [Google Scholar]
- 7.Meerwaldt R, Graaff R, Oomen PHN, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004;47:1324–1330 [DOI] [PubMed] [Google Scholar]
- 8.Heikal AA. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anamolies. Biomarkers Med 2010;4:241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Na R, Stender IM, Ma L, Wulf HC. Autofluorescence spectrum of skin: component bands and body site variations. Skin Res Technol 2000;6:112–117 [DOI] [PubMed] [Google Scholar]
- 10.Conway B, Edmundowicz D, Matter N, Maynard JD, Orchard T. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther 2010;12:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conway BN, Aroda VR, Maynard JD, et al. Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes Care 2011;34:1000–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orchard TJ, Dorman JS, Maser RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care 1990;13:741–747 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maynard JD, Rohrscheib M, Way JF, Nguyen CM, Ediger MN. Noninvasive type 2 diabetes screening: superior sensitivity to fasting plasma glucose and A1C. Diabetes Care 2007;30:1120–1124 [DOI] [PubMed] [Google Scholar]
- 15.Ediger MN, Olson BP, Maynard JD. Noninvasive optical screening for diabetes. J Diabetes Sci Tech 2009;3:776–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hull E, Ediger M, Unione A, Deemer E, Stroman M, Baynes J. Noninvasive, optical detection of diabetes: model studies with porcine skin. Opt Express 2004;12:4496–4510 [DOI] [PubMed] [Google Scholar]
- 17.Maynard J, Matter N, Olson B, Ediger M, Way J, Hull E. Noninvasive Pre-diabetes and Diabetes Screening Using Skin Fluorescence: Clinical Validation in an At-Risk Cohort. Canadian Journal of Diabetes. 2011;35:426 [Google Scholar]
- 18.Atkinson AC. A note on the generalized information criterion for choice of a model. Biometrika 1980;67:413–418 [Google Scholar]
- 19.Peppa M, Uribarri J, Vlassara H. Glucose, Advanced Glycation End Products, and Diabetes Complications: What is New and What Works. Clin Diabetes 2003;21:186–187 [Google Scholar]
- 20.Makita Z, Radoff S, Rayfield EJ, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 1991;325:836–842 [DOI] [PubMed] [Google Scholar]
- 21.Thomas MC, Forbes JM, MacIsaac R, Jerums G, Cooper ME. Low-molecular weight advanced glycation end products: markers of tissue AGE accumulation and more? Ann N Y Acad Sci 2005;1043:644–654 [DOI] [PubMed] [Google Scholar]
- 22.Uhlmann S, Rezzoug K, Friedrichs U, Hoffmann S, Wiedemann P. Advanced glycation end products quench nitric oxide in vitro. Graefes Arch Clin Exp Ophthalmol 2002;240:860–866 [DOI] [PubMed] [Google Scholar]
- 23.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 2003;21:3–12 [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Zalewski A, Liu Y, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation 2003;108:472–478 [DOI] [PubMed] [Google Scholar]
- 25.Schleicher E, Friess U. Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl 2007;72:S17–S26 [DOI] [PubMed] [Google Scholar]
- 26.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006;114:597–605 [DOI] [PubMed] [Google Scholar]
- 27.Jandeleit-Dahm K, Cooper ME. The role of AGEs in cardiovascular disease. Curr Pharm Des 2008;14:979–986 [DOI] [PubMed] [Google Scholar]
- 28.Kilhovd BK, Juutilainen A, Lehto S, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia 2007;50:1409–1417 [DOI] [PubMed] [Google Scholar]
- 29.Ahmed N, Thornalley PJ, Lüthen R, et al. Processing of protein glycation, oxidation and nitrosation adducts in the liver and the effect of cirrhosis. J Hepatol 2004;41:913–919 [DOI] [PubMed] [Google Scholar]
- 30.Conway B, Costacou T, Orchard T. Is glycaemia or insulin dose the stronger risk factor for coronary artery disease in type 1 diabetes? Diab Vasc Dis Res 2009;6:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar A, Klein R, Klein BE, Moss SE. Association between glycosylated hemoglobin level and cardiovascular and all-cause mortality in type 1 diabetes. Am J Epidemiol 2007;166:393–402 [DOI] [PubMed] [Google Scholar]
- 32.Klein BE, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med 2004;164:1917–1924 [DOI] [PubMed] [Google Scholar]
- 33.Lutgers HL, Gerrits EG, Graaff R, et al. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia 2009;52:789–797 [DOI] [PubMed] [Google Scholar]
- 34.Mulder DJ, van Haelst PL, Graaff R, Gans RO, Zijlstra F, Smit AJ. Skin autofluorescence is elevated in acute myocardial infarction and is associated with the one-year incidence of major adverse cardiac events. Neth Heart J 2009;17:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meerwaldt R, Lutgers HL, Links TP, et al. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care 2007;30:107–112 [DOI] [PubMed] [Google Scholar]
- 36.Mulder DJ, van Haelst PL, Gross S, et al. Skin autofluorescence is elevated in patients with stable coronary artery disease and is associated with serum levels of neopterin and the soluble receptor for advanced glycation end products. Atherosclerosis 2008;197:217–223 [DOI] [PubMed] [Google Scholar]
- 37.Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes 2000;49:1571–1578 [DOI] [PubMed] [Google Scholar]
- 38.Felipe DL, Hempe JM, Liu S, et al. Skin intrinsic fluorescence is associated with hemoglobin A(1c) and hemoglobin glycation index but not mean blood glucose in children with type 1 diabetes. Diabetes Care 2011;34:1816–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleary P, Braffett B, Maynard J, et al. Association of skin intrinsic fluorescence with cumulative glycemic exposure in the DCCT/EDIC Study (Abstract). Diabetes 2011;54(Suppl. 1):S146 [Google Scholar]