Abstract
OBJECTIVE
To evaluate whether LY2605541 results in lower fasting blood glucose (FBG) versus insulin glargine (GL).
RESEARCH DESIGN AND METHODS
This 12-week, randomized, open-label, Phase 2 study enrolled patients with type 2 diabetes (hemoglobin A1c [A1C] ≤ 10.5%), taking metformin and/or sulfonylurea with GL or NPH insulin once daily. Patients converted to morning insulin administration during lead-in were randomized 2:1 from GL (n = 248) or NPH insulin (n = 39) to LY2605541 (n = 195) or GL (n = 95) once daily in the morning.
RESULTS
At 12 weeks, FBG (mean ± SE) was similar with LY2605541 and GL (118.2 ± 2.0 mg/dL [6.6 ± 0.1 mmol/L] vs. 116.9 ± 2.7 mg/dL [6.5 ± 0.2 mmol/L], P = 0.433) as was A1C (7.0 ± 0.1 vs. 7.2 ± 0.1%, P = 0.279). Intraday blood glucose variability was reduced with LY2605541 (34.4 vs. 39.1 mg/dL [1.9 vs. 2.2 mmol/L], P = 0.031). LY2605541 patients had weight loss (−0.6 ± 0.2 kg, P = 0.007), whereas GL patients gained weight (0.3 ± 0.2 kg, P = 0.662; treatment difference: −0.8 kg, P = 0.001). The incidence and rate of both total hypoglycemia and nocturnal hypoglycemia were comparable between LY2605541 and GL, although, LY2605541 had a 48% reduction in nocturnal hypoglycemia after adjusting for baseline hypoglycemia (P = 0.021). Adverse events were similar across treatments. Alanine aminotransferase and aspartate aminotransferase remained within normal range but were significantly higher with LY2605541 (P ≤ 0.001).
CONCLUSIONS
In patients with type 2 diabetes, LY2605541 and GL had comparable glucose control and total hypoglycemia rates, but LY2605541 showed reduced intraday variability, lower nocturnal hypoglycemia, and weight loss relative to GL.
Basal insulin in combination with oral antidiabetes medications (OADs) has been a successful initial insulin therapy for type 2 diabetes treatment (1–4). Once-daily analog basal insulins have a comparable glycemic-lowering effect and reduce nocturnal hypoglycemia rates relative to NPH insulin (1,5). This reduced risk for hypoglycemia, as well as a reduction in the need for twice-daily injections, has been attributed to the prolongation of action by retarding subcutaneous absorption, lessening peak activity, and reducing variability of circulating insulin levels. However, the peripheral subcutaneous administration of these insulin analogs does not replicate the twofold higher portal-versus-systemic circulating insulin levels seen with endogenously secreted insulin. Therefore, the net effect of peripheral insulin administration is potentially overstimulation of glucose uptake to compensate for the reduced hepatic insulin action needed to maintain glucose homeostasis.
The basal insulin analog LY2605541 is a novel, long-acting insulin that consists of insulin lispro modified with a 20-kDa polyethylene glycol (PEG) moiety having a large hydrodynamic size which delays insulin absorption and reduces clearance, resulting in prolonged duration of action. The increase in functional molecular size appears to alter the distribution of this insulin to tissues. Hypothetically, the fenestrated hepatic sinusoidal endothelium may facilitate greater transport of LY2605541 into the liver relative to peripheral tissues (i.e., muscle and fat), potentially providing a more preferential hepatic action akin to normal physiology.
This exploratory Phase 2 clinical trial was designed to compare the safety and efficacy of LY2605541 versus insulin glargine (GL) when administered once daily in the morning in combination with OADs for 12 weeks in patients with type 2 diabetes. The primary objective was to test the hypothesis that LY2605541 would result in lower fasting blood glucose (FBG) levels as measured by self-monitored blood glucose (SMBG) at end point (week 12) compared with GL.
RESEARCH DESIGN AND METHODS
This 12-week, randomized, open-label, 3-arm, multinational, parallel group Phase 2 study was conducted in 25 sites (Australia, Hungary, Poland, Puerto Rico, Romania, the Russian Federation, Spain, and the United States). The study was conducted in accordance with International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki (6). All patients provided written informed consent.
Eligible patients were aged 18 to 65 years with a diagnosis of type 2 diabetes for ≥1 year, had a hemoglobin A1c (A1C) ≤10.5% and a BMI between 19 and 45 kg/m2, and had been using metformin and/or a sulfonylurea in combination with GL or NPH insulin once daily (maximum dose <1.0 units/kg/day) for ≥ 3 months. Patients were excluded if they had New York Heart Association Class III or IV cardiac functional status, fasting triglycerides >500 mg/dL (>5.7 mmol/L), liver disease, an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) value more than twofold the upper limit of normal (ULN), renal transplantation or serum creatinine >2.0 mg/dL (>177 μmol/L), ≥1 episode of severe hypoglycemia within the previous 6 months or a diagnosis of hypoglycemia unawareness, participation in a weight loss program, or at least or more than two emergency room visits or hospitalizations as a result of poor glucose control within the past 6 months.
Eligible patients were stratified within country by A1C level (≤8.5%, >8.5%) and baseline basal insulin dose (≤0.4 units/kg, >0.4 units/kg), then randomized 1:1:1 to two different LY2605541 insulin-starting and adjusting algorithms (LY2605541 Algorithm 1 [LY1] or LY2605541 Algorithm 2 [LY2]) or to GL. Nonblinded assignment to treatment groups was determined by a computer-generated random sequence using an interactive voice response system. All treatments were started after a 4-week lead-in period (Supplementary Fig. 1). Two weeks before randomization, those patients administering their basal insulin outside morning hours were converted to prebreakfast administration in the lead-in period. After randomization, patients underwent clinic visits every 2 weeks with weekly telephone visits interspersed. All patients were instructed to continue their prestudy diet and activity levels and maintain their prestudy metformin and/or sulfonylurea doses without modification throughout the study.
The two LY2605541 algorithms differed by both the dose at initiation and the dose increases. Patients randomized to LY1 initiated LY2605541 treatment gradually over 5 days. At randomization, LY1 patients received the converted dose of LY2605541 in addition to the prestudy dose of NPH insulin or GL; thereafter, the prestudy basal insulin dose was reduced by 25% daily. Patients randomized to LY2 transitioned directly to LY2605541 by doubling the converted dose on the first day of administration and stopping prestudy insulin. All LY2605541 patients were converted from their prior dose of NPH insulin or GL, with the use of an initial conversion factor of 6 nmol/unit of prestudy basal insulin. A predetermined interim assessment was performed to evaluate the efficacy and safety of the initial LY2605541 dose conversion and its impact on the two algorithms. After the interim assessment, the conversion factor for LY2605541 was changed to 7 nmol/unit, and the LY1 algorithm was altered to taper NPH insulin or GL over 4 days beginning with 75% of the dose on the first day of administration followed by 25% daily dose decreases. Because the LY2605541 formulation concentration was ∼1,000 nmol/mL, the LY2605541 dose was rounded to the nearest 10 nmol to allow administration in 10-μL increments (therefore, 1 volumetric insulin unit of LY2605541 = 10 nmol of LY2605541) and to facilitate the prescription of patient dosing with units in a U-100 syringe.
Based on time required to achieve steady state circulating LY2605541 levels and prior publication of GL dose titration (1), all study insulins were restricted to increases only at weekly intervals. The LY1 algorithm, adapted from Yki-Järvinen et al. (7), was based on the mean FBG of three consecutive mornings before the visit: for a mean FBG 101 to 180 mg/dL (5.5 to 10.0 mmol/L), the LY2605541 dose was increased by 10 nmol (10 μL or 1 volumetric unit) and for a mean FBG >180 mg/dL (10.0 mmol/L), the LY2605541 dose was increased by 20 nmol (20 μL or 2 volumetric units). The GL dosing algorithm was similar to the Yki-Järvinen et al. (7) algorithm using the same two and four IU increments, respectively. The LY2 algorithm was adapted from Riddle et al. (1) and likewise was based on the mean of three consecutive mornings before the visit: for a mean FBG of 101 to 120 mg/dL (5.5 to 6.7 mmol/L), the LY2605541 dose increased by 10 nmol (10 μL or 1 volumetric unit); for 121 to 140 mg/dL (6.8 to 7.8 mmol/L), the LY2605541 dose increased by 20 nmol (20 μL or 2 volumetric units); for 141 to 180 mg/dL (7.9 to 10.0 mmol/L), the LY2605541 dose increased by 30 nmol (30 μL or 3 volumetric units); and for >180 mg/dL (10.0 mmol/L), the LY2605541 dose increased by 40 nmol (40 μL or 4 volumetric units). After the same interim assessment as above, LY1 and LY2 dosing increment increases were doubled, whereas the GL algorithm remained unchanged.
In all groups, emphasis was placed on optimizing the insulin dose to achieve fasting and preprandial blood glucose (BG) measures between 90 and 130 mg/dL (5.0 and 7.2 mmol/L). However, the titration algorithms targeted an FBG level of ≤100 mg/dL (≤ 5.6 mmol/L). Patients taking GL who experienced at least or more than two hypoglycemic episodes had their dose decreased by 2 IU; and for patients taking LY2605541, the dose was decreased to 80% of the pre-event dose on the first day and 90% thereafter. Patients injected their final treatment-period dose the day before their last office visit. After 12 weeks, patients recommenced basal insulin and returned for a follow-up visit 4 weeks later.
Outcome measures and measurements
The FBG measured by SMBG was collected daily from screening to study completion. The 8-point SMBG profile (measured pre- and 2 h postmeal, at bedtime, and 3 a.m.) was collected for 3 days during the week before each clinic visit. Fasting serum glucose (FSG) was measured at each clinic visit, and A1C was measured every 4 weeks. Laboratory measures as well as adverse events (AEs) were collected at randomization, interim visit(s), and end point and included fasting lipid (i.e., LDL-C and HDL-C), chemistry (e.g., triglycerides and liver enzymes), urinalysis, and hematology panels as well as LY2605541 antibody titers.
Hypoglycemia was defined as BG ≤70 mg/dL (≤3.9 mmol/L) or a sign or symptom associated with hypoglycemia. Severe hypoglycemia was defined as a sign or symptom requiring assistance from another person because of severe neurologic impairment and, in the absence of BG measurement, showed prompt recovery in response to carbohydrate intake or administration of glucagon or intravenous glucose. All severe hypoglycemia episodes were reported as a serious adverse event (SAE).
Statistical methods
Sample size was determined assuming a randomization ratio of 1:1:1 and a mean difference of 18 mg/dL (1.0 mmol/L) with an SD of 44 mg/dL (2.5 mmol/L) to provide 90% statistical power to detect a significant FBG difference between GL and combined LY2605541 using a two-sample t test at an α of 0.10. With the assumption of a 15% drop-out rate, it was calculated that ∼282 patients were needed for randomization to achieve ∼240 completers.
All analyses (SAS Drug Development system [SAS], Cary, NC) were based on all patients who were randomized and took ≥1 dose of study drug, defined as Full Analysis Set, a slightly modified intent-to-treat population. All tests were performed for two-sided tests at α level of 0.10, and the corresponding 90% CIs were calculated. No adjustments for multiplicity were performed.
The FBG at a visit, as the primary efficacy variable, was calculated as the average of daily FBG from SMBG measurements between the current and the previous clinical visit. The daily mean BG was calculated as the average of the three 8-point SMBG profiles at each visit. Three measurements were used to quantify glucose variability: 1) interday FBG variability was quantified as the SD of the daily FBG measurements between the current and previous visit; 2) interday SMBG variability at a visit was calculated as the SD of daily mean glucose; and 3) intraday BG variability at a visit was quantified as the SD of 8-point SMBG profiles. Rate of hypoglycemia events was adjusted for 30 days. Nocturnal hypoglycemia events were also analyzed by adjusting for baseline hypoglycemia events using a negative binomial regression, including treatment as a factor and baseline nocturnal hypoglycemia events as a covariate. Baseline hypoglycemia events were collected during the lead-in period. The relationship between the hypoglycemia event rate during the treatment period and end point A1C was characterized by the negative binomial regression curve of hypoglycemia event rate on the end point A1C.
The primary analysis variable and other continuous variables, excluding weight, lipids, and liver enzymes, were analyzed using mixed model repeated measures (MMRM) with independent variables of treatment, week, dose conversion (interim analysis and postinterim analysis), baseline A1C group, baseline basal insulin dose group, the interaction between treatment and week, and a random effect for patient. Weight, lipids, and liver enzymes were analyzed by ANCOVA with the same variables as MMRM, excluding week, interaction between treatment and week, and random effect for patient. Binary variables were analyzed using Fisher’s exact test. The hypoglycemia rate (per patient/30 days) was analyzed using a negative binomial model with treatment and dose conversion as independent variables.
RESULTS
This trial was conducted between February 2010 and January 2011. Of 289 randomized patients, 288 were included in the Full Analysis Set: 98 patients in LY1, 97 patients in LY2 (totaling 195 patients in the combined LY2605541 group), and 93 patients in GL (Supplementary Fig. 2). Here we report the comparisons between combined LY2605541 and GL. Glycemia data for LY1 and LY2 individual groups are provided in Supplementary Table 1.
Overall, 92.7% (267 of 288) of patients completed the study. Reasons for discontinuation included physician decision 6 (2.1%), protocol violation 4 (1.4%), patient decision 5 (1.7%), AE 4 (1.4%), and sponsor decision 2 (0.7%) (Supplementary Fig. 2). Of the four patients who discontinued because of an AE, two were in the LY1 group (1 because of nonsevere hypoglycemia and 1 because of VII nerve paralysis) and two were in the LY2 group (1 because of an injection site reaction and 1 because of myocardial ischemia). No GL patients discontinued as a result of an AE. There was no statistically significant difference in any individual reported reason for discontinuation between combined LY2605541 and GL. Overall, early discontinuation was significantly greater in the combined LY2605541 group (10%) than the GL group (2%, P = 0.027).
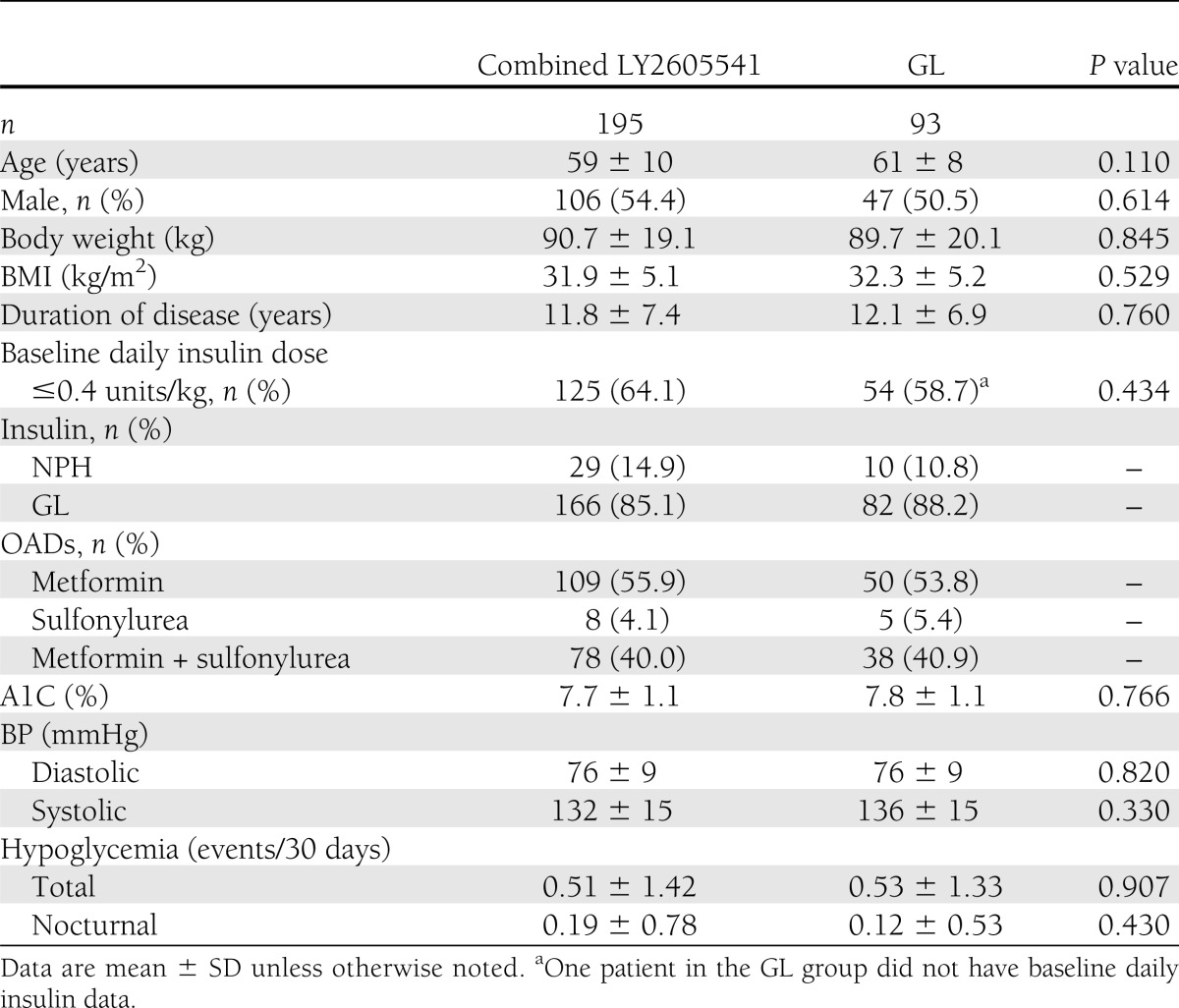
Demographic and baseline characteristics for combined LY2605541 and GL were well balanced (Table 1). Participants had inadequate glycemic control (A1C 7.7 vs. 7.8%), and ∼64% (125 of 195) in combined LY2605541 and 59% (54 of 92) in GL were taking a daily basal insulin dose ≤0.4 units/kg. Overall statin use at baseline was similar between combined LY2605541 (36%) and GL (41%, P = 0.437).
Table 1.
Patient demographics and baseline characteristics
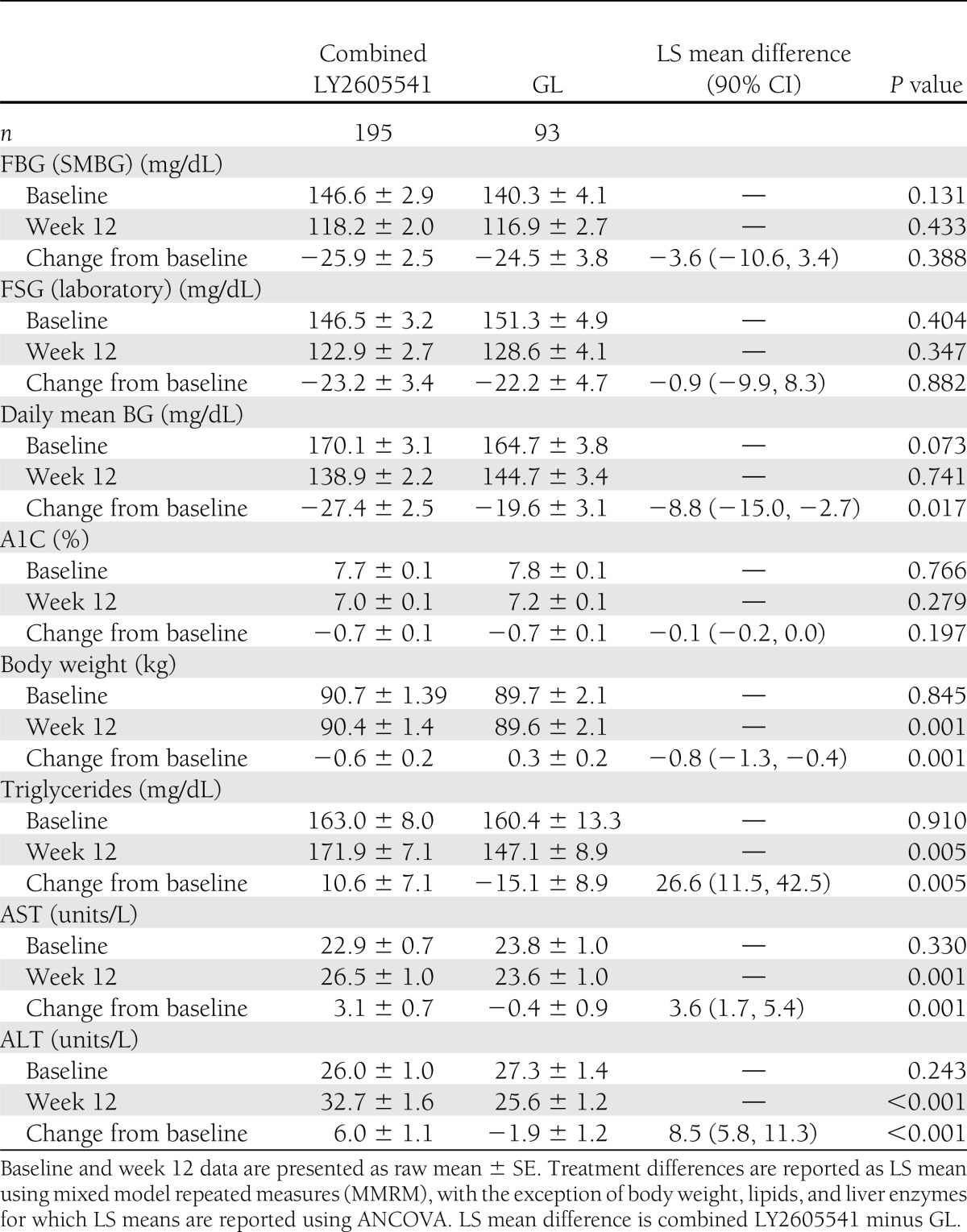
Results are presented as mean (± SE). After 12 weeks, FBG, measured by morning premeal SMBG, showed that combined LY2605541 was similar to GL (118.2 ± 2.0 mg/dL [6.6 ± 0.1 mmol/L] vs. 116.9 ± 2.7 mg/dL [6.5 ± 0.2 mmol/L]) (Table 2) and demonstrated noninferiority to GL (least squares [LS] mean treatment difference: 3.1 mg/dL; 90% CI −3.2, 9.4 [0.2 mmol/L; 90% CI −0.2, 0.5], P = 0.433). The mean FBG (SMBG) showed similar reductions from baseline with combined LY2605541 and with GL (−25.9 ± 2.5 mg/dL [−1.4 ± 0.1 mmol/L] vs. −24.5 ± 3.8 mg/dL [−1.4 ± 0.2 mmol/L], P = 0.388). Mean laboratory-measured FSG also did not differ between combined LY2605541 and GL at 12 weeks (122.9 ± 2.7 mg/dL [6.8 ± 0.2 mmol/L] vs. 128.6 ± 4.1 mg/dL [7.1 ± 0.2 mmol/L], P = 0.347). A1C, at 12-week end point, was not different for patients treated with combined LY2605541 than for patients treated with GL (7.0 ± 0.1 vs. 7.2 ± 0.1%, P = 0.279).
Table 2.
Measures of glycemia and safety at week 12
Based on end point 8-point SMBG profiles, there were no differences between combined LY2605541 and GL daily mean BG (SMBG) (Table 2) or most categorical SMBG time points at end point, although, a significantly greater reduction from baseline in daily mean BG was observed with combined LY2605541 versus GL (P = 0.017). Of the eight categorical time points, only the morning 2-h postprandial time point was significantly different with combined LY2605541 (154.2 ± 2.9 mg/dL [8.6 ± 0.2 mmol/L]) versus GL (165.2 ± 4.3 mg/dL [9.2 ± 0.2 mmol/L], P = 0.034). Intraday and interday mean BG variabilities (SMBG), as measured by SD, were both lower with combined LY2605541 versus GL at week 12 (intraday SD: 34.4 ± 1.1 vs. 39.1 ± 1.8 mg/dL [1.9 ± 0.1 vs. 2.2 ± 0.1 mmol/L], P = 0.031; interday SD: 10.8 ± 0.5 vs. 13.7 ± 1.1 mg/dL [0.6 ± 0.0 vs. 0.8 ± 0.1 mmol/L], P = 0.100) (Supplementary Fig. 3). The interday FBG SD was not significantly different between combined LY2605541 and GL (18.6 ± 0.9 vs. 20.0 ± 1.3 mg/dL [1.0 ± 0.1 vs. 1.1 ± 0.1 mmol/L], P = 0.687).
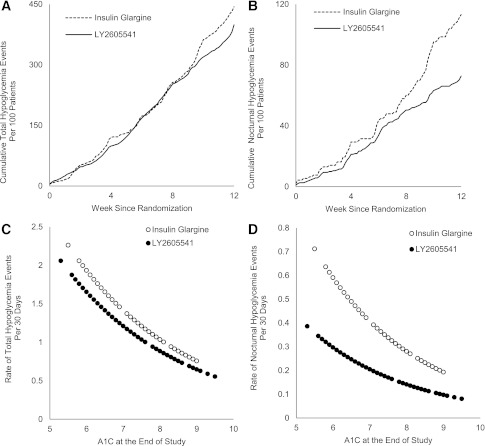
Overall, there were no differences between combined LY2605541 and GL with regard to incidence of total hypoglycemia (54.4 vs. 63.4%, P = 0.162) and nocturnal hypoglycemia (25.6 vs. 34.4%, P = 0.127). For combined LY2605541 versus GL, the mean rates (± SE) of total hypoglycemia (number of events/30 days) (1.34 ± 0.26 vs. 1.52 ± 0.34, P = 0.804) and nocturnal hypoglycemia were similar (0.25 ± 0.07 vs. 0.39 ± 0.12, P = 0.178). When adjusted for baseline, the combined LY2605541 group had a 48% rate reduction in nocturnal hypoglycemia (P = 0.021). No patient in any treatment group experienced a severe hypoglycemia event. Figure 1A and B shows the cumulative incidence of total and nocturnal hypoglycemia events over 12 weeks for LY2605541 and GL. Figure 1C and D shows the rate of total and nocturnal hypoglycemia events as a function of end point A1C.
Figure 1.
A and B: Number of total and nocturnal hypoglycemia events per 100 patients over the 12-week treatment period. C and D: The rate of total and nocturnal hypoglycemia events based on A1C values over 12 weeks. The relationship between the hypoglycemia event rate during the treatment period and the end point A1C was characterized by the negative binomial regression curve of hypoglycemia event rate on the end point A1C. In A and B, the dashed line = insulin glargine and the solid line = LY2605541. In C and D, open circle = insulin glargine and closed circle = LY2605541.
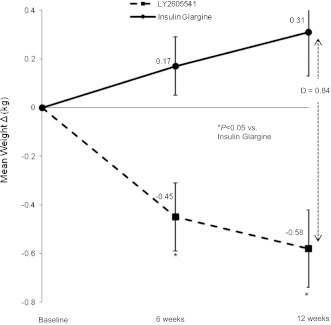
At week 12, combined LY2605541 demonstrated significant mean weight loss (−0.6 ± 0.2 kg, P = 0.007), whereas GL was associated with weight gain (0.3 ± 0.2 kg, P = 0.662) (Fig. 2). Overall, combined LY2605541 had a significantly greater reduction in mean weight versus GL (LS mean treatment difference: −0.8 kg, P = 0.001).
Figure 2.
Mean change in body weight with LY2605541 versus insulin glargine over 12 weeks. D, treatment difference. Solid line with closed circle = insulin glargine; dashed line with closed square = LY2605541.
At 12 weeks, the mean basal insulin dose for combined LY2605541 was 4.46 nmol/kg and was 3.00 nmol/kg for GL. The ratio of the combined LY2605541 dose to the GL dose was 1.483 (90% CI 1.171, 1.879). The estimated ratios were similar after adjusting for end point A1C, FBG (SMBG), and FSG (laboratory).
A total of 47.7% (93 of 195) of combined LY2605541 patients and 48.4% (45 of 93) of GL patients experienced at least or more than one treatment-emergent AE (TEAE). The most frequently occurring TEAEs (≥4%) for combined LY2605541 versus GL, respectively, were nasopharyngitis (4.6 vs. 5.4%), headache (3.5 vs. 4.3%), and back pain (2.1 vs. 4.3%). Gastrointestinal disorders were more frequent with GL (14.0%) than with combined LY2605541 (10.1%), although this difference was not significant (P = 0.145). Skin and subcutaneous tissue disorders were similar with both treatments (combined LY2605541: 4.6%, GL: 4.3%, P = 1.00). The majority (62%) of TEAEs were of mild severity. Seven patients experienced an SAE during the treatment period (5 [2.6%] in the combined LY2605541 group and 2 [2.2%] in the GL group), and two patients reported an SAE during treatment with prestudy insulin in the follow-up period. None of the SAEs were considered by the investigator to be related to treatment. In this study with a 2:1 randomization of LY2605541 to GL, four patients experienced adjudicated cardiovascular events: one case of myocardial infarction and two cases of unstable angina in LY2605541, and one case of myocardial infarction in GL.
Mean increases in ALT and AST were observed for the combined LY2605541 group with the observed mean values remaining within the normal range (Table 2). At end point, AST was similar with combined LY2605541 versus GL for females and males. The treatment difference (combined LY2605541 vs. GL) in ALT was 4.2 units/L (P = 0.037) for female patients and 10.0 units/L (P < 0.001) for male patients. At the 16-week follow-up visit, mean ALT and AST remained higher with combined LY2605541 versus GL (LS mean treatment differences: 5.9 units/L, P = 0.008 and 3.7 units/L, P = 0.017, respectively), but still within normal limits. During this study, two patients had liver enzymes >3× ULN with no change in total bilirubin measurements or alkaline phosphatase at the end of the follow-up period. Both patients had ALT and AST values within normal range at the end of the active treatment period. One of these patients had repeat laboratory measures obtained 4 weeks after the follow-up period ended, which showed liver enzyme elevations had returned to within normal range. The other patient had a different pattern with AST measurement being greater than ALT measurement. No follow-up laboratory measures were obtained.
There were no statistically significant changes for triglycerides, LDL-C, HDL-C, or total cholesterol from baseline to any postbaseline time point for both LY2605541 and GL. However, end point triglycerides were significantly higher in the combined LY2605541 group versus GL (P = 0.005; Table 2). At end point, the LS mean treatment difference (combined LY2605541 vs. GL) in triglycerides was 33.7 mg/dL (0.4 mmol/L, P = 0.029) for male patients and 23.9 mg/dL (0.3 mmol/L, P = 0.051) for female patients. By the 16-week follow-up visit, mean triglycerides were not different between combined LY2605541 and GL (LS mean treatment difference: −5.3 mg/dL [−0.1 mmol/L], P = 0.572). At week 12, mean blood pressure (BP) was higher for combined LY2605541 than GL (diastolic BP: 76 ± 1 vs. 75 ± 1 mmHg, P = 0.030; systolic BP: 132 ± 1 vs. 131 ± 2 mmHg, P = 0.030). No significant change in pulse rate was observed for either group.
Patients with a detectable antibody to LY2605541 at any time during the study were considered antibody positive. At baseline, antibody status was comparable between groups: 9.2% (18 of 195) of combined LY2605541 patients and 9.7% (9 of 93) of GL patients were antibody positive at baseline. The percentages of patients with no detectable antibody at baseline who became antibody positive after 12 weeks of treatment were similar in each group (combined LY2605541: 3.9%; GL: 1.2%) (Supplementary Table 2). A change in antibody status from negative to positive or positive to negative had no apparent influence on glycemic response.
CONCLUSIONS
This study in patients with type 2 diabetes treated previously with basal insulin in combination with metformin and/or sulfonylurea demonstrated that both the novel long-acting basal insulin LY2605541 and GL therapies provided comparable improvement in glucose control when administered in the morning, with the exception that combined LY2605541 demonstrated a greater reduction in change from baseline of daily mean BG versus GL.
The incidences and rates of total hypoglycemia and nocturnal hypoglycemia were comparable between groups, but the baseline nocturnal hypoglycemia rates differed between the different treatment arms. When the difference in baseline hypoglycemia was adjusted, LY2605541 demonstrated a statistically significant 48% reduction in nocturnal hypoglycemia versus GL. Additionally, both intraday and interday glycemic variability were reduced with LY2605541 versus GL. This may be attributed to the longer duration and lower peak-to-trough ratio versus GL (8\x{2013}10). Moreover, in this study, basal insulin was administered in the morning to evaluate potential FBG differences as a result of a considerable difference in the half-lives of the two basal insulins, but FBG measures were similar. Morning administration of GL is expected to have a lower likelihood of nocturnal hypoglycemia, but interestingly, resulted in higher rates versus LY2605541.
This study, along with a type 1 diabetes study, was used to clinically define the molar concentration of an LY2605541 U100 formulation. In both studies, LY2605541 was empirically titrated to treat-to-target, and both studies demonstrated similar results for determining the molar dose ratio of LY2605541 relative to GL. To facilitate dosing, LY2605541 will be formulated as 900 nmol/mL for Phase 3 trials. Comparatively, all U100 insulins, including GL (11), are 600 nmol/mL concentrations, except for U100 insulin detemir at 2,400 nmol/mL (12).
With improved glycemic control, patients treated with GL gained weight as reported previously (13–16); however, patients treated with LY2605541 lost weight. At end point, LY2605541-treated patients had higher triglyceride levels than GL-treated patients. This difference was primarily related to a reduction in triglycerides in the GL group. Both the paradoxical effects on weight and lipids were also noted in a Phase 2 trial in patients with type 1 diabetes (17) and may represent a significantly different physiological action of LY2605541 compared with other insulins. Of note, in a somatostatin and glucagon-infused, conscious dog model, intravenously infused LY2605541 demonstrated a hepatic-preferential and acute-lipolytic effect compared with human insulin (18). It is conceivable that with less peripheral action of LY2605541, patients converted from prior insulin therapy to LY2605541 may experience transiently greater lipolysis, less lipogenesis, increased lipid oxidation, and, ultimately, weight loss.
Overall, mean increases within normal range for serum ALT and AST levels were seen with LY2605541 therapy. Notably, 4 weeks after LY2605541 cessation, while on prestudy insulin, two patients with normal liver function tests during the treatment period experienced elevated liver enzymes (3× ULN). One patient had an abrupt elevation of ALT and AST, which normalized to baseline levels 4 weeks later. The other patient experienced a threefold elevation in an AST elevation 3× ULN, which was much greater than the ALT elevation. Despite higher susceptibility to nonalcoholic fatty liver disease in patients with type 2 diabetes, a similar ALT and AST elevation was observed in a type 1 diabetes study of LY2605541 (17). Thus, increases in mean levels may reflect a hepatic adaptation (19,20) reaction to the PEGylated insulin rather than an increase in hepatic fat content. In future studies, additional hepatic monitoring will occur in a much larger population, and hepatic fat content will also be assessed.
This study was limited in that it was an open-label, Phase 2 clinical trial and was, therefore, predominantly exploratory in nature and of short duration. However, as noted by the end point A1C values, it was well conducted. Although both basal insulins were administered by vial and syringe, LY2605541 required reconstitution, which perhaps further increased dosing variability. Future double-blind studies will be conducted with anticipated commercial formulation.
In conclusion, the use of the long-acting insulin LY2605541 may provide patients with type 2 diabetes a lower risk of nocturnal hypoglycemia, reduced glycemic variability, and a weight advantage for a similar degree of glycemic control compared with GL. The Phase 3 program will assess the risk-benefit of this insulin and elucidate the clinical significance of the liver enzyme and triglyceride changes. Taken together, the body weight, lipid values, and liver function tests suggest that LY2605541 may have a novel mechanism of action distinct from other therapeutic insulins, which will be further investigated in future studies.
Acknowledgments
This study was funded by Eli Lilly and Company.
R.M.B. has served on scientific advisory panels for Abbott Diabetes Care, Amylin Pharmaceuticals, Bayer HealthCare, Eli Lilly and Company, Hygieia, Johnson & Johnson, Roche Pharmaceuticals, sanofi-aventis, and Valeritas; has served as a consultant to Abbott Diabetes Care, Amylin Pharmaceuticals, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Calibra Medical, Eli Lilly and Company, Halozyme Therapeutics, Helmsley Trust, Hygieia, Johnson & Johnson, Medtronic, ResMed, Roche Pharmaceuticals, sanofi-aventis, Takeda Pharmaceutical Company, Valeritas LLC, and Becton, Dickinson and Company; has received research support from Abbott Diabetes Care, Amylin Pharmaceuticals, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Calibra Medical, Dexcom, Eli Lilly and Company, Halozyme Therapeutics, Helmsley Trust, Hygieia, Intarcia Therapeutics, Johnson & Johnson, MannKind Corporation, Medtronic, National Institutes of Health, ResMed, Roche Pharmaceuticals, sanofi-aventis, Takeda Pharmaceutical Company, and Becton, Dickinson and Company; and holds stock in Merck & Co. J.R. has served on scientific advisory panels for Roche Pharmaceuticals, sanofi-aventis, Novo Nordisk, Eli Lilly and Company, MannKind Corporation, GlaxoSmithKline, Takeda Pharmaceutical Company, Daiichi-Sankyo, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Boehringer Ingelheim Pharmaceuticals, Lexicon Pharmaceuticals; has served as a consultant to Roche Pharmaceuticals, sanofi-aventis, Novo Nordisk, Eli Lilly and Company, MannKind Corporation, GlaxoSmithKline, Takeda Pharmaceutical Company, Daiichi-Sankyo, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Boehringer Ingelheim Pharmaceuticals, and Lexicon Pharmaceuticals; and has received research support from Pfizer, sanofi-aventis, Novo Nordisk, Roche Pharmaceuticals, Bristol-Myers Squibb Company, Eli Lilly and Company, Forest Laboratories, GlaxoSmithKline, Takeda Pharmaceutical Company, Novartis Pharmaceuticals Corporation, AstraZeneca LP, Amylin Pharmaceuticals, Johnson & Johnson, Daiichi-Sankyo, MannKind Corporation, Lexicon Pharmaceuticals, and Boehringer Ingelheim Pharmaceuticals. R.F.A. has been a consultant to Novo Nordisk and sanofi-aventis; has received research support from Eli Lilly and Company, sanofi-aventis, Novo Nordisk, Novartis Pharmaceuticals Corporation, AstraZeneca LP, and Reata Pharmaceuticals; and has participated in Speaker’s Bureaus for Amylin Pharmaceuticals and Eli Lilly and Company. M.J.P., Y.Q., and V.P.S. are employees of and hold stock in Eli Lilly and Company. D.C.H. is a retired employee of Eli Lilly and Company and holds stock in Eli Lilly and Company. S.J.J. is an employee of Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
R.M.B., J.R., and R.F.A. participated as investigators and reviewed and edited the manuscript. M.J.P. contributed to the data analysis and reviewed and edited the manuscript. Y.Q. contributed to the study design, implementation, and analysis; wrote the statistical methods; and reviewed and edited the manuscript. V.P.S. contributed to the study design, implementation, and analysis and reviewed and edited the manuscript. D.C.H. contributed to the study design and reviewed and edited the manuscript. S.J.J. contributed to the study design, implementation, and analysis and wrote the manuscript. R.M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Kelly Guerrettaz, of PharmaNet/i3, for writing assistance; Junxiang Luo, of Eli Lilly and Company, for statistical assistance; and Pam Boltz, Lori Rochelle, Barb Jackson, and Teri Tucker, all of PharmaNet/i3, for editorial assistance.
Footnotes
Clinical trial reg. no. NCT01027871, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0060/-/DC1.
A complete list of investigators can be found in the Supplementary Data online.
*Retired.
References
- 1.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 2.Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care 2009;32:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swinnen SG, Hoekstra JB, DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S253–S259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazzano LA, Lee LJ, Shi L, Reynolds K, Jackson JA, Fonseca V. Safety and efficacy of glargine compared with NPH insulin for the treatment of Type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med 2008;25:924–932 [DOI] [PubMed] [Google Scholar]
- 6.World Medical Association Declaration of Helsinki World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2000;284:3043–3045 [PubMed] [Google Scholar]
- 7.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006;49:442–451 [DOI] [PubMed] [Google Scholar]
- 8.Hansen RJ, Cutler GB Jr, Vick A, et al. Leveraging hydrodynamic size to develop a novel basal insulin (Abstract). Scientific Sessions of the American Diabetes Association; 8–11 June 2012; Philadelphia, PA, Poster # 896-P. [Google Scholar]
- 9.Sinha VP, Howey DC, Soon D, et al. Single-dose pharmacokinetics (PK) and glucodynamics (GD) of the novel long-acting basal insulin LY2605541 in healthy subjects (Abstract). Scientific Sessions of the American Diabetes Association; 8–11 June 2012; Philadelphia, PA, Poster # 106-P. [Google Scholar]
- 10.Heise T, Howey DC, Sinha VP, Choi SL, Mace KF. Steady-state pharmacokinetics (PK) and glucodynamics (GD) of the novel, long acting basal insulin LY2605541 dosed once-daily (QD) in patients with type 2 diabetes mellitus (T2DM) (Abstract). Scientific Sessions of the American Diabetes Association; 8–11 June 2012; Philadelphia, PA, Poster # 1000-P. [Google Scholar]
- 11.Lantus (insulin glargine) prescribing information [Internet], 2007. Bridgewater, NJ, sanofi-aventis US. Available from http://products.sanofi.us/lantus/lantus.html Accessed 20 February 2012
- 12.Levemir (insulin detemir) prescribing information [Internet], 2011. Princeton, NJ, Novo Nordisk. Available from http://www.levemir-us.com/ Accessed 20 February 2012
- 13.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with Type II diabetes mellitus. Diabetologia 1999;42:406–412 [DOI] [PubMed] [Google Scholar]
- 14.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 15.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 16.Kudlacek S, Schernthaner G. The effect of insulin treatment on HbA1c, body weight and lipids in type 2 diabetic patients with secondary-failure to sulfonylureas. A five year follow-up study. Horm Metab Res 1992;24:478–483 [DOI] [PubMed] [Google Scholar]
- 17.Rosenstock J, Bergenstal RM, Blevins T, et al. Better glycemic control and weight loss with the novel long-acting basal insulin LY2605541 compared with insulin glargine in patients with type 1 diabetes (Abstract). Scientific Sessions of the American Diabetes Association; 8–11 June 2012; Philadelphia, PA, Poster # 1026-P. [Google Scholar]
- 18.Moore MC, Smith MS, Mace KF, et al. Novel PEGylated basal insulin LY2605541 has a preferential hepatic effect on glucose metabolism (Abstract). doi: 10.2337/db13-0826. Scientific Sessions of the American Diabetes Association; 8–11 June 2012; Philadelphia, PA, Poster # 1609-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Au JS, Navarro VJ, Rossi S. Review article: Drug-induced liver injury—its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther 2011;34:11–20 [DOI] [PubMed] [Google Scholar]
- 20.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006;354:731–739 [DOI] [PubMed] [Google Scholar]