Abstract
OBJECTIVE
In diabetic foot ulcers, wound fluid inflammatory mediators have previously been proposed as surrogate markers for nonhealing. However, currently available wound fluid sampling techniques are not suitable for clinical practice due to low levels of exudate and a high logistical effort. The aim of this investigation was to assess 1) the technique of superficial wound swabbing for harvesting wound fluid; and 2) the quality of the collected fluid for immunoassay analysis of inflammatory mediators.
RESEARCH DESIGN AND METHODS
Both nylon-flocked swabs and film dressings were used to collect wound fluid from foot ulcers of diabetic patients. In randomly selected patients, levels of wound fluid inflammatory mediators and matrix metalloproteases were determined using multiplexed bead-based sandwich immunoassays with respect to both sampling methods. Wound fluid spike-in experiments were performed to evaluate the impact of different sample processing protocols on subsequent immunoassay analysis.
RESULTS
Using the swabbing technique, a median amount of 40 µL (2–120 µL) wound exudate was collected, which allowed the measurement of several multiplex panels. Comparing both sampling methods, a similar qualitative protein recovery was observed with a trend to analyte enrichment by swabbing. Sample processing using swabs did not affect analyte recovery, with the exception of interleukin (IL)-8, thymus and activation-regulated chemokine, IL-17A, interferon-γ–induced protein 10, and IL-4.
CONCLUSIONS
The quality of wound fluid collected by superficial swabbing is not inferior to the current standard technique. Combined with subsequent bead-based sandwich immunoassay analysis, this new method offers a noninvasive technique, suitable for daily clinical routines, for assessment of inflammatory activity in diabetic foot ulcers.
The molecular mechanisms of wound healing and the role of inflammation in this context have been well-described (1). However, it remains unclear why tissue repair in diabetic foot ulcerations does not proceed through the physiological healing cascade in a timely manner. Different attempts have been made to characterize the healing process of the diabetic foot by analyzing serum markers known to be associated with inflammation and tissue repair (2,3). However, serum parameters do not necessarily reflect the wound microenvironment because they can be influenced by multiple wound healing-independent processes. Therefore, assessing markers in wound fluid itself is attractive.
Wound fluid has been investigated in a variety of studies (4–10). Nevertheless, to date, there are neither techniques nor markers that have made their way into clinical routines. Most nonhealing wounds are characterized by a chronic inflammatory microenvironment consisting of inflammatory mediators and proteases (11–13) and persistent inflammatory activity has been linked to the nonhealing status (14). Matrix metalloproteases (MMPs), cytokines, and chemokines have been proposed as the presumably most promising candidate biomarkers (15).
Reproducible sampling of wound fluid, particularly from diabetic foot lesions, is challenging because of low levels of exudate. So far, there is no gold standard to which a new sampling technique could be referenced. Nevertheless, aspiration of wound fluid that has accumulated under occlusive film dressings over time is the most common technique used and might thus be proposed as current standard (6–10,16–18). However, this method requires high logistical effort and is unlikely to work in daily clinical routines (4).
We therefore describe a new method of sampling and processing wound fluid for subsequent immunoassay analysis, which might overcome some of the shortcomings of previously proposed techniques. For this purpose, we investigated whether: 1) superficial wound swabbing is capable of obtaining sufficient sample material for subsequent immunoassay analysis; 2) there is any global or selective loss of relevant proteins comparing superficial wound swabbing with wound fluid aspiration; and 3) the way of sample processing has an impact on protein recovery. Among a wide range of parameters that might be determined in wound fluid, we have chosen to assess a broad panel of immune mediators including macrophage/monocyte-associated cytokines interleukin (IL)-6, IL-1β, tumor necrosis factor-α (TNF-α), and IL-1α, the T-cell–associated cytokines IL-17A, IL-5, IL-4, IL-12p70, interferon-γ (IFN-γ), the regulatory cytokine IL-10, as well as the chemokines IFN-γ–induced protein 10 (IP-10), monocyte chemotactic protein-1 (MCP-1), IL-8, thymus and activation-regulated chemokine (TARC), and MMP1, -2, -3, -7, -8, and -9.
RESEARCH DESIGN AND METHODS
Patient and wound characteristics
Between March and November 2008, 95 consecutive patients with type 2 diabetes attending the outpatient wound care clinic at the Department of Surgery, University Hospital of Tuebingen, were prospectively enrolled. All ulcerations were located below the ankle and assessed by a physician at the initial visit. Wounds were treated according to a comprehensive wound-care protocol (19). Wounds were graded by measuring wound depth with a sterile blunt probe, and the deepest tissue involved was documented. In patients with multiple ulcerations, the wound with the highest grading was selected for analysis. For wounds with identical grading, the larger wound was chosen. Soft-tissue infection was diagnosed clinically if an increase in exudate volume combined with two other local signs (warmth, erythema, lymphangitis, lymphadenopathy, edema, or pain) was present. The sampling protocol was approved by the local university ethics committee, and informed consent was obtained from all patients. All reported investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Technique of wound fluid sampling in diabetic foot ulcerations
Superficial swabbing.
In diabetic patients, wound fluid was collected from the ulceration site using a modified Levine technique (20) with commercially available nylon-flocked swabs (Minitip Flocked Swabs 551C; MicroRheologics, Brescia, Italy, now Copan Diagnostics Inc., Murrieta, CA) after sharp debridement and hemostasis. These particular swabs consist of perpendicular nylon fibers allowing hydraulic uptake of liquid samples by capillary action. In addition, a molded breakpoint allows wound wiping with minimal and constant exertion of pressure. Wound fluid was then recovered from the swab by immediate centrifugation (10,000 rpm for 3 min at room temperature). For centrifugation, the tip of the swab was cut off and inserted into a centrifuge filter (Oxy Fill Centrifugal filter/product discontinued; replaced by centrifuge filter, Ultrafree-MC 0.5 mL; Carl Roth, Karlsruhe, Germany) without the filter membrane placed into an Eppendorf cup. All wound fluid aliquots used in this study were stored at −80°C for subsequent analysis.
Aspiration.
In four randomly selected diabetic patients, wound fluid was additionally collected by covering the wound with an occlusive transparent dressing (Opsite Flexifix; Smith and Nephew, London, U.K.) for 30 min. Fluid that had accumulated beneath the dressing was subsequently aspirated by needle puncture as described previously (7).
Reabsorption of aspirated wound fluid into flocked swabs.
For comparison of both techniques, 50 µL of aspirate was soaked up again with a nylon-flocked swab and then recovered using the above-mentioned protocol, yielding sample material (A/S). By this procedure, we sought to assess: 1) differences in wound fluid quality resulting from the two different wound fluid sampling techniques; and 2) the impact of reabsorbing aspirated wound exudate into flocked swabs on wound fluid quality.
Sampling of acute wound fluid
For analysis of acute wound fluid, drainage liquid was obtained at the first postoperative day in nondiabetic patients undergoing groin hernia repair. Similarly, 50 µL of drainage liquid was soaked up again with a flocked swab (MicroRheologics). Then, wound fluid was recovered by centrifugation as described above.
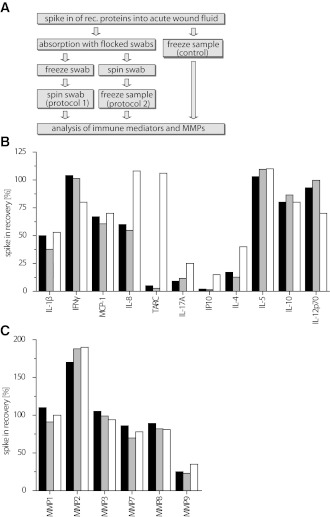
Spike-in experiments and assessment of the impact of the sample-processing procedure
In order to characterize and optimize the sample-processing workflow, commercially available recombinant cytokines, chemokines, and MMPs (all from R&D Systems, Minneapolis, MN) in predefined concentrations were spiked into 1% acute wound fluid supplemented with LowCross-Buffer (CANDOR Bioscience, Wangen, Germany) serving as a matrix. These samples were then either soaked up by swabs (MicroRheologics) with subsequent freezing of the entire swab (protocol 1), or samples were recovered from the swab by centrifugation ahead of freezing (protocol 2). The above-described matrix with spiked-in analytes, which was immediately frozen, served as control. (The workflow is schematically depicted in Fig. 3A.) The relative recovery of spiked-in proteins was determined using multiplexed sandwich immunoassays. Relative analyte recovery values were calculated as: measured concentration × 100/spiked-in concentration.
Figure 3.
Influence of the sample-processing procedure on protein recovery. Recombinant (rec.) immune mediators and MMPs were spiked into 1% acute wound fluid obtained from one patient. The spiked wound fluid sample was absorbed to flocked swabs. Subsequently, samples were either frozen directly on the swabs (protocol 1, gray bars) or after recovery from the swabs by centrifugation (protocol 2, black bars). In control experiments, spiked wound fluid was frozen directly without swab treatment (white bars). After thawing, concentrations of immune mediators and MMPs were determined using multiplexed sandwich immunoassays. A: Experimental workflow. B and C: Recovery of spiked-in immune mediators and MMPs. Shown are mean percentage recovery values averaged from two individual experiments and duplicate measurements. Intra-assay coefficients of variation were <8%. Spiked-in analyte concentrations: cytokines/chemokines: 2.5 ng/mL each; MMP1: 3.4 ng/mL; MMP2: 27.5 ng/mL; MMP3: 4.75 ng/mL; MMP7: 45.75 ng/mL; MMP8: 40 ng/mL; and MMP9: 23.95 ng/mL.
Wound fluid analysis
All samples were cleared by centrifugation at 10,000 × g for 20 min at 4°C ahead of analysis, yielding cell-free supernatants.
LDS-PAGE.
Samples were diluted 1:10,000 in lithium dodecyl sulfate (LDS) sample buffer (Invitrogen, Carlsbad, CA) and boiled immediately. Further deionized water was soaked up by sterile swabs and recovered by centrifugation as described previously; consecutively, the undiluted samples were mixed with an equal volume of LDS sample buffer and boiled instantly. PAGE was subsequently conducted using a standard protocol (Invitrogen). Protein staining was consecutively performed using a Coomassie Brilliant Blue R250 solution (Serva, Heidelberg, Germany).
Multiplexed sandwich immunoassays.
A broad range of immune mediators, including macrophage/monocyte-associated cytokines IL-6, IL-1β, TNF-α, IL-1α, the T-cell–associated cytokines IL-17A, IL-5, IL-4, IL-12p70, IFN-γ, and the regulatory cytokine IL-10, as well as the chemokines IP-10, MCP-1, IL-8, and TARC, were measured. The assays used in return consisted of a set of in-house–developed and thoroughly validated Luminex-based sandwich immunoassay panels, each consisting of commercially available capture and detection antibodies and standard proteins. All assays were thoroughly validated ahead of the study with respect to accuracy, precision, robustness, specificity, and sensitivity (21). Interassay coefficients of variation were <20%. Intra-assay coefficients of variation were <8% over a 2-year period and a total of 35 experiments. LowCross-Buffer (CANDOR Bioscience) was used as assay matrix. The concentrations of the MMPs were determined using a commercially available Fluorokine MAP Kit (R&D Systems). All measurements were performed in duplicate on a Luminex 100 analyzer system, using Luminex IS 2.2 software (Luminex, Austin, TX).
Statistics
Clinical data are presented as median (minimum − maximum) or n (%) unless otherwise stated. Each sandwich immunoassay was calibrated using a seven-point standard curve performed in duplicate. Raw fluorescence intensities were interpreted to final concentrations using a five-parametric fitting model of the respective standard dilution series. Any sample value exceeding the maximum concentration of the calibration curve was excluded from further analysis and marked as above the upper limit of quantification. The lower limit of quantification (LLOQ) was calculated as the blank value +10 × SD. Analyte concentrations below the lower limit of quantification are assigned as <LLOQ.
RESULTS
Patient and wound characteristics
A total of 95 type 2 diabetic patients (64% males and 36% females) with a median age of 73 years (46–89) were included. Median wound size was 4.55 cm2 (0.2–131 cm2). In 46% of the patients, positive probing to bone was observed. In 71% of the patients, pedal pulses were not palpable, and in 43% of patients, soft-tissue infection was diagnosed by clinical assessment. All wounds were located below the ankle (16% toe, 77% foot, and 7% heel), and median wound duration was 29 days (7–103). Multiple ulcers (n ≥ 2) were observed in 35 patients (37%), and median HbA1c was 6.7% (5.0–9.9).
Amount of wound fluid collected by superficial swabbing
Using the swabbing technique, a median sample volume of 40 μL (2–120 µL) could be obtained. In 75% of patients (n = 71), a volume of at least 20 μL could be harvested. In two patients (2.1%), no sample material could be collected. The distribution of wound fluid sample volumes is visualized in Fig. 1. A tendency toward an increase in sample volume with respect to soft-tissue infection (45 [2–100] vs. 30 [0–120] µL; P = 0.625), nonpalpable pulses (40 [0–120] vs. 30 [0–120] µL; P = 0.612), and positive probe to bone (40 [4–100] vs. 30 [3–120] µL; P = 0.785) was observed, even though none of these differences reached statistical significance.
Figure 1.
Amounts of wound fluid obtained from 95 type 2 diabetic patients. Data are given as box plot showing median as well as 25th and 75th quantiles. Whiskers indicate 5th and 95th percentiles. Additionally, the mean (square) and outliers (x) are shown. Single values are represented by circles on the right.
Comparison of wound fluid sampling and processing methods
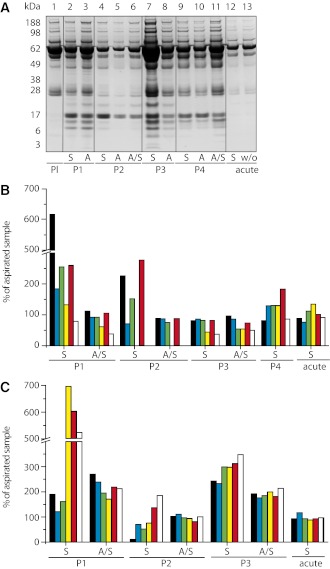
LDS-PAGE analysis of wound fluid from four different diabetic patients (P1–P4) showed no visible loss of protein in a range between 3 and 188 kDa comparing swabbing and aspiration technique (Fig. 2A, lanes 2, 4, 6, 7, 9, 11, and 12). In contrast, a higher amount of protein was collected using flocked swabs, indicated by higher band intensity compared with the aspiration technique. Comparison of diabetic wound fluid (Fig. 2A, lanes 2–11) to blood plasma obtained from one healthy donor (lane 1) or acute wound fluid that was either soaked up by swabs (S) or used without further processing (w/o) (Fig. 2A, lanes 12 and 13) revealed evident differences in protein composition, especially at lower molecular weights (≤49 kDa) irrespective of the sampling technique. Protein fractions observed at low molecular weight in wound fluid of diabetic foot ulcerations appeared neither in acute wound fluid nor in plasma. Further, elution of unused sterile swabs (n = 3) with deionized water by centrifugation and subsequent LDS-PAGE did not show any visible protein bands (results not shown).
Figure 2.
Comparison of wound fluid collection methods. Exudate was sampled from diabetic foot ulcerations of four individual patients (P1–P4) using flocked swabs (S) and from beneath occlusive film dressings (A) of identical wounds in parallel. Additionally, wound exudate collected from under occlusive dressing was soaked up with a nylon-flocked swab and recovered by centrifugation (indicated as A/S). Acute wound fluid was obtained from wound drainage of one nondiabetic patient and processed similarly using swabbing (S). A: The collected sample material was subjected to LDS-PAGE analysis followed by Coomassie protein-staining. Heparinized plasma was used for comparison (Pl). In each sample, immune mediators (B) (IL-1β [black bars]; MCP-1 [blue bars]; TNF-α [green bars]; TARC [yellow bars]; IL-1α [red bars]; and IP-10 [white bars]) and MMPs (C) (MMP1 [black bars]; MMP2 [blue bars]; MMP3 [green bars]; MMP7 [yellow bars]; MMP8 (red bars]; and MMP9 [white bars]) were quantified using multiplex bead-based sandwich immunoassays. The graphs show relative concentration values averaged from technical duplicates and are referenced to concentrations measured in wound fluid collected from beneath occlusive dressings. Intra-assay coefficients of variation were <8%.
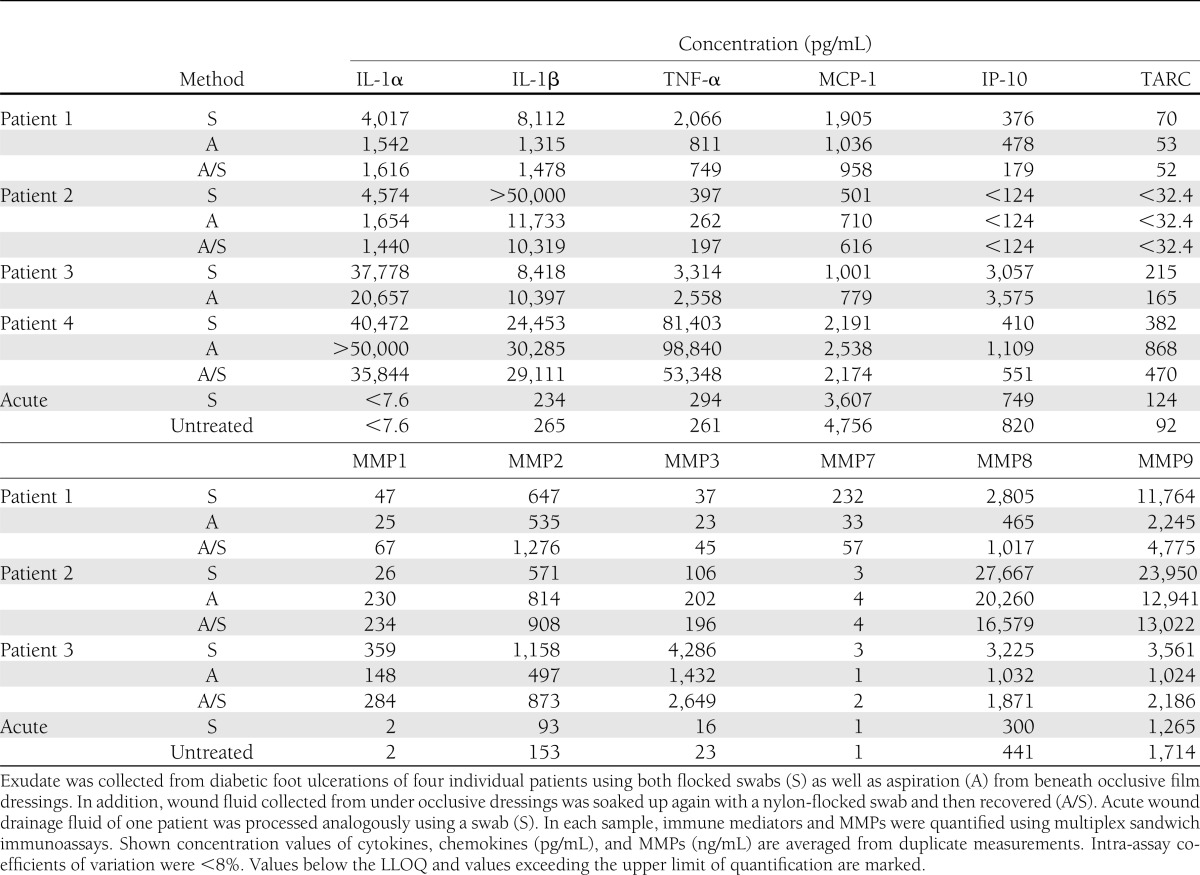
Mean relative differences (in percent) in cytokine and MMP levels determined by bead-based immunoassays are shown in Fig. 2B and C. All measurements were performed in duplicate. In randomly selected patients, wound fluid was harvested both by swabbing as well as aspiration. In addition, the aspirated wound fluid was then reabsorbed into flocked swabs, recovered by centrifugation, and levels of cytokines and MMPs were normalized to analyte concentrations measured in the original wound fluid just as aspirated from beneath occlusive dressings. In wound fluid from patients 1 and 2, the swabbing technique showed up to sixfold higher relative cytokine concentrations as compared with aspiration. In particular, manifold higher concentrations of the proinflammatory cytokines IL-1β, TNF-α, and IL-1α were observed in wound fluid of patients 1 and 2 when collected by swabbing compared with aspiration. The amount of IL-6 and IL-8 generally exceeded the upper limit of quantification (500 ng/mL) in wound fluids of all patients regardless of the technique used (data not shown), whereas IFN-γ and the interleukins IL-17A, IL-10, IL-5, IL-4, and IL-12p70 were not present at detectable concentrations in any sample material (lower limits of quantification: IFN-γ, 9 pg/mL; IL-17A, 35 pg/mL; IL-10, 2 pg/mL; IL-5, 2 pg/mL; IL-4, 7 pg/mL; and IL-12p70, 30 pg/mL, respectively; data not shown). The chemokines TARC and IP-10 were also not detectable in wound fluid derived from patient 2 by neither technique. Analysis of wound fluid derived from patient 3 showed similar levels of IL-1β, MCP-1, TNF-α, and IL-1α when comparing both sampling techniques (swabbing and aspiration), whereas TARC and IP-10 were found in lower concentrations when samples were collected by swabbing. In wound fluid derived from patient 4, higher concentrations of MCP-1, TNF-α, TARC, and IL-1α were measured in samples collected by swabbing. Comparable results were found in the MMP panel in which peak amounts were detectable when superficial wound swabbing was used. This picture was especially evident in patients 1 and 3, in whom protein concentrations of MMPs were clearly elevated when wound fluid was collected by swabbing or aspirated wound fluid processed by flocked swabs. For patient 2, decreased concentrations of MMP1, -2, -3, and -7 were observed when sample material was collected with flocked swabs as opposed to aspiration.
In acute wound fluid, no markedly altered analyte recovery was observed comparing original drainage fluid to soaked-up drainage fluid by nylon-flocked swabs. In this study, analyte recoveries ranged generally ∼100%. Overall, analysis by multiplexed sandwich immunoassays revealed that concentrations of cytokines, chemokines, and MMPs varied strongly in a patient- and collection method-dependent manner (Table 1).
Table 1.
Concentrations of immune mediators and MMPs in chronic wound fluid
Determination of recovery after spike-in following different sample processing protocols
The experimental workflow is illustrated in Fig. 3A. Recovery values were calculated from two individual experiments each measured in duplicate. Dependent on the analyte, recovery values varied with respect to the different sample-processing protocols. These included freezing the sample on the swab (protocol 1), samples recovered first from the swabs and then frozen (protocol 2), and spiked-in samples frozen without further treatment (control). All results were normalized to the initially spiked-in amounts of analytes. Whereas for IFN-γ, IL-10, IL-12p70, and IL-5 values ∼100% recovery was found with only minor variations due to the sample-processing protocols used, recovery of IL-1β, MCP-1, and IL-8 was <75%. However, recovery of IL-17A, IL-4, IP-10, and TARC was <25% using either protocol 1 or protocol 2 (Fig. 3B). Even though IL-8 and TARC were entirely preserved by sample freezing (see control), both sample-processing protocols resulted in a similarly marked loss of those particular analytes. TARC almost entirely diminished using both protocol 1 and 2. IL-17A, IP-10, and IL-4 generally showed relevantly impaired recovery irrespective of sample processing. Nevertheless, in control samples, the recovery of IL-8, TARC, IL-17A, IP-10, and IL-4 was generally higher than in sample material obtained using swabs. As presented in Fig. 3C, recovery of MMP1, -3, -7, and -8 was ∼100%; of MMP2, ∼175%; and of MMP9, <40% regardless of sample processing. Only marginal differences were found between samples that had immediately been frozen together with the entire swab (protocol 1) and those which had been regained prior to freezing (protocol 2), showing variations <20%.
CONCLUSIONS
In this investigation, we technically evaluated a novel method of wound fluid sampling and processing for subsequent analysis by multiplexed sandwich immunoassays in diabetic foot ulcerations. Combining those techniques, we aimed at overcoming limitations caused by low sample volume and assessing its suitability for daily clinical routines. However, it was not within the scope of the study to characterize wound fluid of diabetic patients. Due to the low number of patients included, the study cannot provide statistically reliable data concerning the overall concentrations of cytokines, chemokines, and MMPs in chronic wound fluid.
Using superficial wound swabbing, a volume of at least 20 μL could be obtained in 75% of patients. This is of clinical relevance because 20 μL can still be considered as an adequate volume for multiplexed bead-based array analysis.
We were able to demonstrate that sampling of wound exudate by superficial swabbing and subsequent recovery by centrifugation is not inferior to collecting wound fluid from under occlusive film dressings, which is currently accepted as the standard technique. Furthermore, our data suggest that the swabbing technique works in a major proportion of diabetic foot ulcer patients in whom wound fluid sampling is challenging due to frequently low levels of exudate. In contrast, sampling wound fluid from beneath occlusive dressings can be difficult (4) and laborious when aiming at a high level of standardization (22).
Comparison of levels of cytokines, chemokines, and MMPs demonstrated no relevant loss of analytes when using the swabbing technique compared with the currently available standard method. This was confirmed by LDS-PAGE analysis in which no visible loss of protein occurred at sizes between 3 and 188 kDa. Interestingly, superficial swabbing led to a trend of analyte enrichment rather than depletion when compared with aspiration from beneath occlusive dressings. This effect may result from a more efficient wiping of the wound, causing a higher protein uptake. In addition, there might be interactions of analyte proteins with nylon fibers of the swab and/or the surface of polyurethane film dressings (Opsite), resulting in either enrichment or depletion of wound fluid protein content, whereas elution of sterile swabs with deionized water by centrifugation and subsequent LDS-PAGE analysis ruled out initial protein contamination of swabs. However, we cannot exclude that some proteins are released into the aspirate during sampling that may have been bound to the wound surface. In particular, relevant endogenous levels of macrophage/monocyte-associated mediators IL-1β, MCP-1, TNF-α, IL-1α, IP-10, IL-6, and IL-8 were found in wound fluid of diabetic foot ulcerations, and this is in accordance with earlier findings (11,23). In contrast to our expectations, IFN-γ could not be detected in any sample material. This is interesting because Chow et al. (24) and Grimstad et al. (25) reported high concentrations of IFN-γ in acute wound fluid.
In daily clinical practice, wound fluid collection is associated with a high logistical effort when using currently available techniques (22). Furthermore, these techniques have, at least to our knowledge, never been evaluated with respect to feasibility in a larger patient cohort, and some of them may relevantly interfere with the analytes intended to be measured (5,26). Moreover, an appropriate sampling procedure has to be quick and technically simple. However, reproducibility must be guaranteed by minimizing factors influencing sample quality. This is also a major issue when sampling wound fluid from beneath occlusive dressings, as duration of exudate accumulation is not clearly defined ranging from hours to days (7,27).
Because at least this obstacle is overcome by our sampling technique and can be ruled out as a confounding factor, we investigated the effect of loading nylon-flocked swabs with predefined concentrations of immune mediators using two different consecutive protocols of processing the swabs. Based on the fact that chronic wound fluid is characterized by high endogenous levels of immune mediators and MMPs (6,8,10,12,28,29), together with limitations in sample volumes, we used acute wound fluid as a sample matrix for these spike-in experiments.
No obvious differences in protein recovery could be observed whether sampled wound fluid was directly frozen on the soaked swab (protocol 1) or recovered by centrifugation prior to freezing (protocol 2) (Fig. 3A). Rapid swab freezing after sample collection therefore represents a valuable sample-processing protocol for daily clinical routines in particular with respect to saving handling time. However, comparison of samples obtained by swabbing and untreated controls revealed that a loss of IL-8, TARC, IL-17A, IP-10, and IL-4 can be ascribed to using swabs. Nevertheless, in this context, the recovery values of control samples that had not been treated by any swab indicate that the main part of the observed loss of IL-1β, IL-17A, IP-10, IL-4, and MMP9 is independent of sample processing and therefore caused by the freezing and thawing process. Alterations caused by freezing and thawing might also explain the observed enrichment of MMP2 because without freezing and thawing recovery values were ∼100% (data not shown). Recovery values for all MMPs analyzed present fairly similar throughout all protocols employed, with only little variation, which may even be ascribed to measurement inaccuracy. Therefore, this protocol proves especially worthwhile in this regard, because a wide range of MMPs can be reproducibly sampled and measured. These data show that immediate freezing of the entire swab containing wound fluid allows subsequent recovery and analysis without any relevant loss of analytes. However, whether analytes intended to be measured are stable when undergoing a freeze-and-thaw cycle needs to be checked in vitro.
The method of superficial swabbing has previously been shown to be suitable for wound fluid lactate analysis when sample degradation is of little relevance (30). For MMPs, a good stability was observed in our investigations, comparable to that reported by Muller et al. (31), using a modified Schirmer’s technique for wound fluid sampling. However, this method is technically more demanding, does not preserve the original sample matrix, and is relevantly more time consuming.
Another relevant concern is biofilm production. Therefore, we are convinced that it is useful to clean wounds thoroughly by sharp debridement with adequate hemostasis afterward. This may also trigger cytokine response to bacterial epitopes subsequently measurable in wound fluid. The nylon-flocked swabs allow swabbing with minimal and constant pressure, and wound fluid is absorbed by capillary action. Wound fluid sampled by this particular technique should be most suited to represent the extracellular fluid space constituting a snapshot of the recent biochemical state of the wound.
Notably, attention should be paid to the choice of analytes. Many cytokines may be prone to degradation by wound fluid proteases over time, similar to growth factors (7,16,32). Moreover, different methods of processing wound fluid could particularly influence these analytes. This may therefore relevantly alter wound fluid quality and content. Therefore, when analyzing proteins or cytokines in particular, adequate and standardized techniques for sampling and processing wound fluid are a crucial prerequisite for obtaining meaningful results.
In conclusion, we could show in a fairly large population of 95 diabetic foot ulcer patients that our novel sampling technique does work in the majority of cases. There are qualitative differences in wound fluid when obtained by swabbing compared with aspiration. Moreover, our data suggest that it does matter how samples are processed, and distinct analytes may be instable and are degraded by freezing and thawing, which is not necessarily associated with the sampling technique itself. The swabbing technique introduced in this study represents a real-time snapshot of the local wound microenvironment, overcoming the obstacle of wound fluid being an “undefined soup,” as termed by Trengove et al. (22).
Acknowledgments
This work was supported by a fortune grant (1780-0-0 and 1780-0-1) from Tuebingen University (M.W.L.).
T.O.J. is a member of the scientific advisory board of Luminex, Austin, TX, and Myriad-RBM, Austin, TX. No other potential conflicts of interest relevant to this article were reported.
M.S. researched data and wrote the manuscript. S.B. wrote, reviewed, and edited the manuscript. T.O.J. contributed to discussion and reviewed and edited the manuscript. A.K. contributed to discussion and reviewed the manuscript. N.S.-M. researched data and wrote the manuscript. M.W.L. wrote the manuscript and researched data. M.S. and M.W.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007;127:514–525 [DOI] [PubMed] [Google Scholar]
- 2.Jeandrot A, Richard JL, Combescure C, et al. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia 2008;51:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weigelt C, Rose B, Poschen U, et al. Immune mediators in patients with acute diabetic foot syndrome. Diabetes Care 2009;32:1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gohel MS, Windhaber RA, Tarlton JF, Whyman MR, Poskitt KR. The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J Vasc Surg 2008;48:1272–1277 [DOI] [PubMed] [Google Scholar]
- 5.Schmidtchen A. Degradation of antiproteinases, complement and fibronectin in chronic leg ulcers. Acta Derm Venereol 2000;80:179–184 [DOI] [PubMed] [Google Scholar]
- 6.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen 2000;8:13–25 [DOI] [PubMed] [Google Scholar]
- 7.Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–452 [DOI] [PubMed] [Google Scholar]
- 8.Wallace HJ, Stacey MC. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers—correlations to healing status. J Invest Dermatol 1998;110:292–296 [DOI] [PubMed] [Google Scholar]
- 9.Wysocki AB, Grinnell F. Fibronectin profiles in normal and chronic wound fluid. Lab Invest 1990;63:825–831 [PubMed] [Google Scholar]
- 10.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–748 [DOI] [PubMed] [Google Scholar]
- 11.Fivenson DP, Faria DT, Nickoloff BJ, et al. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen 1997;5:310–322 [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009;32:117–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegand C, Schönfelder U, Abel M, Ruth P, Kaatz M, Hipler UC. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch Dermatol Res 2010;302:419–428 [DOI] [PubMed] [Google Scholar]
- 14.Acosta JB, del Barco DG, Vera DC, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J 2008;5:530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yager DR, Kulina RA, Gilman LA. Wound fluids: a window into the wound environment? Int J Low Extrem Wounds 2007;6:262–272 [DOI] [PubMed] [Google Scholar]
- 16.Chen SM, Ward SI, Olutoye OO, Diegelmann RF, Kelman Cohen I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen 1997;5:23–32 [DOI] [PubMed] [Google Scholar]
- 17.James TJ, Hughes MA, Cherry GW, Taylor RP. Simple biochemical markers to assess chronic wounds. Wound Repair Regen 2000;8:264–269 [DOI] [PubMed] [Google Scholar]
- 18.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen 1999;7:154–165 [DOI] [PubMed] [Google Scholar]
- 19.Coerper S, Wicke C, Pfeffer F, Köveker G, Becker HD. Documentation of 7051 chronic wounds using a new computerized system within a network of wound care centers. Arch Surg 2004;139:251–258 [DOI] [PubMed] [Google Scholar]
- 20.Wound infection in clinical practice. An international consensus. Int Wound J 2008;5(Suppl. 3):iii–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Medicines Agency Guideline on Validation of Bioanalytical Methods. European Medicines Agency, London, United Kingdom, 2010, p. 17 [Google Scholar]
- 22.Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen 1996;4:234–239 [DOI] [PubMed] [Google Scholar]
- 23.Zillmer R, Trøstrup H, Karlsmark T, Ifversen P, Agren MS. Duration of wound fluid secretion from chronic venous leg ulcers is critical for interleukin-1α, interleukin-1β, interleukin-8 levels and fibroblast activation. Arch Dermatol Res 2011;303:601–606 [DOI] [PubMed] [Google Scholar]
- 24.Chow LW, Loo WT, Yuen KY, Cheng C. The study of cytokine dynamics at the operation site after mastectomy. Wound Repair Regen 2003;11:326–330 [DOI] [PubMed] [Google Scholar]
- 25.Grimstad O, Sandanger O, Ryan L, et al. Cellular sources and inducers of cytokines present in acute wound fluid. Wound Repair Regen 2011;19:337–347 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman R, Noble J, Eagle M. The use of proteases as prognostic markers for the healing of venous leg ulcers. J Wound Care 1999;8:273–276 [DOI] [PubMed] [Google Scholar]
- 27.Ono I, Gunji H, Suda K, Iwatsuki K, Kaneko F. Evaluation of cytokines in donor site wound fluids. Scand J Plast Reconstr Surg Hand Surg 1994;28:269–273 [DOI] [PubMed] [Google Scholar]
- 28.Barone EJ, Yager DR, Pozez AL, et al. Interleukin-1alpha and collagenase activity are elevated in chronic wounds. Plast Reconstr Surg 1998;102:1023–1027; discussion 1028–1029 [PubMed] [Google Scholar]
- 29.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 1993;101:64–68 [DOI] [PubMed] [Google Scholar]
- 30.Löffler M, Zieker D, Weinreich J, et al. Wound fluid lactate concentration: a helpful marker for diagnosing soft-tissue infection in diabetic foot ulcers? Preliminary findings. Diabet Med 2011;28:175–178 [DOI] [PubMed] [Google Scholar]
- 31.Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med 2008;25:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bank U, Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J Leukoc Biol 2001;69:197–206 [PubMed] [Google Scholar]