Abstract
OBJECTIVE
To evaluate the rate and determinants of concordance between advanced diabetic retinopathy (DR) and chronic kidney disease (CKD), as assessed by both albuminuria and estimated glomerular filtration rate (eGFR), in the large cohort of the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study.
RESEARCH DESIGN AND METHODS
Patients with type 2 diabetes (n = 15,773) visiting consecutively 19 hospital–based diabetes clinics in years 2007 and 2008 were examined. DR was assessed by dilated fundoscopy. CKD was defined based on albuminuria and eGFR.
RESULTS
CKD was present in 58.64% of subjects with advanced DR, whereas advanced DR was detectable only in 15.28% of individuals with any CKD and correlated with the albuminuric CKD phenotypes more than with the nonalbuminuric phenotype. Age, male sex, diabetes duration, hemoglobin A1c, hypertension, triglycerides, previous cardiovascular disease, and, inversely, HDL-cholesterol correlated independently with the presence of any CKD in individuals with advanced DR; correlates differed according to the presence of albuminuria, reduced eGFR, or both. Conversely, factors associated with the presence of advanced DR in subjects with any CKD were diabetes treatment, previous cardiovascular disease, albuminuria, and, inversely, smoking, eGFR, and age at diagnosis.
CONCLUSIONS
Concordance of CKD with advanced DR is low in subjects with type 2 diabetes, and CKD without advanced DR is more frequent than isolated advanced DR, at variance with type 1 diabetes. Factors independently associated with the presence of any CKD in individuals with advanced DR differ, at least in part, from those correlating with the presence of advanced DR in subjects with any CKD and by CKD phenotype.
Diabetic retinopathy (DR) and diabetic nephropathy (DN) are the main microvascular complications of diabetes, representing the leading cause of blindness (1) and end-stage renal disease (2), respectively. Hyperglycemia is believed to play a central role in the initiation and progression of both complications, as shown by their prevention or retardation by intensive glycemic control in patients with type 1 and 2 diabetes (3,4), thus suggesting common pathogenetic mechanisms. Accordingly, in type 1 diabetes, DR often is associated with albuminuria, and subjects with DN almost always have DR (5), whereas in type 2 diabetes, the concordance rate between these two complications seems to be lower (6), although studies investigating this association within large samples are lacking.
Interestingly, in type 2 diabetes, concordance between reduced glomerular filtration rate (GFR) and albuminuria also is much lower than in type 1 diabetes. In fact, of patients with type 2 diabetes from the UK Prospective Diabetes Study, 67% were normoalbuminuric at the time they developed chronic kidney disease (CKD), as defined by an estimated GFR (eGFR) <60 mL/min/1.73 m2; of these, 51% remained normoalbuminuric, whereas 16% developed albuminuria thereafter (7). Conversely, in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study, only 24% of subjects with type 1 diabetes who developed CKD were normoalbuminuric (8). Thus, in type 2 diabetes, CKD often occurs in the absence of DR or albuminuria, which is the main criteria for differential diagnosis between DN and nondiabetic renal disease. Among subjects with CKD from the Third National Health and Nutrition Examination Survey, 30% had neither DR nor albuminuria and many of them showed only one of these abnormalities in addition to reduced eGFR (9). Unfortunately, previous studies of the association between DN and DR used only albuminuria or proteinuria as CKD markers and, hence, did not consider eGFR, and most of them did not distinguish advanced sight-threatening lesions from nonadvanced or no DR (6,10–12).
This study aimed to evaluate the rate and determinants of concordance between advanced DR with CKD, as evaluated by both albuminuria and eGFR, in the large cohort of patients with type 2 diabetes from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study.
RESEARCH DESIGN AND METHODS
Patients
We used the data collected during the baseline visit for the RIACE Italian multicenter study (registered with clinicaltrials.gov, NCT00715481; URL http://clinicaltrials.gov/ct2/show/NCT00715481), an observational, prospective cohort study of the impact of eGFR on the morbidity and mortality of cardiovascular disease (CVD) in subjects with type 2 diabetes.
The RIACE cohort consisted of 15,933 Caucasian patients with type 2 diabetes (defined by the American Diabetes Association criteria) presenting consecutively to 19 hospital-based diabetes clinics of the National Health Service throughout Italy (see the list of participating diabetes centers in the Supplementary Data online) during the years 2007 and 2008. Exclusion criteria were dialysis or renal transplantation. The study protocol was approved by the locally appointed ethics committees. The quality and completeness of data were controlled and 160 patients were excluded because of missing or implausible values; data from the remaining 15,773 patients were subsequently analyzed.
Measurements
All patients underwent a structured interview to collect the following information: age; smoking status; known diabetes onset and duration; and current glucose, blood pressure (BP), and lipid-lowering therapies, with indication of the class of drug. Weight and height were assessed and BMI was calculated, then BP was measured with a sphygmomanometer after 5 min of rest. Hemoglobin A1c (HbA1c) was measured with high-performance liquid chromatography using methods aligned with those in the Diabetes Control and Complications Trial; triglycerides and total and HDL cholesterol were determined by standard analytical methods; LDL cholesterol was calculated using the Friedwald formula.
The presence of DR was assessed by an expert ophthalmologist using dilated fundoscopy. Patients were classified into the following categories: absent DR; mild, moderate, or severe nonproliferative DR (non-PDR); proliferative DR (PDR); or maculopathy, according to the Global Diabetic Retinopathy Project Group (13). Patients were classified on the basis of the actual fundus appearance or the retinal disease condition that had eventually required previous photocoagulation or surgical treatment. For further analysis, patients with a mild (microaneurysms only) or moderate (microaneurysms and other microvascular lesions) degree of non-PDR were classified as having nonadvanced DR, whereas those with severe non-PDR or pre-PDR (i.e., microaneurysms/hemorrhages in four quadrants, or venous beadings in two quadrants, or intraretinal microvascular abnormalities in one quadrant); PDR (i.e., neovascularization from the disc or from elsewhere, vitreous hemorrhages, or tractional retinal detachment); maculopathy (retinal thickening or hard exudates distant from, approaching, or involving the center of the macula); or blindness (if less than 1/10 normal vision or 20/200 on the Snellen test) either were grouped into the advanced, sight-threatening DR category or were considered separately as patients with severe non-PDR or PDR and patients with maculopathy. Subjects with maculopathy and nonadvanced DR were classified as having maculopathy, whereas those with maculopathy and severe non-PDR or PDR were classified as having one of the latter conditions. DR grade was assigned based on the worst eye.
The presence of CKD was assessed by measuring albuminuria and serum creatinine. As previously reported in detail (14), albumin excretion rate (AER) was obtained from timed (24 h) urine collections or calculated from the albumin-to-creatinine ratio (A:C) in early morning, first-voided urine samples in the absence of symptoms and signs of urinary tract infection or other interfering clinical conditions. Albuminuria was measured in one, two, or three fresh urine samples for each patient using immunonephelometry or immunoturbidimetry and, in cases of multiple measurements, the geometric mean was used for analysis. In subjects with multiple measurements (4,062 with at least two and 2,310 with three values), the concordance rate between the first value and the geometric mean was >90% for all classes of albuminuria (14). Patients then were assigned to one of the following categories of albuminuria (milligrams per 24 h): normoalbuminuria (AER <30), microalbuminuria (AER 30–299), or macroalbuminuria (AER ≥300). In addition, normoalbuminuric subjects were further classified as having normal (AER <10) or low albuminuria (AER <10–29), according to the recent definition of the National Kidney Foundation (15). Serum (and urine) creatinine was measured using the modified Jaffe method. One to three measurements were obtained for each patient and eGFR was calculated using the four-variable equation from the Modification of Diet in Renal Disease study (16) or the equation from the Chronic Kidney Disease Epidemiology Collaboration (17), and the mean serum creatinine value was used in cases of multiple measures, as reported in previous publications (14,18). Patients then were assigned to one of the following categories of eGFR: 1 (≥90), 2 (60–89), 3 (30–59), 4 (15–29), and 5 (<15 mL/min/1.73 m2). Finally, subjects were classified as having no CKD or CKD stages 1–5 on the basis of the presence or absence of micro- or macroalbuminuria and the value of eGFR, according to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (19). Patients assigned to CKD stages (and GFR classes) 4 and 5 were pooled together. As previously reported (18), patients with CKD were further classified as having one of the following CKD phenotypes: albuminuria alone (CKD stages 1–2), reduced eGFR alone (CKD stage ≥3 without albuminuria), or both (CKD stage ≥3 with albuminuria).
Prevalent CVD was assessed from the medical history by recording documented previous major acute CVD events, including myocardial infarction; stroke; foot ulcer or gangrene; amputation; coronary, carotid, and lower-limb revascularization; and surgery for aortic aneurysm. CVD events were adjudicated based on hospital discharge records or specialist visits by an ad hoc committee at each participating center (20).
Statistical analysis
Data are expressed as median (interquartile range) for continuous variables and as numbers of cases and percentages for categorical variables. Patients were stratified by DR grade and CKD phenotype, and prevalence rates were calculated for each combination.
Logistic regression analyses with stepwise variable selection were performed to assess the independent association of CKD phenotypes with nonadvanced or advanced DR compared with no DR; advanced DR was further divided into severe non-PDR plus PDR and maculopathy. Covariates were age, sex, smoking status, known diabetes duration or age at diabetes diagnosis, HbA1c, hypertension, dyslipidemia, triglycerides, HDL cholesterol, and previous major acute CVD events. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, antihypertensive treatment, or all three. Dyslipidemia was defined as high LDL cholesterol, lipid-lowering treatment, or both, whereas high triglycerides and low HDL cholesterol were considered separately. Results of these analyses were expressed as odd ratios (ORs) with their 95% CIs.
Then, subjects with no or advanced DR were stratified by the presence or absence of DR and CKD, and groups were compared using the following statistical tests: one-way ANOVA and the Kruskall-Wallis test for parametric and nonparametric continuous variables, respectively, and Pearson χ2 for categorical variables.
Further analyses were applied to identify variables independently associated with the presence versus absence of CKD (either any or by phenotype) in subjects with advanced DR and with the presence versus absence of advanced DR in subjects with CKD. Covariates were risk factors, previous major acute CVD events, and, in the latter instance, albuminuria and eGFR.
All P values were two-sided, and a P value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL).
RESULTS
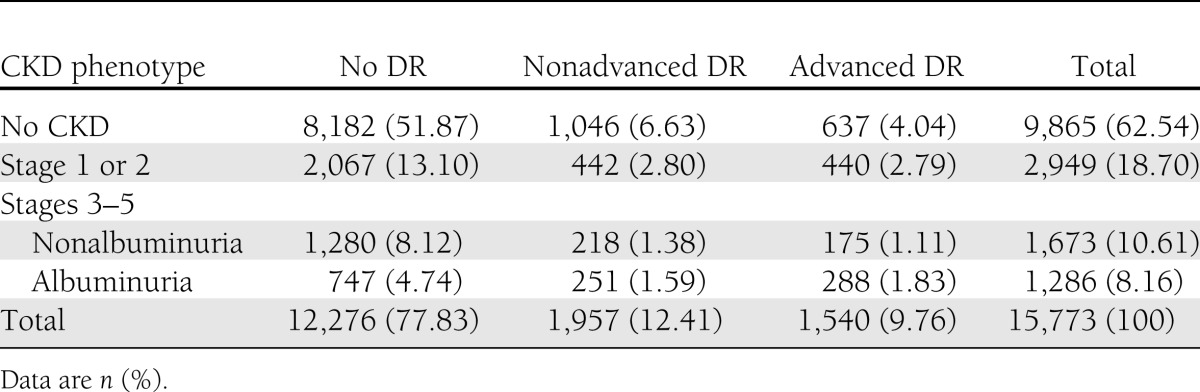
Prevalence rates of CKD phenotypes according to DR grading are shown in Table 1. The majority of subjects (8,182; 51.87%) had neither DR nor CKD, whereas concordance between DR and CKD was found only in 1,814 individuals (11.50%). Of these subjects, 911 (50.22%) had nonadvanced DR and 903 (49.78%) had advanced DR, whereas 882 patients (48.62%) had stage 1 or 2 CKD, 393 (21.66%) had nonalbuminuric stage ≥3 CKD, and 539 (29.72%) had albuminuric stage ≥3 CKD, thus indicating that DR is more frequent in the albuminuric than in the nonalbuminuric CKD phenotypes. Overall, advanced DR was found in 15.28% of the 5,908 individuals with any CKD, and CKD was detected in 58.64% of subjects with advanced DR. Discordance between DR and CKD was observed in 5,777 subjects (36.63%). Of these, 1,683 (10.67%) had only DR, 1,046 (62.15%) had nonadvanced DR, and 637 (37.85%) had advanced DR; 4,094 (25.96%) had only CKD, 2,067 (50.49%) had stage 1 or 2 CKD, 1,280 (31,27%) had nonalbuminuric stage ≥3 CKD, and 747 (18.25%) had albuminuric stage ≥3 CKD. In addition, of the 2,959 patients with reduced eGFR, 1,498 (50.63%) had neither albuminuria nor advanced DR, whereas, of the 4,235 subjects with albuminuria, 2,509 (59.24%) had neither reduced eGFR nor advanced DR, thus indicating that both isolated reduced eGFR and albuminuria often occurred without advanced DR (Supplementary Figure 1). The albuminuric CKD phenotypes without (OR 2.142 [95% CI 1.858–2.468]) and with (OR 2.967 [2.473–3.559]) reduced eGFR, were associated more strongly with advanced DR than the nonalbuminuric one (OR 1.290 [1.059–1.570]).
Table 1.
Prevalence of CKD phenotypes according to DR grade
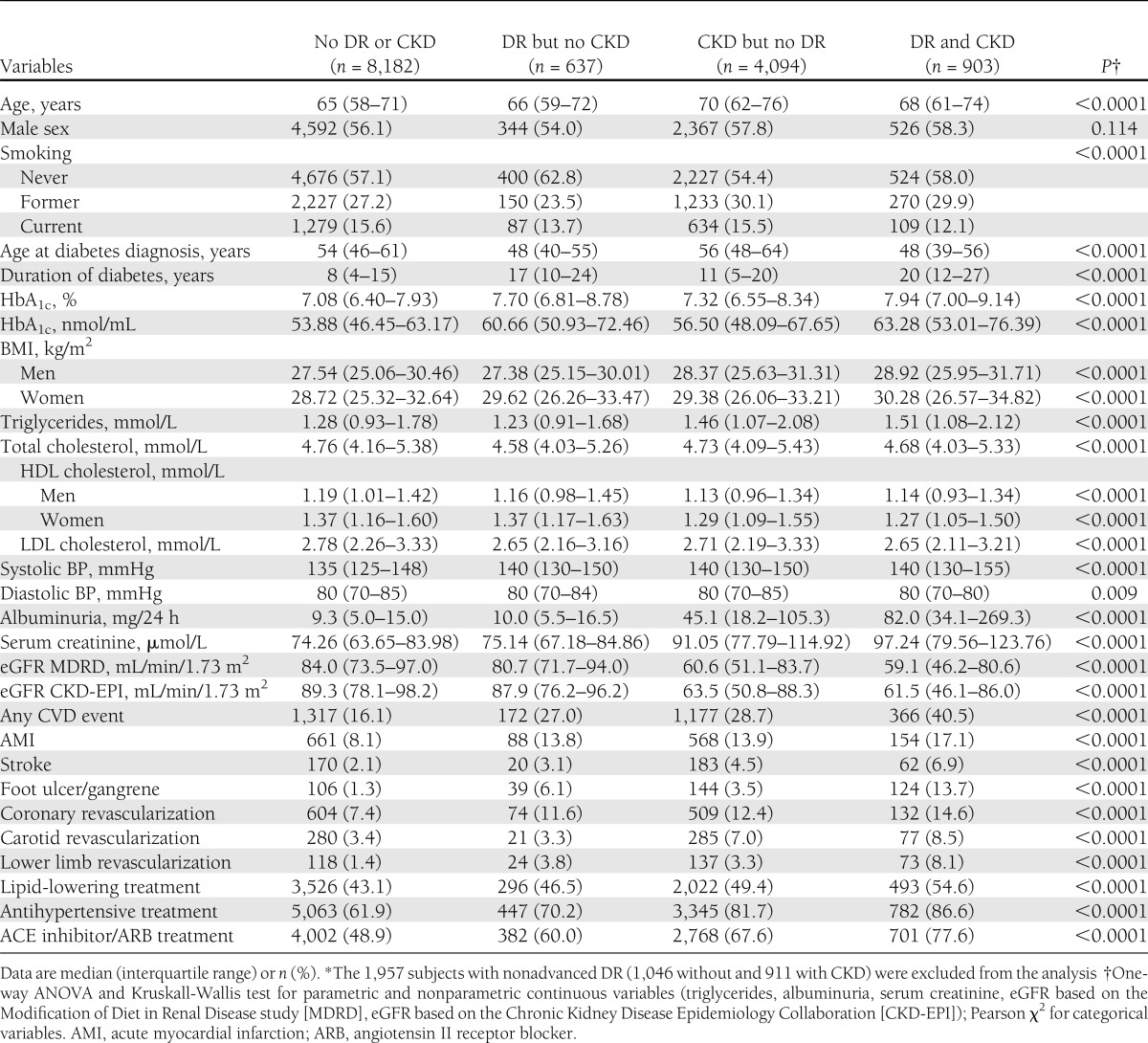
Further analyses were conducted in subjects with no or advanced DR, stratified by presence or absence of any CKD, thus excluding the 1,957 subjects with nonadvanced DR (1,046 without and 911 with CKD). The clinical characteristics of these patients are presented in Table 2. Subjects with advanced DR, either alone or associated with CKD, were less frequently smokers and had a younger age at diabetes diagnosis, longer duration of diabetes, and higher HbA1c. Patients with CKD, either alone or associated with advanced DR, were older and had higher triglyceride and lower HDL cholesterol levels. Systolic but not diastolic BP was higher in individuals with advanced DR, CKD, or both. In the presence of CKD, albuminuria was much higher in subjects with than in those without advanced DR, whereas serum creatinine and eGFR did not differ between these two groups. CVD event and treatment rates were higher in subjects with advanced DR (particularly peripheral events) or CKD (especially coronary and cerebrovascular events and treatments) than in those without, and the rates increased markedly when both complications were present.
Table 2.
Clinical characteristics of study subjects with no or advanced DR, stratified by presence or absence of any CKD*
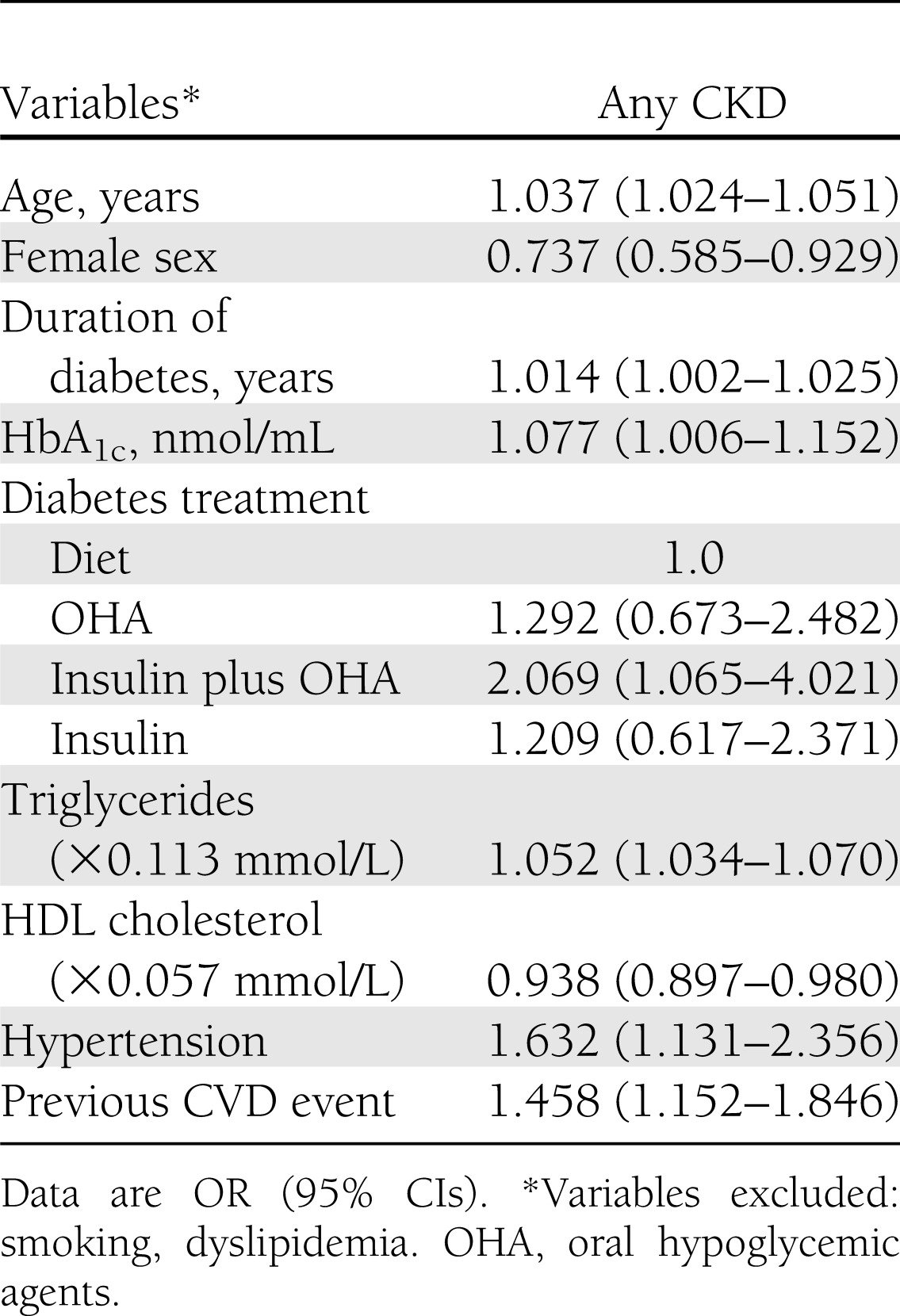
Logistic regression analysis carried out in individuals with advanced DR (Table 3) showed that factors independently associated with any CKD versus no CKD were age, male sex, HbA1c, duration of diabetes, combined therapy with insulin and oral agents, triglycerides, hypertension, previous CVD event, and, inversely, HDL cholesterol. Conversely, smoking status, dyslipidemia, and— when substituted for age and duration of diabetes—age at diabetes diagnosis (data not shown) were excluded from the model. Moreover, correlates differed according to the CKD phenotype: stage 1 or 2 CKD was associated with male sex, HbA1c, hypertension, and previous CVD; albuminuric stage ≥3 CKD was associated with age, former smoking, duration of diabetes, hypertension, and previous CVD; and nonalbuminuric stage ≥3 CKD was associated with age and female sex. Triglycerides correlated with all three phenotypes (data not shown).
Table 3.
Logistic regression analysis with stepwise variable selection of independent correlates of any CKD vs. no CKD in subjects with advanced DR
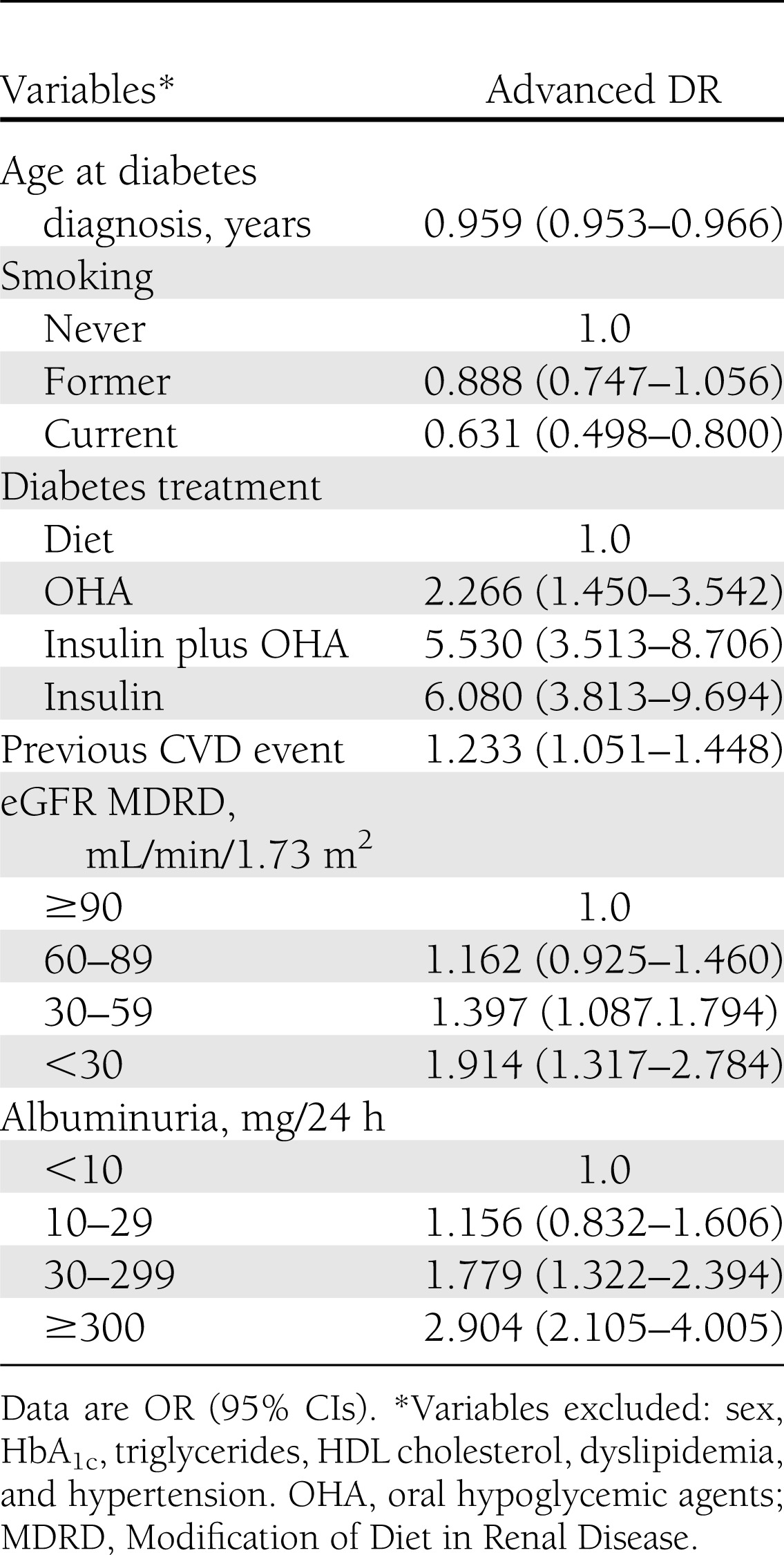
Logistic regression analysis performed for individuals with CKD (Table 4) showed that diabetes treatment (particularly with insulin, either alone or combined with oral agents), previous CVD, reduced eGFR, albuminuria, and, inversely, age at diabetes diagnosis (or age; data not shown) and smoking correlated with presence of advanced DR, whereas sex, HbA1c, hypertension, and lipid abnormalities were excluded from the model.
Table 4.
Logistic regression analysis with stepwise variable selection of independent correlates of advanced DR vs. no DR in subjects with any CKD
Results did not change when subjects with nonadvanced DR were taken into consideration and pooled with individuals with no DR as opposed to those with advanced DR and when patients with maculopathy alone were excluded from the analysis (data not shown).
CONCLUSIONS
This is the first study investigating the association between advanced DR and CKD in a large sample of subjects with type 2 diabetes with combined assessment of albuminuria and eGFR and stratification of patients by CKD phenotype. Main findings are that 1) the two complications coexist in only a small percentage of these patients; 2) CKD is present in approximately 60% of subjects with advanced DR, whereas advanced DR is detectable in approximately 15% of individuals with any CKD; 3) the albuminuric CKD phenotypes are stronger correlates of advanced DR than the nonalbuminuric one; and 4) factors independently associated with the presence of any CKD in individuals with advanced DR differ from those correlated with presence of advanced DR in subjects with any CKD and differ according to the CKD phenotype.
The finding that only 11.50% of individuals from the RIACE cohort had both complications is consistent with previous reports showing a lower concordance rate in type 2 than in type 1 diabetes (5,6). The percentage of subjects with advanced DR showing any CKD (64%) is lower in type 2 diabetes than that observed in type 1 diabetes (5) but similar to that reported by Gall et al. (6) and Magri et al. (11) in type 2 diabetes, thus confirming that DR is a risk factors for DN in these individuals (8). Moreover, the majority of subjects with advanced DR and CKD had increased albuminuria with or without reduced eGFR, which is in keeping with the observation that DR is a risk factor for albuminuria but not for lower eGFR in individuals with type 2 diabetes (8). On the contrary, the prevalence of advanced DR in patients with CKD was quite low (15.28%) in our study, indicating that concordance between advanced DR and CKD is different in type 2 diabetes compared with type 1 diabetes. In fact, the finding that detection of any or advanced DR in subjects with CKD was less frequent than the presence of CKD in subjects with any or advanced DR is at odds with the observation that, in type 1 diabetes, DR occurs almost invariably in individuals with CKD, whereas the latter does not always develop in patients with DR (5). This finding is also in contrast with the report that, in subjects with type 2 diabetes, DR was present in the majority of those with DN and in none or few of those without DN based on renal biopsy (21,22) and, hence, with the concept that DR represents a major criterion for the diagnosis of DN. The reason for this discrepancy is that considering CKD (i.e., increased albuminuria, reduced eGFR, or both) instead of albuminuria alone as a marker of DN implies the inclusion of individuals with nonalbuminuric CKD (i.e., reduced eGFR alone), an increasingly frequent clinical phenotype of CKD that shows a weak relationship with DR.
A growing body of evidence indicates that this CKD phenotype has become the predominant form of renal impairment (i.e., eGFR <60 mL/min/1.73 m2) in subjects with type 2 diabetes (8,18,23), at variance with type 1 diabetes (7). In the RIACE cohort, the prevalence of the nonalbuminuric form was 56.6%, and this phenotype correlated with any or advanced DR less strongly than the albuminuric phenotypes (18) and vice versa. In addition, DR is a risk factor for albuminuria but not for reduced eGFR (8), and a recent prospective study showed that albuminuria with nonreduced eGFR is a stronger predictor of DR than reduced eGFR without albuminuria in subjects with type 2 diabetes (24). These findings, together with the lack of association of nonalbuminuric renal impairment with HbA1c, prompted the hypothesis that macroangiopathy, rather than microangiopathy, is the predominant underlying renal pathology in subjects with nonalbuminuric CKD (18), which might explain, at least in part, the low concordance rate between advanced DR and CKD (particularly the nonalbuminuric phenotype) in individuals with type 2 diabetes. This explanation is consistent with the more marked anatomical heterogeneity of DN in type 2 than type 1 diabetes. In fact, although typical diabetic glomerulopathy almost invariably underlies DN in subjects with type 1 diabetes (25), it can be detected in only one third of patients with type 2 diabetes and microalbuminuria; the remaining two thirds show prevailing vascular and/or tubulointerstitial injury or no significant lesions (26). The increasing use of blockers of the renin-angiotensin system (RAS) also has been claimed to explain the weaker association of the nonalbuminuric CKD phenotype with DR (18,24). In recent large trials, these agents were shown to be effective in preventing renal disease in individuals with diabetes and DR (27). The antiproteinuric effect of RAS blockers might contribute to protection beyond the reduction of BP levels, not only from DN (28) and CVD (29), but also from DR, as suggested by the strong correlation between this complication and albuminuria. However, the percentage of subjects with the albuminuric phenotype who were taking RAS blockers was higher than that of patients with the nonalbuminuric phenotype, though this might simply be an indication effect (18).
An intriguing finding of this study is that variables independently associated with the presence of any CKD in individuals with advanced DR (i.e., age, male sex, duration of diabetes, HbA1c, hypertension, high triglycerides, and low HDL cholesterol) differed from those correlating with the presence of advanced DR in subjects with any CKD (i.e., diabetes treatment, particularly with insulin alone or combined with oral agents; micro-/macroalbuminuria more than reduced eGFR, and, inversely, age/age at diabetes diagnosis and smoking), thus suggesting that different risk factors are involved in these two complications. Interestingly, classic risk factors correlated with CKD, whereas DR was predicted by insulin treatment, which likely is a marker of worst control or most complicated disease, and albuminuria more than eGFR, which is in keeping with the different relation of these two markers of CKD with DR. DR correlated inversely with age/age at diabetes diagnosis. Only previous CVD event(s) correlated independently with both advanced DR and CKD, suggesting that CVD risk associated with DR is not driven entirely by CVD risk factors and the presence of DN or CKD. Our study also showed that factors independently associated with the presence of CKD in individuals with advanced DR differed according to the CKD phenotype, in keeping with previous reports from the RIACE and other cohorts of subjects with type 2 diabetes, showing that nonalbuminuric renal impairment is characterized by distinct clinical features compared with the albuminuric forms, and particularly by the association with female instead of male sex and no or weaker correlation with HbA1c and hypertension (8,18,30).
Strengths of this study include the large size of the cohort, the completeness of data, and the analysis of a contemporary dataset. Limitations include the lack of data on visual acuity and, in particular, the use of fundoscopy instead of the reference method for DR diagnosis, that is, multifield stereoscopic retinal photography. However, fundus examination by an ophthalmologist is the most used method for DR screening and diagnosis in Italy, whereas fundus photography is rarely used because of the lack of trained and qualified personnel and the abundance of ophthalmologists. On the other hand, the use of fundoscopy instead of fundus photography did not allow centralized evaluation of DR and, hence, another potential limitation of the study was assessment by different ophthalmologists, although they were asked to complete a standardized report format for classifying the RIACE participants. To reduce potential errors due to the lower sensitivity of fundoscopy and noncentralized fundus evaluation, the analysis of concordance between DR and CKD considered only individuals with advanced DR. Finally, potential limitations concerning the assessment of DN have been addressed in previous RIACE reports (14,18,20).
In conclusion, the data from this large cohort of subjects with type 2 diabetes show that 1) the concordance rate between advanced DR and CKD is low and 2) CKD without advanced DR is more frequent than isolated advanced DR because of the weaker association with reduced eGFR, which often occurs in the absence of albuminuria and is at variance with type 1 diabetes. Moreover, factors independently associated with the presence of any CKD in individuals with advanced DR differ, at least in part, from those that correlate with the presence of advanced DR in subjects with any CKD and according to the clinical CKD phenotype.
Acknowledgments
This work was supported by the Research Foundation of the Italian Society of Diabetology (Fo.Ri.SID) and the Diabetes, Endocrinology, and Metabolism (DEM) Foundation, and by unconditional grants from Eli Lilly, Takeda, Chiesi Farmaceutici, and Boehringer-Ingelheim. The sponsors played no role in the conduct of the study, collection of data, management of the study, analysis of data, interpretation of the data, or preparation of the manuscript. No other potential conflicts of interest relevant to this article were reported.
G.Pe., A.S., and A.N. researched data and reviewed and edited the manuscript; G.Zo., E.O., G.Ze., R.T., G.G., F.C., L.L., and S.M. researched data and contributed to the discussion. G.Pu. researched data and wrote the manuscript. G.Pu. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the Annual Meeting of the European Diabetic Nephropathy Study Group, Dublin, Ireland, 17–19 May 2012, and at the Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
The authors thank the RIACE Investigators for participating in this study (see the complete list in the Supplementary Data online).
Footnotes
Clinical trial reg. no. NCT00715481, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0628/-/DC1.
A complete list of the members of the Renal Insufficiency And Cardiovascular Events (RIACE) Study Group can be found in the Supplementary Data online.
A slide set summarizing this article is available online.
References
- 1.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003;290:2057–2060 [DOI] [PubMed] [Google Scholar]
- 2.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis 1999;34:795–808 [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications (DCCT) Research Group Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 1995;47:1703–1720 [DOI] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 5.Parving HH, Hommel E, Mathiesen E, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed) 1988;296:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gall MA, Rossing P, Skøtt P, et al. Prevalence of micro- and macroalbuminuria, arterial hypertension, retinopathy and large vessel disease in European type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1991;34:655–661 [DOI] [PubMed] [Google Scholar]
- 7.Molitch ME, Steffes M, Sun W, et al. Epidemiology of Diabetes Interventions and Complications Study Group Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 2010;33:1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, UKPDS Study Group Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006;55:1832–1839 [DOI] [PubMed] [Google Scholar]
- 9.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273–3277 [DOI] [PubMed] [Google Scholar]
- 10.Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am 2004;88:1001–1036, xi [DOI] [PubMed] [Google Scholar]
- 11.Magri CJ, Calleja N, Buhagiar G, Fava S, Vassallo J. Factors associated with diabetic nephropathy in subjects with proliferative retinopathy. Int Urol Nephrol 2012;44:197–206 [DOI] [PubMed] [Google Scholar]
- 12.Rani PK, Raman R, Gupta A, Pal SS, Kulothungan V, Sharma T. Albuminuria and diabetic retinopathy in type 2 diabetes mellitus. Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular Genetic Study (SN-DREAMS, report 12). Diabetol Metab Syndr 2011;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 14.Pugliese G, Solini A, Fondelli C, Trevisan R, Vedovato M, Nicolucci A, Penno G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) study. Nephrol Dial Transpl 2011;26:3950–3954 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2009;54:205–226 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penno G, Solini A, Bonora E, et al. Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 2011;29:1802–1809 [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl 1):S1–S266 [PubMed] [Google Scholar]
- 20.Solini A, Penno G, Bonora E, et al. Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Diabetes Care 2012;35:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parving HH, Gall MA, Skøtt P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992;41:758–762 [DOI] [PubMed] [Google Scholar]
- 22.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 2000;58:1719–1731 [DOI] [PubMed] [Google Scholar]
- 23.Ninomiya T, Perkovic V, de Galan BE, et al. ADVANCE Collaborative Group Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YH, Chen HS, Tarng DC. More impact of microalbuminuria on retinopathy than moderately reduced GFR among type 2 diabetic patients. Diabetes Care 2012;35:803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 1994;43:1358–1364 [DOI] [PubMed] [Google Scholar]
- 26.Fioretto P, Mauer M, Brocco E, et al. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 1996;39:1569–1576 [DOI] [PubMed] [Google Scholar]
- 27.Sjølie AK, Dodson P, Hobbs FR. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int J Clin Pract 2011;65:148–153 [DOI] [PubMed] [Google Scholar]
- 28.de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004;65:2309–2320 [DOI] [PubMed] [Google Scholar]
- 29.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004;110:921–927 [DOI] [PubMed] [Google Scholar]
- 30.Thomas MC, Macisaac RJ, Jerums G, et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 2009;32:1497–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]