Abstract
OBJECTIVE
Available evidence supports the emerging hypothesis that metabolic syndrome may be associated with the risk of some common cancers. We did a systematic review and meta-analysis to assess the association between metabolic syndrome and risk of cancer at different sites.
RESEARCH DESIGN AND METHODS
We conducted an electronic search for articles published through October 2011 without restrictions and by reviewing reference lists from retrieved articles. Every included study was to report risk estimates with 95% CIs for the association between metabolic syndrome and cancer.
RESULTS
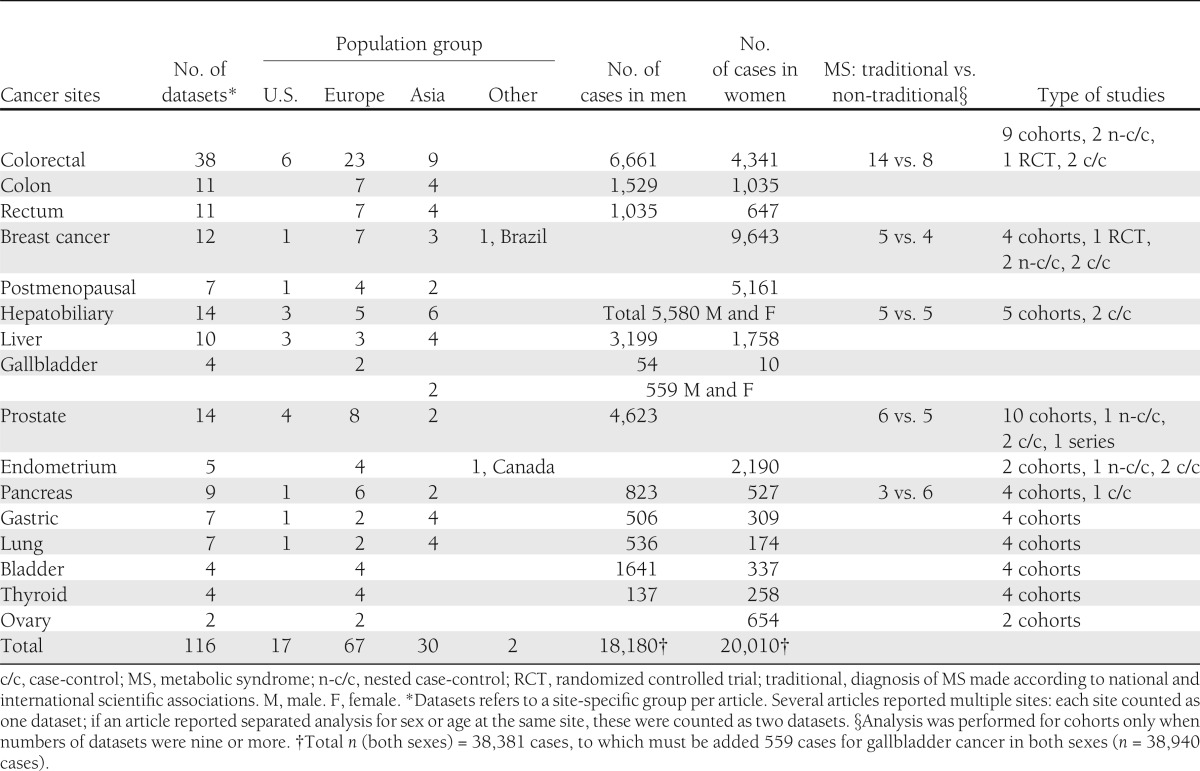
We analyzed 116 datasets from 43 articles, including 38,940 cases of cancer. In cohort studies in men, the presence of metabolic syndrome was associated with liver (relative risk 1.43, P < 0.0001), colorectal (1.25, P < 0.001), and bladder cancer (1.10, P = 0.013). In cohort studies in women, the presence of metabolic syndrome was associated with endometrial (1.61, P = 0.001), pancreatic (1.58, P < 0.0001), breast postmenopausal (1.56, P = 0.017), rectal (1.52, P = 0.005), and colorectal (1.34, P = 0.006) cancers. Associations with metabolic syndrome were stronger in women than in men for pancreatic (P = 0.01) and rectal (P = 0.01) cancers. Associations were different between ethnic groups: we recorded stronger associations in Asia populations for liver cancer (P = 0.002), in European populations for colorectal cancer in women (P = 0.004), and in U.S. populations (whites) for prostate cancer (P = 0.001).
CONCLUSIONS
Metabolic syndrome is associated with increased risk of common cancers; for some cancers, the risk differs betweens sexes, populations, and definitions of metabolic syndrome.
The metabolic syndrome is a cluster of risk factors for cardiovascular disease and type 2 diabetes and constitutes a growing problem worldwide (1). These factors include obesity (particularly central adiposity), dysglycemia, raised blood pressure, elevated triglyceride levels, and low HDL cholesterol levels. On the basis of the most recent epidemiological analysis using the American Heart Association/National Heart, Lung, and Blood Institute 2005 guidelines, similar to those of National Cholesterol Education Program/Adult Treatment Panel III, slightly more than one-third (35%) of adults in the U.S. could be characterized as having the metabolic syndrome (1). This translates to nearly 80 million U.S. adults affected by the syndrome (calculated from U.S. Bureau of the Census data for 2007, with an adult resident population of 228 million). A higher percentage (40.1%) of prevalence occurred with revised International Diabetes Federation 2005 criteria, which use a lower cutoff point for waist (≥94 cm in men and ≥80 cm in women).
Available evidence from epidemiologic investigations and experimental, translational, and clinical studies supports the emerging hypothesis that metabolic syndrome may be an important etiologic factor for the development and progression of certain types of cancer and also for overall cancer mortality (2). Differences in the study populations, length of follow-up, sample sizes, frequency of events, study end points, and statistical adjustment for confounding may all have contributed to the conflicting patterns of association seen in earlier studies. Moreover, both obesity (3) and diabetes (4) have repeatedly been associated with increased incidence for some common cancers, and both conditions represent two important factors contributing to the prevalence of the metabolic syndrome. There is also some evidence that dyslipidemia (low HDL cholesterol levels and/or raised triglyceride) is associated with some cancers (5). It therefore remains possible that some of the associations between metabolic syndrome and cancer risk may be mediated by the coexistence of obesity and overt diabetes.
A systematic and quantitative assessment of published studies is not available. Therefore, we conducted a meta-analysis to summarize all published studies to date on the incidence of cancer associated with metabolic syndrome.
RESEARCH DESIGN AND METHODS
Data sources
We followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) checklist for reporting systematic reviews and meta-analyses (6). We systematically searched Medline, Embase, CENTRAL, CINAHL, and Web of Science through October 2011 for studies in humans of the association between metabolic syndrome and cancer. Our core search consisted of the terms metabolic syndrome, insulin resistance syndrome, and syndrome X, combined with specific terms for each cancer site: colorectal (colon and rectum), gastric, esophageal, hepatobiliary (liver and gallbladder), pancreas, lung, bladder, thyroid, renal, leukemia, malignant melanoma, multiple myeloma, and non-Hodgkin lymphoma for both sexes and prostate, breast, ovary, and endometrium for single sex. Relevant journals, bibliographies, reviews, and personal files were hand searched for additional articles. The search had no language restriction. The last search was performed on 31 October 2011. The electronic database search strategy for Medline is available in Supplementary Table 1.
Study selection
We included studies if 1) their aim was to assess the effect of metabolic syndrome on risk of cancer or association with cancer, 2) they reported the definition of metabolic syndrome according to criteria of national or international scientific associations, federations, or organizations (traditional definitions) or if they used proxy indicators in the absence of the original data (nontraditional definitions), and 3) they included at least three factors, even in the absence of others. We included cohort studies, nested case-control studies, control arms from clinical trials, case-control studies, patient series, and mortality studies. We specified that every study must either report risk estimates (relative risks [RRs], odds ratios, hazard ratios, and standardized incidence ratio) with 95% CIs separately for men, women, or both or must report sufficient data to estimate these. If a site-specific dataset had been published more than once, we used the most recent publication. We included a specific cancer site in the analysis if there were at least two cohort datasets. We excluded studies that were not published as full reports, such as conference abstracts and letters to editors, and studies of cancer precursors (e.g., colorectal adenoma).
Data extraction
From each retrieved article, we extracted the following data: name of the first author, year of publication, country where the study was performed, specific outcomes, follow-up time, proportion of men and women, total number of individuals, number of cases, and risk estimates and their 95% CIs (presence versus absence of metabolic syndrome). We collected data for the most adjusted model. Populations were categorized into four groups: U.S., Europe, Asia, and other. Returned articles were reviewed against inclusion and exclusion criteria by three reviewers (D.G., K.E., and P.C.) until interrater reliability (κ ≥ 0.60) was established. Methodological quality of each study was assessed according to three study components that might affect the strength of the association between metabolic syndrome and cancer risk: length of follow-up for cohort studies, whether metabolic syndrome definition was traditional or nontraditional, and the extent of adjustments for potential confounding factors. We also collected, where available, risk estimates of the association with cancer for each single factor of the syndrome taken at its highest level.
Data synthesis and analysis
The primary end point was to assess the association between metabolic syndrome and cancer risk in cohort studies. For the main outcome at each cancer site, we graded the evidence for study quality and for the risk of bias: study quality was based on the number of datasets, number of events, width of CIs, and heterogeneity; risk of bias was mainly based on type of study and adjustment for confounders. Unless otherwise stated, we used the most adjusted risk estimate from each study. Heterogeneity of the effect across studies was assessed by Q2 statistics, which is distributed as χ2 statistics (7). A value of P < 0.10 was used to indicate lack of homogeneity (heterogeneity) among effects. I2 statistics were provided to quantify the percentage of total variation across studies that was attributable to heterogeneity rather than to chance. I2 values of 25, 50, and 75% correspond to cutoff points for low, moderate, and high degrees of heterogeneity. We used a fixed-effects model if I2 value significance was >0.1; otherwise, we used a random-effect model. We did subgroup analyses for each site to identify study-level factors that modify the association between the presence of metabolic syndrome and cancer risk: these factors include sex, subsite (e.g., colon and rectum), definition of metabolic syndrome (traditional versus nontraditional), and design; for incidence of cancer, we considered cohort studies, nested case-control studies, and control arms of clinical trials. Sensitivity analyses evaluated whether the results could have been affected markedly by a single study and were repeated using a fixed-effects model. Publication bias was examined in funnel plots and with a regression asymmetry test: the Egger test is best for cancer sites with 10 or more datasets. We used STATA, version 9.0 (STATA, College Station, TX), to analyze data.
RESULTS
We screened 2,628 potentially relevant, nonduplicate articles. The κ score for concordance between reviewers rating the articles was 0.62–0.77. The final number of articles (8–50) included in the meta-analysis was 43 (Supplementary Fig. 1), which reported on 116 datasets (Supplementary Table 2). All articles were published in English. The characteristics of included studies are summarized in Table 1 and Supplementary Table 3. The analysis included 38,940 cancer cases (18,180 men and 20,201 women, plus 559 cases for gallbladder cancer not divided by sex). The median follow-up per cohort studies and per cancer site varied from 3 years (endometrium) to 12.2 years (prostate). Notably, no North American population data contributed to the summaries for gallbladder, ovary, thyroid, and bladder cancers. The proportion of studies in which the definition of metabolic syndrome was traditional varied by cancer sites: higher for colorectal (14 vs. 8 nontraditional); approximately equal for breast, hepatobiliary and prostate; and lower for pancreas. No further differentiation was made when the number of datasets for cancer site was four or fewer. The number of potential confounding factors (cancer-site–specific risk factors) included in the adjusted analyses also varied (Supplementary Table 3).
Table 1.
Baseline characteristics of studies included in meta-analysis
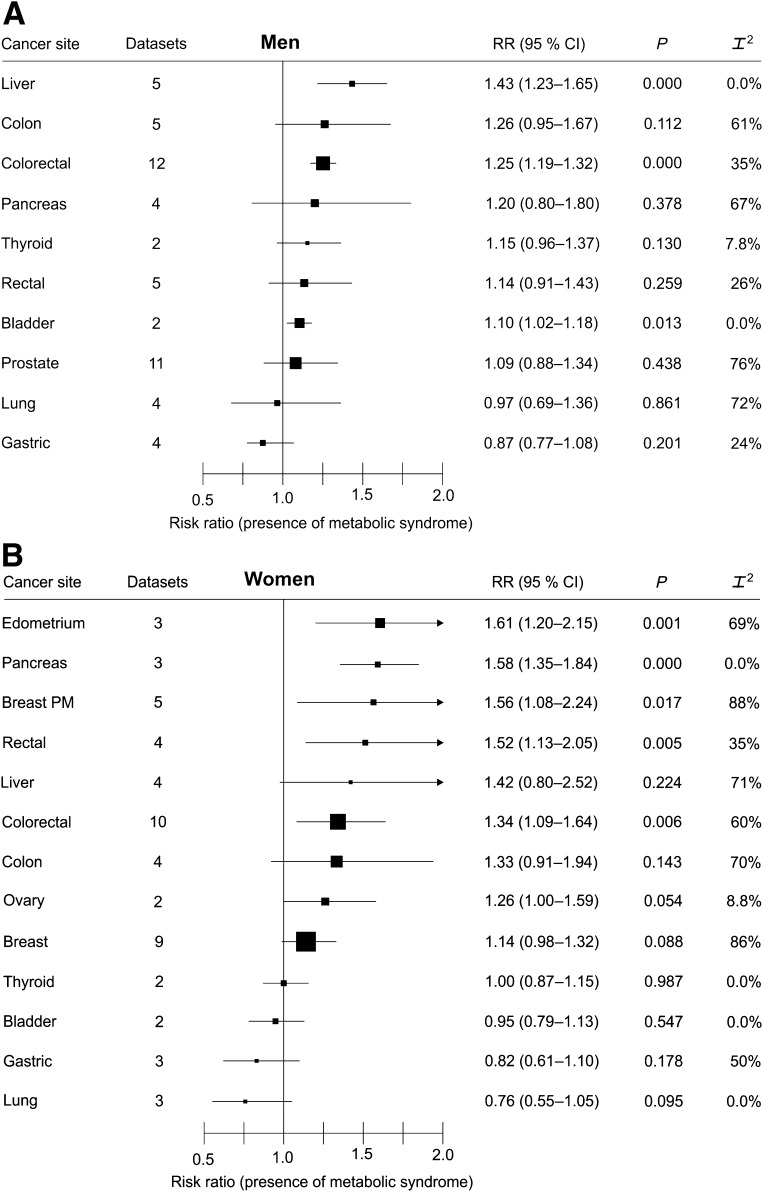
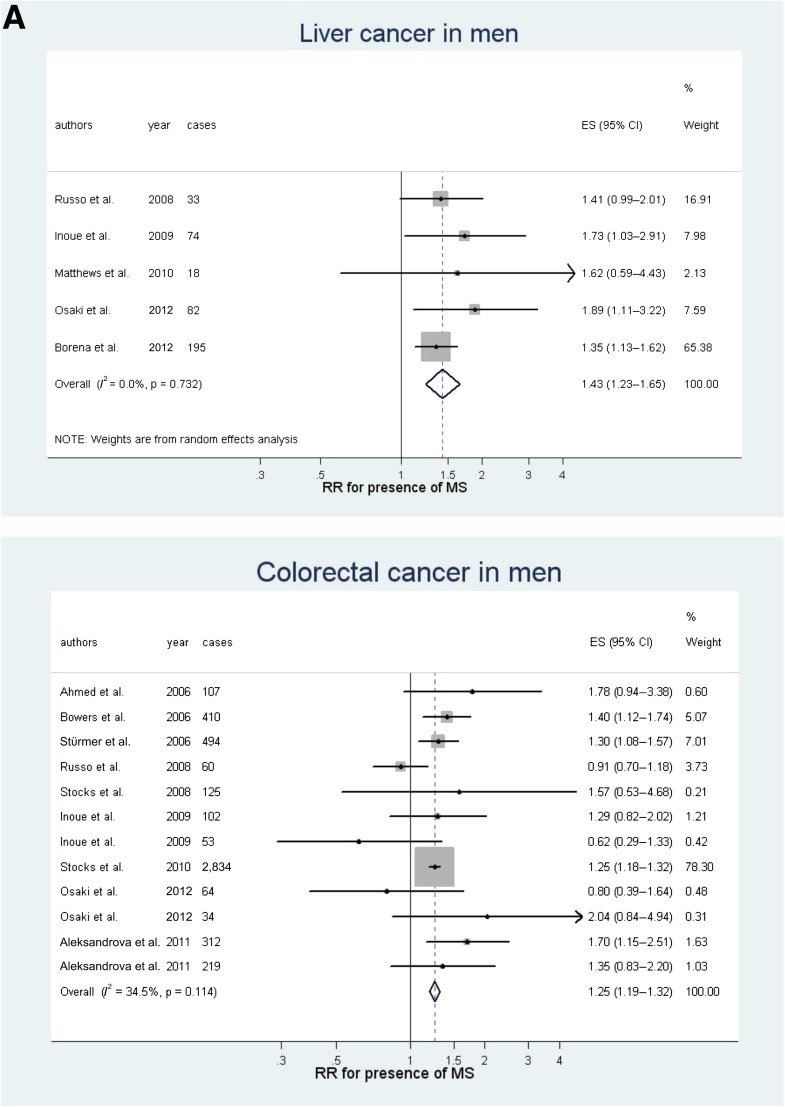
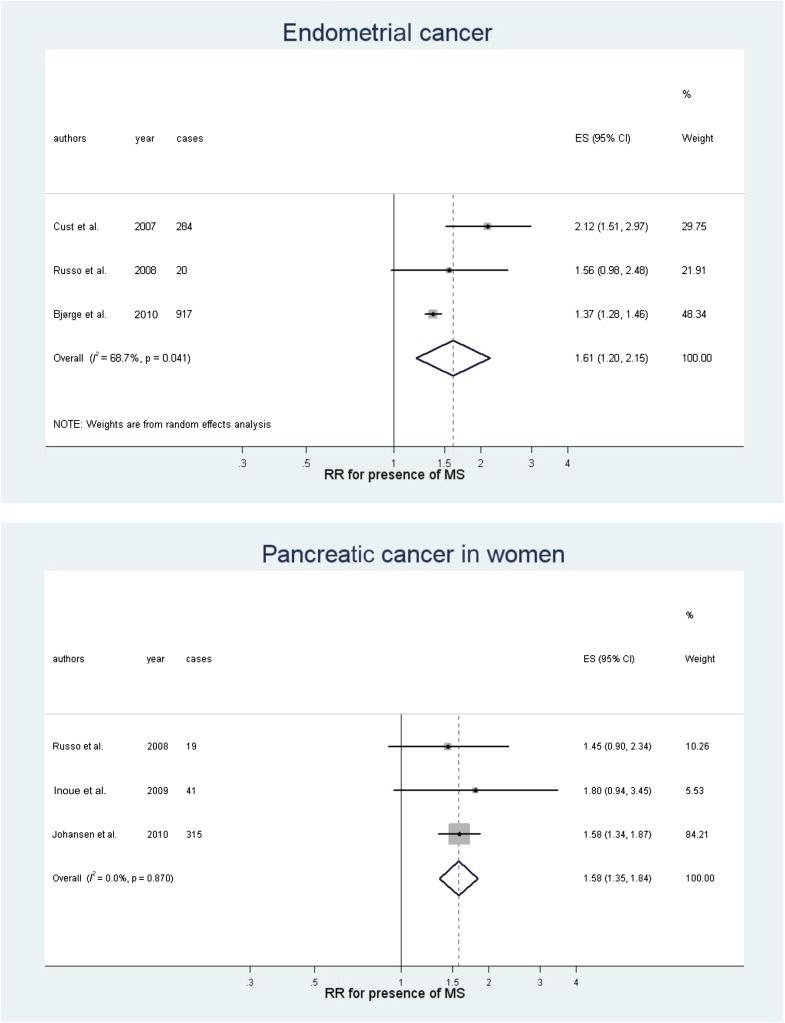
Fig. 1A and B shows the results of meta-analyses of RR (for presence of metabolic syndrome) in men and in women, respectively, for cohort studies only. Separate meta-analyses for some relevant sites and for sex are given in Supplementary Figs. 1–6. In men, the presence of metabolic syndrome was associated with liver (Fig. 2; RR 1.43, P < 0.0001) and colorectal (Fig. 2A; 1.25, P < 0.001) cancers and weakly associated with bladder cancer (1.10, P = 0.013). Between-study heterogeneity was low or moderate for liver, colorectal, and bladder cancer (I2 = 0.0, 35, and 0.0%, respectively) (Fig. 1A). The quality of the evidence was high for the association with colorectal cancer (high number of datasets and events, narrow CIs, and low heterogeneity), moderate for liver cancer, and low for bladder cancer. The overall risk of bias was low, as all studies were prospective cohort studies and most adjusted for many confounders.
Figure 1.
Summary risk estimates by cancer sites in men (A) and in women (B).
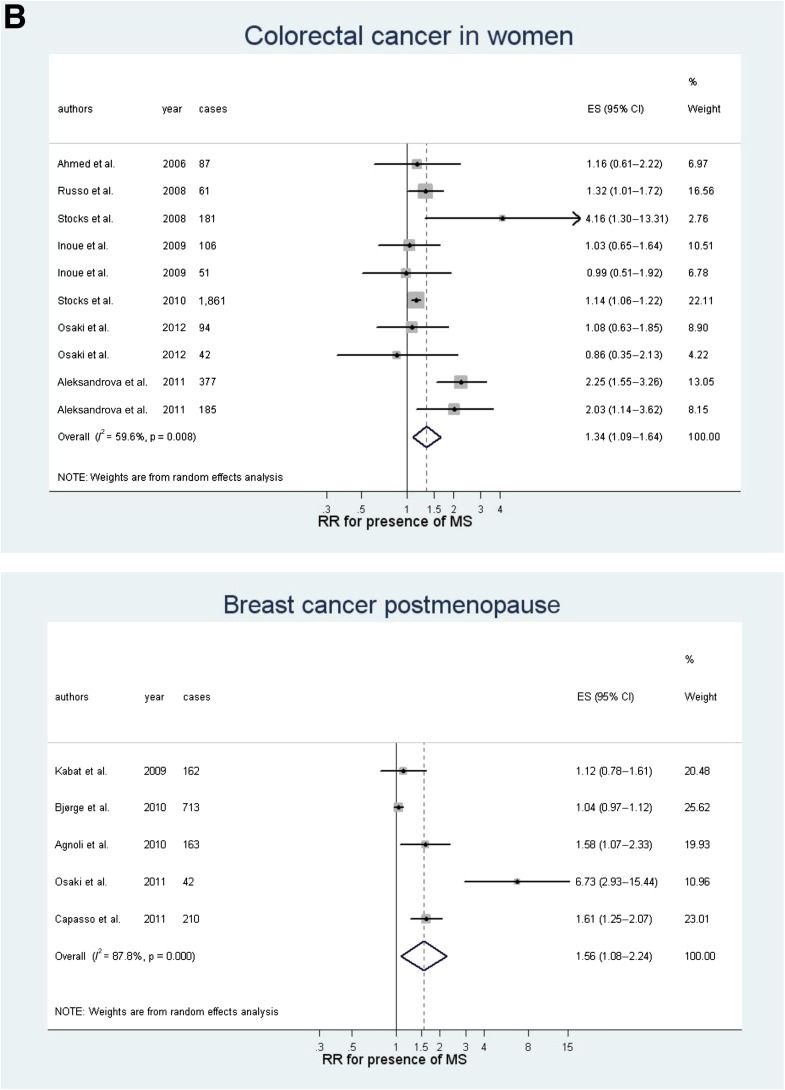
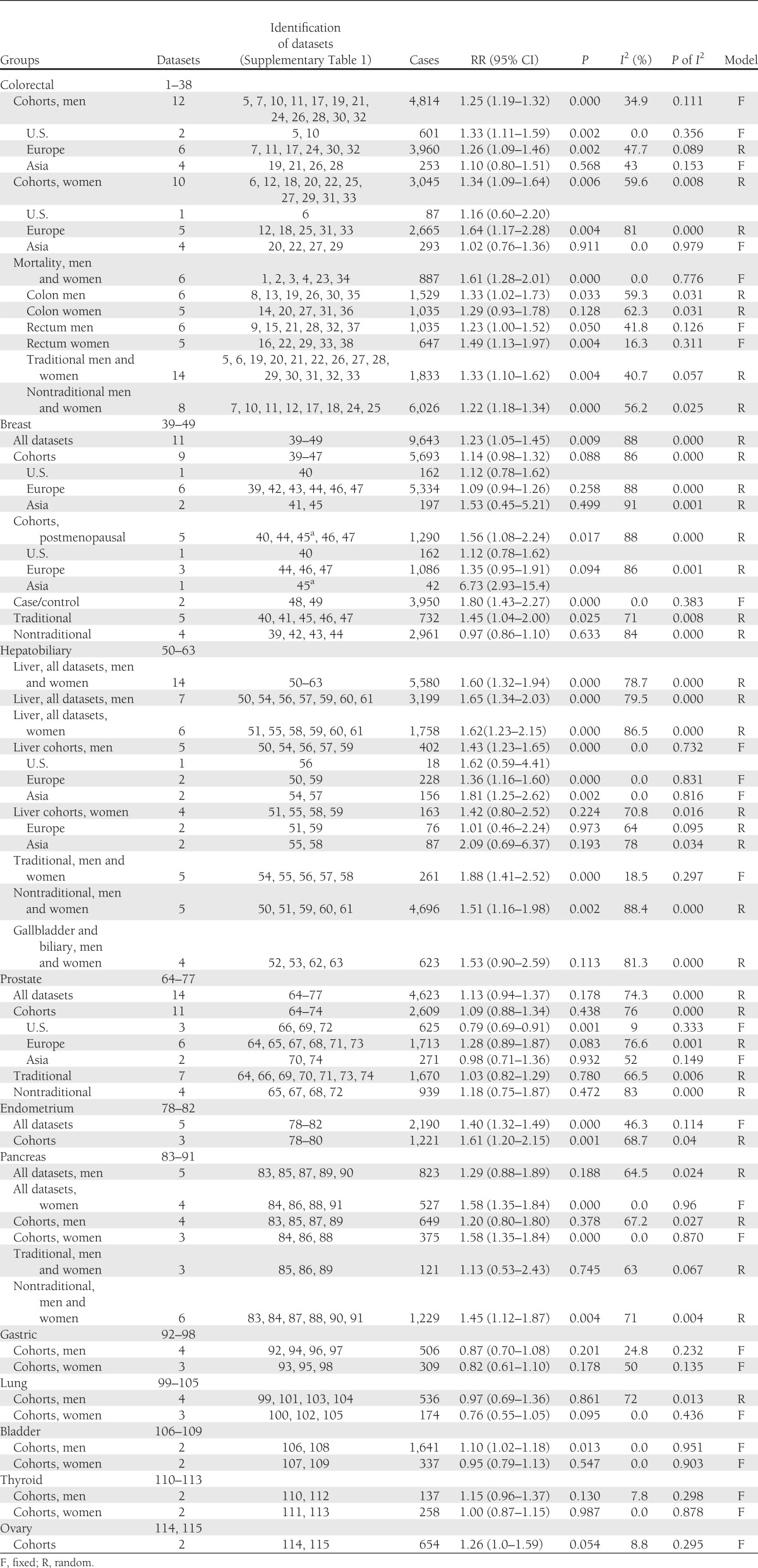
Figure 2.
Meta-analyses for some common cancer sites in both sexes: colorectal and liver cancer in men (A) and colorectal, breast postmenopausal, endometrial, and pancreatic cancer in women (B). ES, effect size; MS, metabolic syndrome.
In women, the presence of metabolic syndrome was associated with endometrial (Fig. 2B; RR 1.61, P = 0.001), pancreas (Fig. 2B; 1.58, P < 0.0001), breast postmenopausal (Fig. 2B; 1.56, P = 0.017), rectal (1.52, P = 0.005), and colorectal (Fig. 2B; 1.34, P = 0.006) cancers; the association with ovary cancer (1.26) was of borderline significance (P = 0.054). Between-study heterogeneity was high for endometrial, breast postmenopausal, and colorectal cancers and moderate or low for rectal (I2 = 35%), pancreas (0.0%), and ovary cancers (8.8%) (Fig. 1B). The quality of the evidence was moderate for the association with colorectal and pancreas cancers and low for endometrium and breast postmenopausal cancers. The overall risk of bias was low, as all studies were prospective cohort studies and most adjusted for many confounders. Associations with metabolic syndrome were stronger in women than in men for pancreas (P = 0.01), rectal (P = 0.01), and bladder (P = 0.01) cancers.
We also examined whether estimates varied between populations in cancer sites for which we had at least two datasets from the main geographical regions (Table 2). For colorectal cancer, for example, we recorded a positive association in U.S. and Europe populations for men and in Europe populations for women; for postmenopausal breast cancer, the positive association was lost in Europe populations; for liver cancer, the association remained significant in Europe and Asia populations for men only; and for prostate cancer the association became negative in U.S. populations (almost exclusively whites, RR 0.79, P = 0.001, I2 = 9%). This last figure was also significant if a mortality study (dataset n = 69) was excluded (RR 0.75 [95% CI 0.60–0.94], P = 0.011, I2 = 0.0%).
Table 2.
Main analyses and prespecified subgroup analyses for cancer sites
We also examined mortality from cancer in the available studies for which we had at least two datasets (Table 2). There were three cohort studies from the U.S. (two for both sexes, one for men only) and a case-control study from China (both sexes) for colorectal cancer only (8,9,16,20). Risk estimate for cancer mortality was 1.61 (P < 0.0001), with no heterogeneity (I2 = 0.0%).
We also examined whether results for cancer association differed according to whether studies of different design (case-control and patient series) were included in the full analysis (Table 2). For breast cancer, the inclusion of two case-control studies (26,27) with 3,950 cases produced a significant overall association (11 datasets, 9,643 cases) of 1.23 (P = 0.009) with high heterogeneity (88%). For liver cancer, the inclusion of two large case-control studies (29,30), with an additional 4,951 cases, produced a significant association for women (RR 1.62, P < 0.0001).
The definitions used for diagnosis of metabolic syndrome affected estimates of the association between metabolic syndrome and cancer risk (Table 2). For both sexes, the estimates remained similar for colorectal cancer (RR 1.33 and 1.22 for traditional versus nontraditional definitions); for liver cancer, both definitions achieved significant associations (1.88 and 1.51); for pancreas cancer, there was no association with traditional definitions (RR 1.13, P = 0.745); for prostate cancer, both definitions gave similar results; and for breast cancer, there was an association with traditional definitions only (1.45, P = 0.025).
To summarize the results for cohort studies with available data (32 cohorts), risk estimates for single factors were equal to metabolic syndrome in 15 cohorts, higher in 11 cohorts, and lower in 6 cohorts. For colorectal cancer, for example, all increased cancer risk was explained by diabetes alone (9,10), diabetes and waist (10), diabetes and BMI (12), and triglycerides >150 mg/dL (15); other single or combined factors explained part (from 30 to 50%) of the increased risk conveyed by metabolic syndrome: BMI (11,21), waist (16,19), BMI and lipid (17), BMI and dysglycemia (14), and hypertension (21).
Influence analysis showed that no single study affected the sex-specific summary estimates for most sites. Moreover, we did not note funnel plot asymmetry for cancer sites where a sufficient number of datasets exited to run the Egger test (colorectal cohorts men, P = 0.912; colorectal cohorts women, P = 0.201; and prostate cancer cohorts, P = 0.085).
CONCLUSIONS
Our results from meta-analyses of prospective cohort studies indicate that metabolic syndrome is consistently associated with an increased risk of several cancers in adults. However, many of the reported associations are small (RR between 1.1 and 1.6) and might differ between sexes for some sites and also across populations. In particular, the associations were stronger in women for some cancers (pancreas and rectal), and the magnitude of the associations was highest for sex-specific cancers (endometrial and breast postmenopausal). Moreover, from analyses in which sufficient datasets existed, the association was stronger for colorectal cancer in female European populations (RR 1.64 [95% CI 1.17–2.28]; five datasets with 2,665 incident cancers) and became protective for prostate cancer in the white U.S. populations, which needs confirmation from future studies. Given the widespread diffusion of metabolic syndrome (1) and the increased cancer mortality associated with metabolic syndrome (2), the findings of the present meta-analysis may have a clinical significance. At least for some common cancer sites (colorectal cancer in both sexes, liver cancer in men, and pancreas cancer in women), we are confident that the results are real, as the grading for study quality was moderate to high and overall risk of bias was low. Moreover, the inclusion of the few case-control studies did not change the overall estimates significantly. In general, the most robust association seems to be with colorectal cancer in both sexes and liver cancer in men. However, part of the association may be explained by the presence of obesity and overt hyperglycemia.
Mechanisms that link metabolic syndrome and cancer risk are not fully understood. Metabolic syndrome may be a surrogate marker for other cancer risk factors, such as decreased physical activity, consumption of high–calorie dense foods, high dietary fat intake, low fiber intake, and oxidative stress (1). Excess adiposity, in particular visceral obesity, results in a state of chronic systemic low-grade inflammation, attributed to production of inflammatory cytokines by both adipocytes and infiltrating immune cells creating a protumorigenic environment (51). By contrast, adiponectin levels are inversely associated with risk of some cancer, and some polymorphisms of adiponectin and its receptor genes are associated with multiple cancer risk (52). The altered balance between proinflammatory and antiinflammatory cytokines driven by central obesity might contribute to insulin resistance, a core component of the metabolic syndrome. The IGF-1 axis has also been implicated in the progression of breast, pancreatic, and esophageal cancer (53): levels of IGF are influenced by circulating insulin levels, with increasing insulin leading to decreased levels of IGF-binding proteins 1 and 2, thus increasing the bioavailability of IGF.
There were some limitations to this meta-analysis. Although not suggested by the formal statistical tests that we undertook, there is still a possibility of publication bias considering that the tests were likely to be underpowered. Moreover, we cannot exclude the possibility of residual confounding and bias because of misclassification. Although the included studies attempted to control for various known risk factors, the possibility of residual or unmeasured confounding cannot be ruled out. Single-point measurement increases the chance of random measurement error, which may underestimate the reported associations. Studies on the association between metabolic syndrome and cancer risk used different factors and cutoff points, which complicate comparisons between studies. Additionally, metabolic factors were not directly measured in some cohorts but replaced either by proxy indicators of the factor (i.e., hypercholesterolemia as a proxy indicator of high triglyceride and/or low HDL cholesterol levels), self-reported diagnosis of diseases (i.e., diabetes, hypertension), or specific drug use (antidiabetic, antihypertensive, and antidyslipidemic). However, there was a consistent positive association between studies, despite the use of different definitions.
There were also strengths to this analysis. Our pooled estimates for the primary end point were based on prospective analyses with detailed adjustment for a wide range of variables. We used uniform methods and subgroup analyses to better define associations across cancer types between sexes, populations, cancer subsites, and definitions of metabolic syndrome. Moreover, this is the first meta-analysis that entailed a comprehensive search for all studies that assessed association between metabolic syndrome and cancer risk.
Findings from this meta-analysis, which includes many recently published studies, suggest that metabolic syndrome is associated with increased risk of common cancers. The excess risk of cancer conferred by metabolic syndrome is low to moderate and in part explained by accompanying obesity of hyperglycemia. Nevertheless, the increasing prevalence of metabolic syndrome worldwide and the high incidence of some malignancies, particularly colorectal and breast cancers, imply that every year many cases of cancer are attributable to metabolic syndrome. Preventive strategies (primary prevention and early detection of cancer) are urgently needed, as has been suggested for patients affected by fully developed diseases, such as diabetes (54). Moreover, patients with the metabolic syndrome, even in the absence of obesity or diabetes, should be encouraged to undergo appropriate cancer screenings, at least for some more frequently involved sites, as recommended for all people of their age and sex. More importantly, we need evidence of whether effective interventions to reduce the prevalence of metabolic syndrome in adult populations (55) will reduce cancer risk. The formulation of public health strategies based on sustained and bearable lifestyle changes can hopefully obtain significant results in the fight against cancer at the population level.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
K.E. participated in the study conception and design; analyzed and interpreted data; drafted the manuscript; critically revised the manuscript for important intellectual content; had final approval of the manuscript; provided study materials; obtained funding; provided administrative, technical, or logistic support; and collected and assembled data. P.C. analyzed and interpreted data, critically revised the manuscript for important intellectual content, had final approval of the manuscript, and provided statistical expertise. A.C. and A.L. analyzed and interpreted data, critically revised the manuscript for important intellectual content, and had final approval of the manuscript. D.G. participated in the study conception and design; analyzed and interpreted data; drafted the manuscript; critically revised the manuscript for important intellectual content; had final approval of the manuscript; provided study materials; provided statistical expertise; obtained funding; provided administrative, technical, or logistic support; and collected and assembled data. D.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0336/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 2.Zhou JR, Blackburn GL, Walker WA. Symposium introduction: metabolic syndrome and the onset of cancer. Am J Clin Nutr 2007;86:s817–s819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–578 [DOI] [PubMed] [Google Scholar]
- 4.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol 2010;47:87–95 [DOI] [PubMed] [Google Scholar]
- 5.Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol 2010;55:2846–2854 [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 8.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F, Risk Factors and Life Expectancy Research Group Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev 2001;10:937–941 [PubMed] [Google Scholar]
- 9.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev 2002;11:385–391 [PubMed] [Google Scholar]
- 10.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006;107:28–36 [DOI] [PubMed] [Google Scholar]
- 11.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 2006;164:652–664 [DOI] [PubMed] [Google Scholar]
- 12.Stürmer T, Buring JE, Lee IM, Gaziano JM, Glynn RJ. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev 2006;15:2391–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer 2008;44:293–297 [DOI] [PubMed] [Google Scholar]
- 14.Stocks T, Lukanova A, Johansson M, et al. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond) 2008;32:304–314 [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Noda M, Kurahashi N, et al. Japan Public Health Center-based Prospective Study Group Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev 2009;18:240–247 [DOI] [PubMed] [Google Scholar]
- 16.Matthews CE, Sui X, LaMonte MJ, Adams SA, Hébert JR, Blair SN. Metabolic syndrome and risk of death from cancers of the digestive system. Metabolism 2010;59:1231–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocks T, Lukanova A, Bjørge T, et al. for the Metabolic Syndrome Cancer Project (Me-Can) Group Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2010;117:2398–2407 [DOI] [PubMed] [Google Scholar]
- 18.Osaki Y, Taniguchi S, Tahara A, Okamoto M, Kishimoto T. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol 2012;36:141–147 [DOI] [PubMed]
- 19.Aleksandrova K, Boeing H, Jenab M, et al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res (Phila) 2011;4:1873–1883 [DOI] [PubMed] [Google Scholar]
- 20.Shen Z, Wang S, Ye Y, et al. Clinical study on the correlation between metabolic syndrome and colorectal carcinoma. ANZ J Surg 2010;80:331–336 [DOI] [PubMed] [Google Scholar]
- 21.Pelucchi C, Negri E, Talamini R, et al. Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer 2010;46:1866–1872 [DOI] [PubMed] [Google Scholar]
- 22.Kabat GC, Kim M, Chlebowski RT, et al. A longitudinal study of the metabolic syndrome and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2046–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjørge T, Lukanova A, Jonsson H, et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomarkers Prev 2010;19:1737–1745 [DOI] [PubMed] [Google Scholar]
- 24.Agnoli C, Berrino F, Abagnato CA, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis 2010;20:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capasso I, Esposito E, Pentimalli F, et al. Metabolic syndrome affects breast cancer risk in postmenopausal women: National Cancer Institute of Naples experience. Cancer Biol Ther 2011;10:1240–1243 [DOI] [PubMed] [Google Scholar]
- 26.Porto LAM, Lora KJB, Soares JCM, Costa LOBF. Metabolic syndrome is an independent risk factor for breast cancer. Arch Gynecol Obstet 2011;284:1271–1276 [DOI] [PubMed] [Google Scholar]
- 27.Rosato V, Bosetti C, Talamini R, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol 2011;22:2687–2692 [DOI] [PubMed] [Google Scholar]
- 28.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer 2012;131:193–200 [DOI] [PubMed] [Google Scholar]
- 29.Shebl FM, Andreotti G, Meyer TE, et al. Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer 2011;105:1424–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011;54:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev 2004;13:1646–1650 [PubMed] [Google Scholar]
- 32.Lund Håheim L, Wisløff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol 2006;164:769–774 [DOI] [PubMed] [Google Scholar]
- 33.Tande AJ, Platz EA, Folsom AR. The metabolic syndrome is associated with reduced risk of prostate cancer. Am J Epidemiol 2006;164:1094–1102 [DOI] [PubMed] [Google Scholar]
- 34.Tuohimaa P, Tenkanen L, Syvälä H, et al. Interaction of factors related to the metabolic syndrome and vitamin D on risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2007;16:302–307 [DOI] [PubMed] [Google Scholar]
- 35.Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer 2009;45:1831–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control 2009;20:1181–1192 [DOI] [PubMed] [Google Scholar]
- 37.Wallner LP, Morgenstern H, McGree ME, et al. The effects of metabolic conditions on prostate cancer incidence over 15 years of follow-up: results from the Olmsted County Study. BJU Int 2011;107:929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grundmark B, Garmo H, Loda M, Busch C, Holmberg L, Zethelius B. The metabolic syndrome and the risk of prostate cancer under competing risks of death from other causes. Cancer Epidemiol Biomarkers Prev 2010;19:2088–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beebe-Dimmer JL, Nock NL, Neslund-Dudas C, et al. Racial differences in risk of prostate cancer associated with metabolic syndrome. Urology 2009;74:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelucchi C, Serraino D, Negri E, et al. The metabolic syndrome and risk of prostate cancer in Italy. Ann Epidemiol 2011;21:835–841 [DOI] [PubMed] [Google Scholar]
- 41.De Nunzio C, Freedland SJ, Miano R, et al. Metabolic syndrome is associated with high grade gleason score when prostate cancer is diagnosed on biopsy. Prostate 2011;71:1492–1498 [DOI] [PubMed] [Google Scholar]
- 42.Cust AE, Kaaks R, Friedenreich C, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer 2007;14:755–767 [DOI] [PubMed] [Google Scholar]
- 43.Bjørge T, Stocks T, Lukanova A, et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol 2010;171:892–902 [DOI] [PubMed] [Google Scholar]
- 44.Rosato V, Zucchetto A, Bosetti C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol 2011;22:884–889 [DOI] [PubMed] [Google Scholar]
- 45.Friedenreich CM, Biel RK, Lau DCW, et al. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 2011;20:2384–2395 [DOI] [PubMed] [Google Scholar]
- 46.Johansen D, Stocks T, Jonsson H, et al. Metabolic factors and the risk of pancreatic cancer: a prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol Biomarkers Prev 2010;19:2307–2317 [DOI] [PubMed] [Google Scholar]
- 47.Rosato V, Tavani A, Bosetti C, et al. Metabolic syndrome and pancreatic cancer risk: a case-control study in Italy and meta-analysis. Metabolism 2011;60:1372–1378 [DOI] [PubMed] [Google Scholar]
- 48.Häggström C, Stocks T, Rapp K, et al. Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 2011;128:1890–1898 [DOI] [PubMed] [Google Scholar]
- 49.Almquist M, Johansen D, Björge T, et al. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control 2011;22:743–751 [DOI] [PubMed] [Google Scholar]
- 50.Bjørge T, Lukanova A, Tretli S, et al. Metabolic risk factors and ovarian cancer in the Metabolic Syndrome and Cancer project. Int J Epidemiol 2011;40:1667–1677 [DOI] [PubMed] [Google Scholar]
- 51.Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci 2011;1229:45–52 [DOI] [PubMed] [Google Scholar]
- 52.Kaklamani VG, Wisinski KB, Sadim M, et al. Variants of the adiponectin (ADIPOQ) and adiponectin receptor 1 (ADIPOR1) genes and colorectal cancer risk. JAMA 2008;300:1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab 2006;17:328–336 [DOI] [PubMed] [Google Scholar]
- 54.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giugliano D, Ceriello A, Esposito K. Are there specific treatments for the metabolic syndrome? Am J Clin Nutr 2008;87:8–11 [DOI] [PubMed] [Google Scholar]