Abstract
Elongation rates of barley (Hordeum vulgare L. cv Hanna) leaves decreased with decreasing soil water content, whereas the pH of xylem sap increased from 5.9 to 6.9 over 6 d as the soil dried. The reduction in leaf-elongation rate (LER) was correlated with the increase in sap pH. Artificial sap buffered to different pH values was fed via the subcrown internode to derooted seedlings. Although leaves elongated at in planta rates when fed artificial sap at a well-watered pH of 6.0, LER declined with increasing sap pH. This effect persisted in the light and in the dark. pH had no effect on the relative water content or the bulk abscisic acid (ABA) concentration of the growing zone of these leaves. LERs of the ABA-deficient mutant Az34 were uniformly high over the pH range tested, whereas those of its isogenic wild-type cultivar Steptoe were reduced as the artificial sap pH was increased from 6.0 to 7.0. However, supplying a well-watered concentration of ABA (3 × 10−8 m) in the artificial xylem sap restored the pH response of the Az34 mutant. The results suggest that increased xylem sap pH acts as a drought signal to reduce LER via an ABA-dependent mechanism.
In fertile soils the growth and yield of plants is more sensitive to water availability than to any other environmental factor (Kramer, 1974; Boyer, 1982). The direct relationship between crop yield and the rate of leaf production and expansion (Monteith, 1977), coupled with the high sensitivity of the leaf growth process to drought (Hsiao, 1973), provide both academic and economic motives to further our understanding of the mechanism by which leaf expansion is restricted during drought.
Although the basis of leaf expansion has been subject to investigation for many years (e.g. Hales, 1727; Sachs, 1887; Begg and Wright, 1962; Baker et al., 1985; Palmer and Davies, 1996), substantial questions concerning its control and regulation remain unanswered (Dale, 1988). The mechanistic basis by which leaf expansion is restricted during drought has received comparatively little attention. Clearly, leaf water relations can and do play a vital role in the determination of the rate of leaf growth, but reductions in growth during drought frequently occur in the absence of any decline in leaf turgor (Passioura, 1988; Gowing et al., 1990; Puliga et al., 1995). Michelena and Boyer (1982) demonstrated that although LERs of maize growing in drying soil were significantly reduced, sufficient solutes accumulated in the leaf-elongation zone to maintain virtually constant turgor. This suggests the involvement of regulatory processes aside from leaf water relations that can control the rate of leaf expansion during drought.
Increases in xylem sap pH during soil drying and other stresses are frequently observed (Hartung and Radin, 1989; Gollan et al., 1992; Wilkinson and Davies, 1997; Wilkinson et al., 1998), which may give rise to increases in the apoplastic (wall) pH of leaf tissues (Hoffmann and Kosegarten, 1995). The importance of pH in mediating physiological responses to stress has long been recognized (Hartung and Radin, 1989) and more recently it was shown to form a stress signal that controls gas exchange in leaves of plants growing in drying soil (Wilkinson and Davies, 1997; Wilkinson et al., 1998).
An early indication that drought may control leaf growth via its effects on apoplastic pH was given by Van Volkenburgh and Boyer (1985). They reported that reductions in leaf growth of maize plants subjected to a soil-drying treatment correlated with a reduced ability of the leaf to acidify a weakly buffered droplet of solution placed on its surface and assumed to be in contact with the apoplast. The pH of the droplet on the leaf surface of plants growing in drying soil was initially higher (pH 6.3) than that from well-watered plants (pH 5.9), and plants growing in drying soil showed a reduced ability to acidify the droplet in response to illumination. Van Volkenburgh and Boyer (1985) demonstrated the possibility of a drought-induced increase in apoplastic sap pH and provided the first suggestion that there is a possible link between apoplastic pH and the rate of leaf expansion during drought.
In intact leaves, increases in xylem sap pH cause closure of stomata (Wilkinson and Davies, 1997). This pH response was shown to require the presence of ABA, but only at the very low concentration found in the xylem sap of most well-watered plants. This is a concentration that does not affect stomatal aperture in a well-watered environment (Wilkinson et al., 1998). Rather than having a direct effect on stomatal guard cell turgor, increased pH mediates an accumulation of ABA in the apoplast surrounding the guard cells (Hartung et al., 1998). ABA receptors on the external plasma membrane of the stomatal guard cells (Hartung, 1983) are thus stimulated to induce changes in guard cell turgor and hence in stomatal aperture via effects on membrane ion channels (see MacRobbie, 1991). In this paper we investigate the possibility that high pH could induce reductions in leaf expansion via a mechanism that involves ABA.
A role for ABA in the control of leaf expansion during water deficit has already been suggested (e.g. Zhang and Davies, 1990a, 1990b; Dodd and Davies, 1996). Increased concentrations of this hormone will reduce leaf elongation in vivo and in vitro (Zhang and Davies, 1990a, 1990b). In some cases, however, reduced rates of leaf expansion have been observed in plants growing in dry soil before the xylem sap (or bulk leaf) ABA concentration was increased (Dodd and Davies, 1996), and it has been forcibly argued that for many species, xylem sap of droughted plants may not contain enough ABA to explain the physiological and developmental responses to soil drying (Munns and King, 1988). The mechanistic basis of the ABA-induced restriction of leaf growth also remains to be discovered.
The use of ABA-deficient mutants may further our understanding of the involvement of ABA in the control of leaf expansion (Mulholland et al., 1996a, 1996b). Az34 is a molybdenum cofactor mutant of barley that is isogenic to the Steptoe cultivar (Walker-Simmons et al., 1989). Mutation-induced deficiencies in the molybdenum cofactor-requiring enzyme aldehyde oxidase prevent oxidation of an ABA precursor, such that plants exhibit reduced ABA concentrations under well-watered conditions and are unable to produce as much extra ABA as the wild type in response to soil drying.
In this paper we report a role for pH and ABA in the control of leaf expansion during water deficit in the unrelated barley cvs Hanna and Steptoe, and in the Az34 mutant of the latter. We have observed a significant effect of increased xylem sap pH on leaf expansion, and we demonstrate a fundamental requirement for ABA for this response to occur. To our knowledge, this is the first demonstration that ABA is required to mediate pH-regulated cell expansion in droughted plants.
MATERIALS AND METHODS
Plant Material
For leaf-elongation bioassays, approximately 100 seeds of barley (Hordeum vulgare L. cv Hanna, mutant Az34, and the isogenic wild-type cv Steptoe) were sown in large pots (170 mm diameter, 3.0 dm3 in capacity) containing John Innes No. 2 commercial potting compost (Keith Singleton's Seaview Nurseries, Cumbria, UK). The potting compost provided the nutrient requirements for all cultivars during the period of experimentation. Nitrogen was available in the form of both nitrate and ammonium. Seeds were deep sown 10 cm below the surface of the compost to allow the development of a subcrown internode. Plants were raised in a controlled-environment cabinet with a day/night temperature of 24°C/15°C, RH of 30%/55%, under a photoperiod of 12 h and a light intensity of 400 μmol m−2 s−1 provided by eight 400-W tungsten halide lamps (Powerstar HQI-T, Osram, Frankfurt, Germany). Plants were watered daily to the drip point and were ready for use when the third main leaf had emerged and was expanding at a constant rate (3 weeks old).
For other measurements (soil moisture, LER, and xylem sap pH), no more than 15 seeds of cv Hanna were sown near the surface in 140-mm-diameter plastic pots, 1.0 dm3 in capacity. Plants were raised as described above. Experimentation was initiated soon after the emergence of the fifth main leaf (4 weeks old).
Measurement of Soil Moisture, LER, and Xylem Sap pH during Soil Drying
Water was withheld from half of the pots containing 15 cv Hanna plants and the rest were watered as normal for the next 6 d. The low plant-to-soil ratio ensured a slow soil-drying treatment. Although not measured in this investigation, previous work has estimated that this soil-drying treatment provides a decline in soil water potential to circa −1.0 MPa after 6 d of soil drying (Bacon, 1998). Three determinations of soil moisture content (v/v) within one drying and one well-watered pot were carried out every day using a Theta soil-moisture probe (Delta-T Devices, Cambridge, UK). The length of the expanding fifth leaf from 10 different plants in these pots was measured at the same time every day to determine the LER (in millimeters per hour). Xylem sap pH of 10 plants from an equivalent pot was measured halfway through the photoperiod daily for the entire soil-drying cycle. Measurement of xylem pH was obtained by severing the plant through the base approximately 1 cm above the surface of the soil (halfway through the elongation zone of the currently expanding leaf). The cut surface was immediately wiped with absorbent tissue to remove contaminants from cut cells. Plants were allowed to stand for a short time (5–30 min) until root pressure resulted in the appearance of xylem sap as a droplet at the cut surface. During this time pots were covered with aluminum foil to prevent evaporation from the droplet forming at the cut surface. The pH was determined by placing an Orion combination needle pH electrode (Orme Scientific, Manchester, UK) into the droplet.
Although whole-pot or isolated root-system pressurization is commonly used to express sap from plants, these techniques are fraught with potential complexities that may lead to artifactual results. Whole-pot pressurization may force soil moisture into the roots of the plants (Salim and Pitman, 1984), which could rehydrate roots growing in drying soil and reduce any dry-soil-induced pH changes that had developed in the unpressurized plant. Pressurization of isolated root tissue causes dehydration and affects xylem sap composition regardless of the initial water status of the tissue (Hartung and Radin, 1989). For these reasons we chose to exude sap under root pressure, as described above. Although this takes a little longer and yields only small amounts of sap at the cut surface of plants growing in drying soil, any concentration of the sap constituents that might occur within 30 min is very small and is more likely to acidify the droplet than to artifactually increase its pH (Schurr and Schulze, 1995). These authors found that it took 9 h for the pH of the sap exuding under root pressure to be decreased by 0.5. Presumably, any changes within the 5 to 30 min after the pH of the sap was determined would therefore be very small.
Leaf-Elongation Bioassays
The development of a subcrown internode on the plants as a result of deep sowing allows the removal of the root system and access to the transpiration stream while leaving the elongation zones of the leaves intact and undamaged. The severed internode is a suitable means by which to introduce solutions directly into the transpiration stream of the plant (Munns, 1992; Dodd and Davies, 1994, 1996).
Plants at the correct developmental stage (third leaf with a constant LER) were transferred to the dark for at least 1 h before use. This reduced the transpiration rate and the chance of embolism on cutting the xylem tissue. Plants were excavated in darkness and the internode was cut under distilled water 2 cm below the crown. Each plant was rapidly transferred to a vial containing 10 cm3 AS: 1.0 mm KH2PO4, 1.0 mm K2HPO4, 1.0 mm CaCl2, 0.1 mm MgSO4, 3.0 mm KNO3, and 0.1 mm MnSO4 (from Wilkinson and Davies, 1997). The AS was adjusted to the appropriate pH with 1 m HCl or KOH, which resulted in increases in the concentrations of K+ and Cl− 1 order of magnitude lower than those determined within the xylem sap of growing plants (e.g. Schurr and Schulze, 1995) and total K+ and Cl− concentrations within the AS that have no effect on leaf expansion (Dodd, 1996).
The H2PO4/HPO4− buffering system present within the AS is the same system found within the xylem sap of sunflower seedlings (Gollan et al., 1992). No data on the buffering system within barley were found in the literature. In some cases ABA (Lancaster Synthesis, Morecambe, UK) was added to the AS to give a final concentration of 0.03 μm, the approximate concentration found in the xylem sap of a well-watered monocot (Zhang and Davies, 1990b). The vials containing the seedlings were left in darkness for another hour. They were then randomized and transferred back to the controlled-environment cabinet in the light at 21°C to 24°C. For experiments with cv Hanna involving a dark period, the plants were placed in the light at a higher temperature of 28°C to 30°C to ensure rapid delivery of AS before the dark period, when sap flow rates become much slower. Similar experiments with Az34 were carried out over a longer period and therefore did not require this initial increase in temperature. In all experiments the length of the third leaf was measured immediately and thereafter every 2 h for up to 14 h using graph paper photocopied onto acetate. LER was calculated in millimeters per hour.
At the end of some experiments the basal 30 mm of the experimental leaf, which constitutes the elongation zone (Palmer and Davies, 1996), was dissected and weighed. It was then placed into a vial containing distilled water, sealed, and kept on ice overnight to hydrate the tissue. Storage on ice has been determined to inhibit any measurable increase in length (Milnes et al., 1998). After reweighing, the tissue section was freeze-dried for 48 h to obtain its dry weight. Relative water content was calculated at the end of the bioassay as the water content at that point relative to the fully hydrated water content.
At the end of other experiments, the bulk ABA concentration of the leaf bases was determined using a RIA (for method, see Quarrie et al., 1988). Three bases were required to give enough material for one replicate in the RIA. These were freeze-dried, ground, and incubated at 5°C overnight in tubes containing distilled water at a tissue-to-solvent ratio of 1:20 (w/v). The tubes were centrifuged and the supernatant was removed for RIA analysis.
RESULTS
Effects of Soil Water Deficit on LER and pH
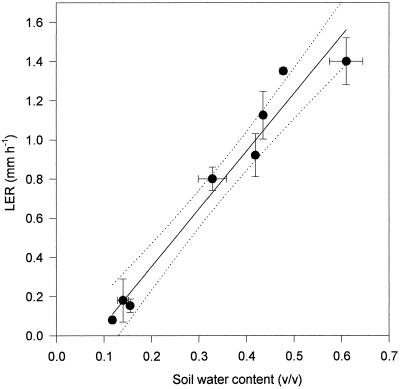
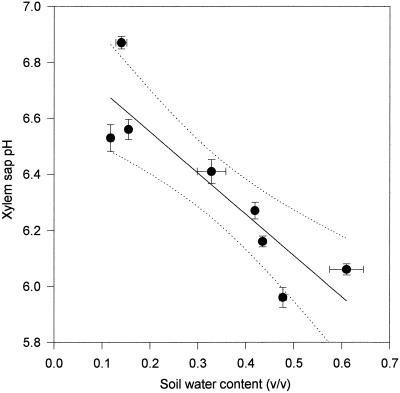
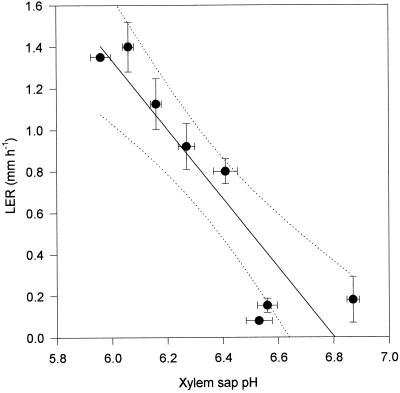
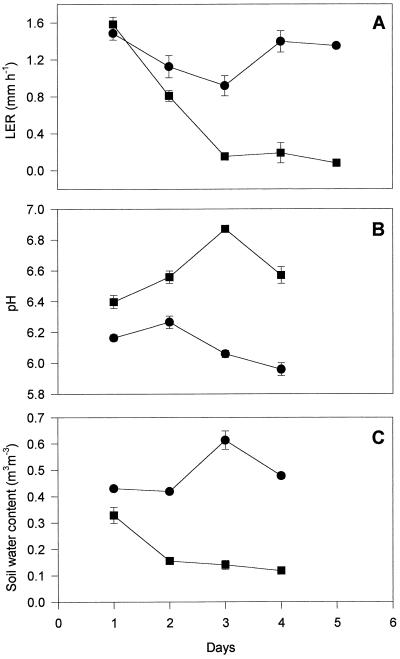
As the soil dried, LER of the fifth leaf of the cv Hanna plants declined with the water content of the soil (Fig. 1). There was a clear correlation (r2 = 0.96) between the soil water content (v/v) in which the plants were growing and the LER of those plants during this time (Fig. 1). There was also a correlation (r2 = 0.8) between the leaf xylem sap pH of barley plants and the water content of the soil in which the plants were growing (Fig. 2). As soil water content declined, xylem sap pH increased. The pH of the xylem sap correlated (r2 = 0.8) with the LER of plants growing in drying soil (Fig. 3). After only 1 d of exposure to drying soil the leaf xylem sap pH increased by about 0.2, and the LER decreased in concert with soil water content over the next 24 h (Fig. 4). The second series of experiments was conducted to determine whether pH and LER were causally related.
Figure 1.
Correlation between LER of the fifth leaf of barley cv Hanna and the water content of the soil in which the plants were growing. Each point is the mean of at least 10 LER determinations and three determinations of soil moisture content. A first-order regression line is fitted to the data (r2 = 0.96) together with lines indicating the 95% confidence interval (dotted lines).
Figure 2.
Correlation between the pH of xylem sap expressed from the leaf bases of cv Hanna and the water content of the soil in which the plants were growing. Each point is the mean of at least 15 pH determinations and three determinations of soil water content ± se. A first-order regression line is fitted to the data (r2 = 0.80) together with lines indicating the 95% confidence interval (dotted lines).
Figure 3.
Correlation between the pH of xylem sap expressed from cv Hanna leaves and the LER of the fifth leaf. Each point is the mean of at least 15 pH determinations and 10 determinations of LER ± se. A first-order regression line is fitted to the data (r2 = 0.90) together with lines indicating the 95% confidence interval (dotted lines).
Figure 4.
Time course of mean LER (A), pH of expressed xylem sap (B), and soil water content (C) in cv Hanna growing in either well-watered (•) or drying soil (▪) ± se.
Effects of Varying the pH of Fed AS on Leaf Growth, Relative Water Content, and Bulk [ABA] in cv Hanna Plants
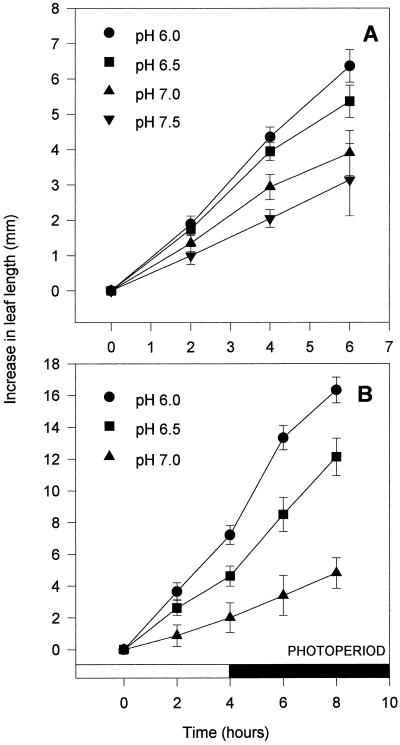
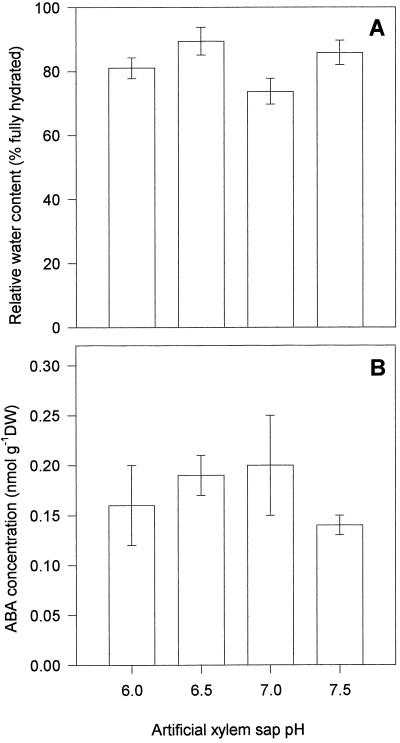
Figure 5A shows that varying the pH of AS fed to derooted seedlings of cv Hanna plants via the subcrown internode induced differential rates of leaf expansion. LER declined with increasing pH. Leaves fed with sap buffered to a well-watered pH of 6.0 (see Fig. 2) expanded the most rapidly (at circa 1.1 mm h−1), whereas those fed sap buffered to a stressed pH of 7.0 or above expanded much more slowly, at a rate 50% lower than those fed at pH 6.0. This trend appeared within 2 to 4 h of feeding and became increasingly pronounced over the experimental growth period. Feeding in the dark resulted in similar pH-dependent reductions in growth, ruling out any stomatal contribution to the observed pH dependence of leaf expansion of cv Hanna (Fig. 5B). Varying the pH of the AS fed to the leaves had no effect on the water content or the bulk [ABA] of the leaf-elongation zone (Fig. 6, A and B).
Figure 5.
The effect of feeding AS buffered to different pH values to derooted cv Hanna seedlings via the subcrown internode on the elongation rate of the third leaf. Each point is the mean of 10 determinations of LER ± se. A, In the light for 6 h at 21°C to 24°C. B, In the light for 4 h at 28°C to 30°C, then in the dark for another 4 h at 20°C.
Figure 6.
Effect of feeding AS buffered to pH values of 6.0, 6.5, 7.0, and 7.5 on the relative water content (A) and bulk ABA concentration (nmol g−1 dry weight [DW]) (B) in the elongation zone of the third leaf of cv Hanna monitored in a leaf-elongation bioassay.
Effects of Varying the pH of AS on the LER of Az34, an ABA-Deficient Mutant, and Its Wild Type (cv Steptoe)
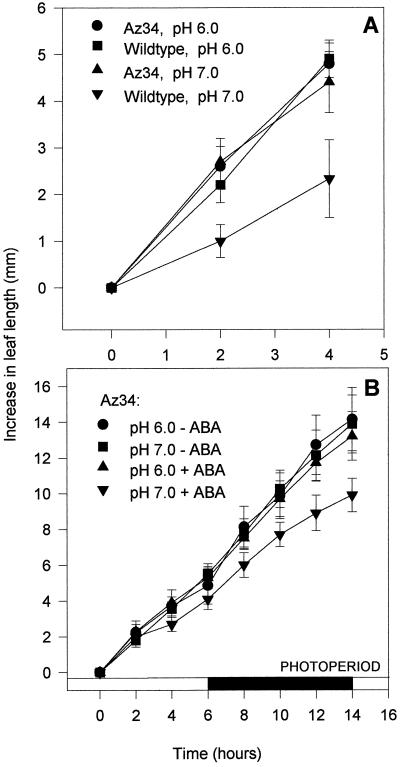
LER declined as described above (Fig. 5) when the pH of the AS fed to cv Steptoe was increased from a well-watered pH of 6.0 to a stressed pH of 7.0 (Fig. 7A). However, changes in the pH of the fed AS had no effect on the growth of Az34: leaves continued to grow at the faster rate observed at pH 6.0 in cv Steptoe and cv Hanna, even when xylem sap buffered to a pH of 7.0 was fed via the subcrown internode.
Figure 7.
A, The effect of feeding AS buffered to pH 6.0 or 7.0 at 21°C to 24°C via the subcrown internode of barley on the elongation rate of the third leaf of Az34 and its isogenic wild-type cv Steptoe over 4 h. Each point is mean of 10 determinations of LER ± se. B, The effect of feeding AS buffered to pH 6.0 or 7.0 with (+) or without (−) 3 × 10−8 m ABA on the elongation rate of the third leaf of Az34 over 14 h in periods of both light and dark. Each point is the mean of 10 determinations of LER ± se.
When a low well-watered concentration of ABA (0.03 μm), which is characteristic of the concentration found in well-watered monocot species (Zhang and Davies, 1990b), was supplied in the AS, the ability of Az34 to respond to the more alkaline stressed pH was restored over 14 h of feeding (Fig. 7B): pH 7.0 only reduced the growth rate of the third leaf of Az34 seedlings when ABA was present. This concentration of ABA was itself too low to cause a reduction in growth when fed via the subcrown internode at a well-watered pH of 6.0 (Fig. 7B). This effect persisted in the dark.
DISCUSSION
It has long been known that LER decreases as soil dries and it is now established that the pH of xylem sap expressed from plants growing in dry soil can be more alkaline than that from plants growing in wet soil (Hartung and Radin, 1989; Gollan et al., 1992; Wilkinson and Davies, 1997). Conditions other than drought can also increase xylem sap pH (flooding, Jackson et al., 1996; nitrate availability, Dannel et al., 1995; and time of day/year, Ferguson et al., 1983; Fromard et al., 1995; Schurr and Schulze, 1995).
The mechanism by which sap pH is increased by stresses such as drought is still unclear (see Wilkinson et al., 1998, and refs. therein). There is some evidence that a reduction in ATPase activity in the cells surrounding the xylem vessels of droughted plants could influence xylem sap pH (Hartung and Radin, 1989; Fromard et al., 1995). Other possibilities involve changes in nitrate reductase activity in roots and/or stems of stressed plants (Raven and Smith, 1976). Alternatively, stress-induced changes in xylem sap ionic composition (e.g. Schurr et al., 1992) could change the pattern of ionic exchange with walls of cells adjacent to the xylem vessels, and hence change sap pH (Ryan et al., 1992). Whether the mechanism proposed to account for increases in xylem sap pH seen during drought is the same as the mechanism that induces increased xylem sap pH in response to flooding or changes in nitrate availability remains to be discovered (see Wilkinson, 1998).
In several experiments using the root pressure vessel, often seen as the more desirable technique for collection of xylem sap (but see Methods), substantial increases in sap pH were reported when the soil surrounding the roots was allowed to dry (e.g. Gollan et al., 1992). Schurr and Schulze (1995) have shown that the method used in the present investigation to express sap will result in a reduction in the sap flow rate and the potential concentration of substances within the forming droplet. However, such concentration of sap constituents results in acidification of the droplet (Schurr and Schulze, 1995). In this study, therefore, we speculate that the difference in pH that we detected between well-watered plants and those growing in drying soil is, if anything, smaller than that occurring in planta.
Reductions in LER correlate with increases in xylem sap pH in plants growing in dry soil (Fig. 3) in a manner similar to that reported by Van Volkenburgh and Boyer (1985), who found that the reduced ability of the apoplast to acidify a weakly buffered droplet of solution placed on the surface of the leaf-elongation zone correlated with reductions in LER in maize plants growing in drying soil. In subsequent experiments (Fig. 5) we have been able to demonstrate that the relationship between xylem sap pH and LER (Fig. 3) is indeed causal and not just correlative.
Several possibilities exist for the mechanism by which high pH might reduce leaf growth. We know that increased xylem sap pH is a signal that closes stomata in the leaves of plants in drying soil (Wilkinson and Davies, 1997; Wilkinson et al., 1998). To determine if such an effect on stomatal aperture had any effect on growth (i.e. via changes in the flux of water and solutes into the growing cells), it was demonstrated that leaf growth limitation by more alkaline pH continued in the dark when stomata were closed (Fig. 5B). Alternatively, increased pH itself could change the water status of the leaf-elongation zone. However, Figure 6A shows that pH had no effect on the relative water content of the leaf bases. Because it has been demonstrated that ABA can reduce growth, it was also necessary to rule out the possibility that the introduction of AS buffered to a high pH somehow increased the ABA content of the base of the third leaf of the barley seedlings. This might have occurred if, for example, high pH inhibited ABA metabolism or prevented its transport out of the elongation zone. Figure 6B shows that the pH of the AS had no effect on the ABA concentration of fed leaves.
The reduction in leaf expansion by high pH observed in this investigation may have been caused by a shift in the pH of the solution around the walls of growing leaf cells out of the range suggested for auxin-stimulated growth (e.g. Cleland, 1991). Auxin is believed to cause acidification of the wall and activation of so-called “cell wall-loosening factors,” which mediate expansion of the cell wall. Expansins, a recently discovered set of cell wall proteins with acidic pH optima (circa 4.5–5.5; see McQueen-Mason, 1995), may be mediators of auxin-induced elongation because they exhibit reduced activity at the more alkaline pH values administered in this investigation (McQueen-Mason, 1995). However, the fact that high pH did not reduce growth in Az34 unless ABA was provided (Fig. 7) suggests that a direct effect of pH on cell expansion cannot account for the observed growth reductions.
Recently, Wilkinson and Davies (1997) demonstrated that high pH reduces stomatal aperture in leaves of plants containing only low well-watered concentrations of ABA, via an accumulation of this ABA in the apoplast adjacent to the stomatal guard cells. Experiments reported here would suggest that such a mechanism might also account for the influence of increased xylem sap pH on expanding cells within the elongation zone. We are already well aware of the ability of ABA to reduce the rate of leaf expansion (Zhang and Davies, 1990b; Munns and Sharp, 1993; Dodd and Davies, 1994, 1996); now, as a result of the present investigation, we suggest that high apoplastic pH may cause an accumulation of ABA in this compartment to a high-enough concentration to inhibit growth. In Commelina communis L. leaves ABA accumulated in the apoplast as a result of the reduced ability of both mesophyll and epidermal cells to remove the ABA from the passing transpiration stream (Wilkinson and Davies, 1997). This was a consequence of the imposed reduction in the pH gradient across the plasma membrane of these cells.
We were able to demonstrate that ABA is required for high pH buffers to reduce LER (Fig. 7). There is a direct parallel here with the requirement for ABA for high pH to close the stomata of tomato leaves (Wilkinson et al., 1998). Whereas pH 7.0 reduced the LER of the barley cv Steptoe in a manner similar to its effects in cv Hanna, LERs of the Az34 mutant of cv Steptoe were equally high at both pH 6.0 and pH 7.0 (Fig. 7). One difference between cv Steptoe and Az34 is that the latter contains a much lower endogenous concentration of ABA (Walker-Simmons et al., 1989). When levels of ABA in Az34 were artificially increased by supplying a concentration of ABA in the transpiration stream equivalent to that presumably found in the xylem sap of the well-watered wild-type cultivar (3 × 10−8 m), pH 7.0 was able to reduce the rate of growth (Fig. 7B). In the mechanism that we propose to be operating, as the sap pH increases, symplastic uptake into the cells within the growing zone is reduced and ABA accumulates around the actively expanding cells. The putative increase in apoplastic ABA concentration is not detectable as an increase in bulk leaf base ABA concentration (Fig. 6B), because the ABA that would have been taken up by the leaf cells is simply left in the apoplast such that the reduction in the symplastic ABA concentration compensates for the apoplastic increase. The experiments with Az34 determined that a high apoplastic wall pH alone cannot reduce leaf growth and confirmed that we are not dealing with a direct effect of pH on the wall or its enzymatic complement.
We are assuming that the buffered solution (or the xylem sap moving from roots of plants in dry soil; Figs. 2 and 3) is carried acropetally with the transpiration stream so that it reaches the leaf-elongation zone to impose a new wall pH around the expanding cells. In broad-leaved species this concept is easier to accept because root and shoot have a continuous xylem connection. Sap travels directly from the root to the shoot and eventually to the apoplast (Canny, 1990). However, understanding the precise nature of xylem transport through grass leaf-elongation zones is a long-standing problem (Esau, 1943) that continues to be addressed (e.g. Paolillo, 1995). No permanent xylem vessel can supply an actively expanding elongation zone. Living protoxylem with very different properties than mature vessels allow transport to and through the elongation zone of an actively expanding grass leaf (Evert et al., 1996). As the leaf nears the end of its expansion period, true metaxylem vessels mature basipetally. Only when leaf expansion is complete does a continuous xylem connection between root and shoot exist.
The precise physical nature of the apoplast/xylem connection in the elongation zone remains unknown. However, this investigation demonstrates that both the pH signal and the ABA required for any pH response to operate must in some way reach those cells that are actively expanding. We may therefore predict with some confidence that, in general, the xylem vessel supply and the apoplastic connection to such vessels do not differ functionally from those present in the fully expanded leaf. If a symplastic pathway were operating, the implication is that applied buffers would arrive at the site of action with a completely different composition. Because it is obvious that applied buffers do affect growing cells at a site distant from their application (Figs. 5 and 7), we must assume that they travel with the transpiration stream to the walls of actively expanding cells in the leaf-elongation zone via an apoplastic pathway.
It is interesting that the most alkaline pH measured in the sap of plants growing in drying soil (pH 6.6–6.8) did not have as significant an effect on LER when applied as AS to freshly detached leaves. It is possible that an effect of drought on leaf water potential may have increased the sensitivity of the growth process to either ABA or pH. Clearly, several factors may contribute to a restriction in leaf expansion during drought. The drought treatment may be expected to increase the amount of ABA within the leaf-elongation zone. Such relatively higher apoplastic concentrations of ABA in droughted plants, together with an increase in xylem pH, may explain the magnitude of the LER response seen in plants growing in drying soil relative to that observed in the bioassay system.
Although we are aware of a reduced level of nitrate reductase activity in the Az34 mutant (Walker-Simmons et al., 1989), nitrogen was supplied to seedlings in the form of both nitrate and ammonium. Although nitrate reductase may also have a role in controlling the pH of the xylem under stress conditions (Wilkinson, 1998), the observation that added ABA restores the ability of high pH to reduce leaf expansion suggests that the nitrate reductase deficiency does not contribute to the lack of pH-regulated cell expansion normally exhibited by Az34.
Although we propose that the pH-induced reduction in leaf expansion is the result of increased apoplastic accumulation of ABA in the elongation zone, we must address the question of how the local wall ABA concentration is detected and consequently results in the restriction of cell expansion. We do not yet know the position of any specific ABA-binding sites in the leaf other than in stomatal guard cells (Hartung, 1983). Binding sites of growing cells could exist in the wall, in the plasma membrane oriented externally or internally, or in an inner membrane. In stomatal guard cells, functional receptors may reside externally in the outer plasma membrane and/or inside the cell (Hartung, 1983; Hetherington and Quatrano, 1991; Allan and Trewavas, 1994). In light of recent work on the stomatal response to increased xylem sap pH (Wilkinson and Davies, 1997; Wilkinson et al., 1998), which demonstrated that apoplastic accumulation of ABA was responsible for the observed responses, we would question the relevance of internal receptors for responses to signals from the transpiration stream.
CONCLUSIONS
We have shown that drying soil increases the pH of barley xylem sap from about 6.0 to 7.0. We suggest that this pH increase persists as the transpiration stream carries the sap acropetally to the apoplast surrounding the growing cells of the barley leaf-elongation zone. This pH increase may cause an accumulation of ABA present in the transpiration stream within the apoplast surrounding the cells of the elongation zone. We speculate that in well-watered plants ABA arriving in this vicinity is taken up by the leaf cells of the growing zone when the pH gradient between the symplast and the apoplast is high, at a well-watered sap pH of 6.0. The increased ABA concentration in the apoplast that results from the increased pH of this compartment in the droughted plant reduces the rate of cell expansion by reducing cell wall extensibility and/or the turgor of these cells.
We propose that increased xylem sap pH is a measure of soil water status developed in the grass roots in contact with drying soil. This pH signal mediates an ABA-dependent restriction in leaf cell expansion, which together with pH- and/or ABA-induced stomatal closure limits the plant's evaporative water loss during drought. It is important to note that although the pH-induced reduction in stomatal aperture and leaf expansion are ABA dependent, no extra ABA (only that concentration present in the well-watered plant) is required for this mechanism to operate. This suggests that the plant always has enough ABA to explain the ABA-induced restriction in stomatal opening and leaf expansion, although synthesis in response to drought will strengthen the drought signal. In some species, xylem sap pH may not be sensitive to soil drying (e.g. Ricinus communis; Schurr and Shulze, 1995). We would predict that in these circumstances, even when endogenous ABA concentrations also increase in response to drought, stomata and leaf expansion may be relatively insensitive to this change. The observation that leaves of the ABA-deficient mutant Az34 elongate rapidly at alkaline pH levels shows that, in itself, pH may not be a primary factor in regulating cell expansion.
ACKNOWLEDGMENTS
We thank Professor R.L. Warner (Washington State University) and Dr J. Roberts (Nottingham University, UK) for supplying barley seed, and we are also grateful to Dr. S.A. Quarrie (John Innes Institute, Norwich, UK) for the MAC252 ABA antibody used in the RIA.
Abbreviations:
- AS
artificial xylem sap
- LER
leaf-elongation rate
- RIA
radioimmunoassay
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council.
LITERATURE CITED
- Allan AC, Trewavas AJ. Abscisic acid and gibberellin perception: inside or out? Plant Physiol. 1994;104:1107–1108. [PMC free article] [PubMed] [Google Scholar]
- Bacon MA (1998) Control of leaf expansion in Lolium temulentum L. and Hordeum vulgare L. growing in drying soil. PhD thesis. University of Lancaster, UK
- Baker NR, Davies WJ, Ong CK, eds (1985) Control of Leaf Growth. Society for Experimental Biology Seminar Series 27. Cambridge University Press, Cambridge, UK
- Begg JE, Wright MJ. Growth and development of leaves from intercalary meristems in Phalaris arunidaceae L. Nature. 1962;194:1097–1098. [Google Scholar]
- Boyer JS. Plant productivity and the environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Canny MJ. What becomes of the transpiration stream? New Phytol. 1990;114:341–368. doi: 10.1111/j.1469-8137.1990.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Cleland RE. Auxin-induced growth of Avena coleoptiles involves two mechanisms with different pH optima. Plant Physiol. 1991;99:1556–1561. doi: 10.1104/pp.99.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale JE. The control of leaf expansion. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:267–295. [Google Scholar]
- Dannel F, Pfeffer H, Marscher H. Isolation of apoplasmic fluid from sunflower leaves and its use for studies on influence of nitrogen supply on apoplasmic pH. J Plant Physiol. 1995;146:273–278. [Google Scholar]
- Dodd I, Davies WJ. Leaf growth responses to ABA are temperature dependent. J Exp Bot. 1994;45:903–907. [Google Scholar]
- Dodd IC (1996) Sensitivity of gramineous leaf growth to abscisic acid. PhD thesis. University of Lancaster, UK
- Dodd IC, Davies WJ. The relationship between leaf growth and ABA accumulation in the grass leaf elongation zone. Plant Cell Environ. 1996;19:1047–1056. [Google Scholar]
- Esau K. Ontogeny of the vascular bundle in Zea mays. Hilgardia. 1943;15:327–368. [Google Scholar]
- Evert RF, Russin WA, Bosabalidid AM. Anatomical and ultrastructural changes associated with sink-to-source transition in developing maize leaves. Int J Plant Sci. 1996;157:247–261. [Google Scholar]
- Ferguson AR, Eiseman JA, Leonard JA. Ann Bot. 1983;51:823–833. [Google Scholar]
- Fromard L, Babin V, Fleurat-Lessard P, Fromont J-C, Serrano R, Bonnemain J-L. Control of vascular sap pH by the vessel-associated cells in woody species. Plant Physiol. 1995;108:913–918. doi: 10.1104/pp.108.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan T, Schurr U, Shulze E-D. Stomatal response to drying soil in relation to changes in the xylem sap concentration of Helianthus annuus 1. The concentration of cations, anions, amino acids in, and pH of, the xylem sap. Plant Cell Environ. 1992;15:551–559. [Google Scholar]
- Gowing DJG, Davies WJ, Jones HG. A positive root-sourced signal as an indicator of soil drying in apple, Malus × domestica Borkh. J Exp Bot. 1990;41:1535–1540. [Google Scholar]
- Hales S (1727) Vegetable Staticks. W and J Inneys, London. Reprint, 1961
- Hartung W. The site of action of abscisic acid at the guard cell plasmalemma of Valerianella locusta. Plant Cell Environ. 1983;6:427–428. [Google Scholar]
- Hartung W, Radin JW. Abscisic acid in the mesophyll apoplast and in the root xylem sap of water-stressed plants: the significance of pH gradients. Curr Top Plant Biochem Physiol. 1989;8:110–124. [Google Scholar]
- Hartung W, Wilkinson S, Davies WJ. Factors that regulate abscisic acid concentrations at the primary site of action at the guard cell. J Exp Bot. 1998;51:361–367. [Google Scholar]
- Hetherington AM, Quatrano RS. Mechanisms of action of abscisic acid at the cellular level. New Phytol. 1991;119:9–32. doi: 10.1111/j.1469-8137.1991.tb01004.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Kosergarten H. FITC-dextran for measuring apoplastic pH and apoplastic pH gradients between various cell types in sunflower leaves. Physiol Plant. 1995;95:327–335. [Google Scholar]
- Hsiao TC. Plant responses to water stress. Annu Rev Plant Physiol. 1973;24:519–570. [Google Scholar]
- Jackson MB, Davies WJ, Else MA. Pressure-flow relationships, xylem solutes and root hydraulic conductance in flooded tomato plants. Ann Bot. 1996;77:17–24. [Google Scholar]
- Kramer PJ. Fifty years of progress in water relations research. Plant Physiol. 1974;54:463–471. doi: 10.1104/pp.54.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC (1991) Effect of ABA on ion transport and stomatal regulation. In WJ Davies, HG Jones, eds, Abscisic Acid: Physiology and Biochemistry. Environmental Plant Biology Series. BIOS Scientific Publishers, Oxford, UK, pp 153–167
- McQueen-Mason SJ. Expansins and cell wall expansion. J Exp Bot. 1995;46:1639–1650. [Google Scholar]
- Michelena VA, Boyer JS. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982;69:1145–1149. doi: 10.1104/pp.69.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes K, Davies WJ, Rodwell JS, Francis B (1998) The responses of Briza media and Koleria macrantha to drought and re-watering. Funct Ecol (in press)
- Monteith JL. Climate and the efficiency of crop production on Britain. Philos Trans R Soc B. 1977;54:1069–1081. [Google Scholar]
- Mulholland BJ, Black CR, Taylor IB, Lenton JR. Effects of soil compaction on barley (Hordeum vulgare L.) growth. I. Possible role for ABA as a root-sourced chemical signal. J Exp Bot. 1996a;47:539–549. [Google Scholar]
- Mulholland BJ, Taylor IB, Black CR, Roberts JA. Effects of soil compaction on barley (Hordeum vulgare L.) growth. II. Are increased xylem sap ABA concentrations involved in maintaining leaf expansion in compacted soils? J Exp Bot. 1996b;47:551–556. [Google Scholar]
- Munns R. A leaf elongation bioassay detects an unknown growth inhibitor in xylem sap from wheat and barley. Aust J Plant Physiol. 1992;19:127–135. [Google Scholar]
- Munns R, King RW. Abscisic acid is not the only stomatal inhibitor in the transpiration stream of wheat plants. Plant Physiol. 1988;88:703–708. doi: 10.1104/pp.88.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Sharp RE. Involvement of abscisic acid in controlling plant growth in soils of low water potential. Aust J Plant Physiol. 1993;20:425–437. [Google Scholar]
- Palmer S, Davies WJ. An analysis of relative elemental growth rate, epidermal cell size and xyloglucan endotransglycosylase activity through the growing zone of aging maize leaves. J Exp Bot. 1996;47:339–347. [Google Scholar]
- Paolillo DJ. Protoxylem maturation in the seedling wheat leaf. Am J Bot. 1995;82:337–345. [Google Scholar]
- Passioura JB. Root signals control leaf expansion in wheat seedlings growing in drying soil. Aust J Plant Physiol. 1988;15:687–693. [Google Scholar]
- Puliga S, Vassana C, Davies WJ. Control of leaf growth of Mediterranean forages by chemical and hydraulic influences. J Exp Bot. 1995;47:529–537. [Google Scholar]
- Quarrie S, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. A monoclonal antibody to (S)-abscisic acid: its characterisation and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Raven JA, Smith FA. Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytol. 1976;76:415–431. [Google Scholar]
- Ryan PR, Newman IA, Arif I. Rapid calcium exchange for protons and potassium in cell walls of Chara. Plant Cell Environ. 1992;15:675–683. [Google Scholar]
- Sachs J. Lectures on the Physiology of Plants. Translated by M Ward. Oxford, UK: Clarendon; 1887. [Google Scholar]
- Salim M, Pitman MG. Pressure-induced water and solute flow through plant roots. J Exp Bot. 1984;35:869–881. [Google Scholar]
- Schurr U, Gollan T, Schulze E-D. Stomatal response to soil drying in relation to changes in the xylem sap composition of Helianthus annuus. 2. Stomatal sensitivity to abscisic acid imported from the xylem sap. Plant Cell Environ. 1992;15:561–567. [Google Scholar]
- Schurr U, Schulze E-D. The concentration of xylem sap constituents in root exudate, and in sap from intact, transpiring castor bean plants (Ricinus communis L.) Plant Cell Environ. 1995;18:409–420. [Google Scholar]
- Van Volkenburgh E, Boyer JS. Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 1985;77:190–194. doi: 10.1104/pp.77.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M, Kudrna DA, Warner RL. Reduced accumulation of ABA during water stress in a molybdenum cofactor mutant of barley. Plant Physiol. 1989;90:728–733. doi: 10.1104/pp.90.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S (1999) pH as a stress signal. Plant Growth Regul (in press)
- Wilkinson S, Corlett JE, Oger L, Davies WJ. Effects of xylem sap pH on transpiration from wild-type and flacca mutant tomato leaves: a vital role for abscisic acid in preventing excessive water loss from well-watered plants. Plant Physiol. 1998;117:703–709. doi: 10.1104/pp.117.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. Xylem sap pH increase: a drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiol. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies WJ. Changes in the concentration of ABA in the xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ. 1990a;13:277–285. [Google Scholar]
- Zhang J, Davies WJ. Does ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants? J Exp Bot. 1990b;41:765–772. [Google Scholar]