Abstract
OBJECTIVE
Adverse microbial exposures might contribute to diabetogenesis. We hypothesized that clinical periodontal disease (a manifestation of microbial exposures in dysbiotic biofilms) would be related to insulin resistance among diabetes-free participants. The roles of inflammatory mediation and effect modification were also studied.
RESEARCH DESIGN AND METHODS
The continuous National Health and Nutrition Examination Survey 1999–2004 enrolled 3,616 participants (51% women) who received a periodontal examination and fasting blood draw. Participants were mean age (± SD) 43 ± 17 years and 28% Hispanic, 52% Caucasian, 17% African American, and 3% other. Log-transformed values of the homeostasis model assessment of insulin resistance (HOMA-IR) or HOMA-IR ≥3.30 (75th percentile) were regressed across full-mouth periodontal probing depth (PD) levels using linear and logistic models. White blood cell (WBC) count and C-reactive protein (CRP) were considered as either mediators or effect modifiers in separate analyses. Risk ratios (RRs) stem from marginal predictions derived from the logistic model. Results were adjusted for multiple periodontal disease and insulin resistance risk factors.
RESULTS
In linear regression, geometric mean HOMA-IR levels increased by 1.04 for every 1-mm PD increase (P = 0.007). WBC mediated 6% of the association (P < 0.05). Among participants with WBC ≤6.4 × 109, PD was unrelated to HOMA-IR ≥3.30. Fourth-quartile PD was associated with HOMA-IR ≥3.30 among participants with WBC >7.9 × 109; RR 2.60 (1.36–4.97) (P for interaction = 0.05). Findings were similar among participants with CRP >3.0 mg/L (P for interaction = 0.04).
CONCLUSIONS
Periodontal infection was associated with insulin resistance in a nationally representative U.S. sample of diabetes-free adults. These data support the role of inflammation as both mediator and effect modifier of the association.
Type 2 diabetes mellitus (T2DM) is a prominent public health problem (1) currently affecting at least 17.7 million individuals in the U.S. The hallmark of T2DM is chronically elevated fasting plasma glucose secondary to insulin resistance and impaired pancreatic β-cell function. Adverse microbial exposures, such as those observed in dysbiotic periodontal biofilms, have been suggested as a potential risk factor for insulin resistance and T2DM development. This notion is bolstered by previous research suggesting a bidirectional relationship between periodontal disease and glycemic control among individuals with diabetes (2,3). However, research exploring periodontal infection as a diabetes risk factor among diabetes-free adults has only been initiated recently.
Two previous publications reported that the presence of periodontal disease predicted 1) a twofold increase in incident T2DM during 20 years of follow-up in a nationally representative sample of ∼9,000 initially diabetes-free men and women (4); and 2) an approximately fivefold increase in the progression of A1C among 2,700 diabetes-free participants arising from a randomly selected population-based sample (5). The latter publication also reported that the infection-associated risk related to A1C progression was stronger among participants with evidence of elevated C-reactive protein (CRP) (5). These studies were unable to specifically examine the association between periodontal disease and insulin resistance.
The potential for periodontal infections to contribute to insulin resistance and overt T2DM is biologically plausible (2,6,7), and one specific causal pathway linking infections and T2DM risk is chronically elevated systemic inflammation. Systemic inflammation is known to be elevated among participants with periodontal infections and has also been shown to predict the progression of insulin resistance (8) as well as the development of T2DM (9,10).
In this study, we explored the association between clinically assessed periodontal disease (a clinical manifestation of adverse microbial exposures in dysbiotic biofilms) and insulin resistance in the continuous National Health and Nutrition Examination Survey (NHANES). We also studied whether or not there was evidence that systemic inflammation either mediated or modified the association between periodontal disease and insulin resistance.
RESEARCH DESIGN AND METHODS
The continuous NHANES began in 1999 and consists of six unique data sets that have been generated in 2-year cycles (i.e., 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, and 2009–2010). The survey examines a nationally representative sample of ∼5,000 people each year and collects a variety of health-related data via questionnaire, physical examination, and laboratory assessments. The current analysis uses the NHANES 1999–2000, 2001–2002, and 2003–2004 cross-sections and includes n = 3,616 diabetes-free men and women aged 20–85 years who received a clinical periodontal examination and fasting glucose and insulin assessments. The NHANES protocol was approved by the National Center for Health Statistics institutional review board, and written informed consent was obtained from all participants.
Oral examination
The NHANES oral health examination (OHE) has been previously described (11,12). Trained dentists performed a full-mouth tooth count as well as a periodontal examination according to a random half-mouth method (excluding third molars). Periodontal probing depth (PD) and clinical attachment loss (AL) measurements were performed at two sites per tooth (mid- and mesio-facial) in the 1999–2000 cross-section (i.e., up to 28 possible sites per participant). In the 2001–2002 and 2003–2004 periodontal examinations, a third distal surface (disto-facial) measurement was added. OHE 1999–2002 used National Institute for Dental Research periodontal probes, and the 2003–2004 OHE used a technically similar Hu-Friedy PCP2 probe (12). Both instruments were color banded with PD graduations at 2, 4, 6, 8, 10, and 12 mm. When the examiner was equivocal as to the best value to assign, measurements were rounded to the next lowest band. Interexaminer reproducibility ranged from good to very good with κ scores ranging from 0.64 to 0.82 (11,12).
Fasting glucose and insulin assessments
Fasting glucose and insulin were measured at the same central laboratory. Glucose was determined according to a hexokinase enzymatic method (13) and insulin according to a radioimmunoassay (14). Glucose and insulin were used to calculate the homeostasis model assessment of insulin resistance (HOMA-IR) as previously described (15). In brief, HOMA-IR is defined as (insulin in μU/mL × glucose in mmol/L)/22.5.
Risk factor assessments
A comprehensive set of questionnaires to assess risk factors relevant to both periodontal disease and insulin resistance was administered. The demographic variables age, race/ethnicity, sex, education, and poverty-to-income ratio (calculated by dividing family income by the poverty guidelines, specific to family size, as well as the appropriate year and state according to Department of Health and Human Services guidelines) were collected. Behavioral risk factor assessments included physical activity level, cigarette smoking duration and intensity, alcohol consumption, and caloric intake. Waist, weight, height, and blood pressure measurements were performed by trained research assistants according to standardized protocols. Triglycerides, total and HDL cholesterol, CRP, and white blood cell (WBC) count were measured from fasting blood samples.
Statistical analysis
Survey procedures in SAS version 9.2 and SAS-callable SUDAAN version 10 were used for all analyses.
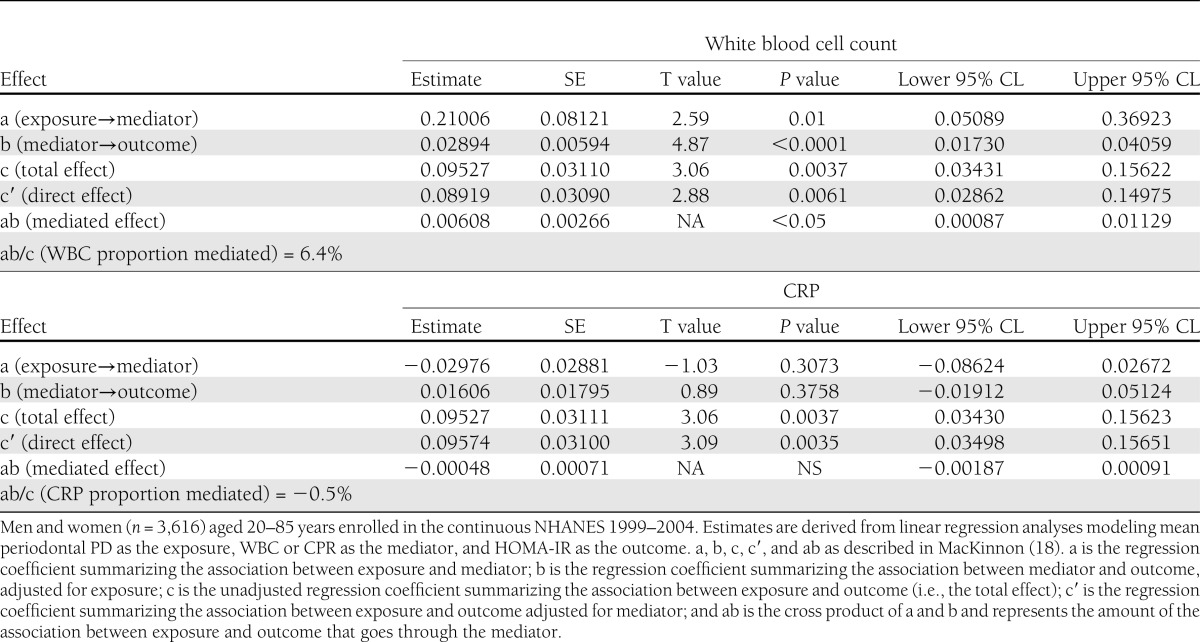
Periodontal disease was defined according to quartiles of either mean full-mouth PD or AL values to obtain a balanced categorization of the periodontal exposure and to enable the assessment of dose responsiveness. The Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) working group definition was also considered (16). The outcomes analyzed were fasting glucose, insulin, and HOMA-IR. The probability of HOMA-IR ≥3.30 (the population-specific 75th percentile of HOMA-IR) was regressed on quartiles of periodontal disease in logistic regression models using SAS PROC SURVEYLOGISTIC. PROC RLOGIST in SUDAAN was used to obtain multivariable-adjusted risk ratios (RRs) from fitted logistic regression models by obtaining point estimates of model-adjusted RRs as functions of average marginal predictions (17). Linear regression (SAS PROC SURVEYREG) modeled either the mean values of continuous glucose, insulin, or HOMA-IR across quartiles of periodontal disease. For insulin and HOMA-IR, both untransformed and natural log–transformed values were considered (no meaningful differences were noted). Both arithmetic- and geometric-adjusted mean values of insulin and HOMA-IR are presented in the results. All P values presented for linear trend were based on models using a continuous periodontal exposure variable. Multivariable models were adjusted for confounding by the following variables: continuous age, poverty-to-income ratio, caloric intake, BMI, blood pressure, triglycerides, total cholesterol-to-HDL cholesterol ratio, and inflammatory biomarkers as well as race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or other), sex, education (<high school, high-school graduate, or >high school), smoking status (never, former, or current in addition to pack-years), physical activity level in the past 30 days (none, moderate, or vigorous), and alcohol consumption (drinks/day). Mediation analyses were performed to estimate whether or not the association between periodontal infection and insulin resistance was mediated by inflammatory biomarkers (i.e., WBC or CRP) (Table 1) (18). Interactions between either WBC or CRP and periodontal infection were also examined. Categories of systemic inflammation were based on either quartiles of WBC or alternatively on CRP defined by the CDC/American Heart Association (AHA) statement on inflammatory markers in cardiovascular disease (19).
Table 1.
Inflammatory mediation of the association between periodontal infection and insulin resistance
RESULTS
General characteristics
Supplementary Table 1 presents general characteristics of study participants both weighted and unweighted to the U.S. population. Before applying NHANES weights, participants included in this analysis were aged 44 ± 17 years (age ± SD) and 51% were women. Whites, Hispanics, and blacks represent 52, 28, and 17% of the sample (3% reported other race/ethnicity). Participants had 28 teeth on average (including third molars), and the mean PD and mean AL values were 1.2 ± 0.5 and 0.8 ± 0.9 mm, respectively. The cumulative prevalence estimates of moderate or severe periodontitis were 11 and 1%, respectively.
Higher levels of PD were associated with several demographic, SES, and lifestyle variables as summarized in Supplementary Table 1, and although many of these associations were statistically significant in this large sample, trends across PD quartiles were generally weak in comparison with trends across quartiles of AL. Mean age varied from 44 to 45 years across PD quartiles (P for trend = 0.08), whereas mean age varied from 34 to 56 years across AL quartiles (P < 0.01). Similarly, mean pack-years in the first versus fourth AL quartile varied from 2 to 12, respectively (P < 0.0001), an increase threefold greater than observed for PD quartiles as described in Supplementary Table 1.
The arithmetic mean ± SD fasting plasma glucose, insulin, and HOMA-IR values were 95 ± 10 mg/dL and 66 ± 48 and 2.6 ± 2.1 pmol/L. Geometric mean values of insulin and HOMA-IR were 54 and 2.1 pmol/L, respectively.
Association between periodontal disease, glucose, insulin, and insulin resistance
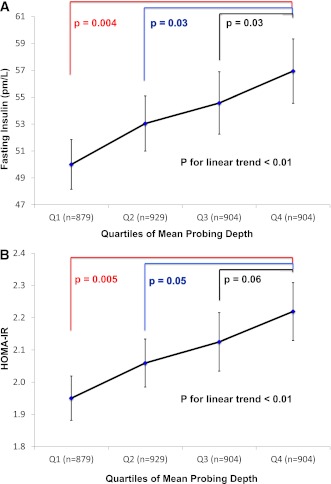
Values of mean fasting glucose (mg/dL) ± SE across quartiles of mean PD were 95.3 ± 0.4, 94.8 ± 0.4, 95.1 ± 0.4, and 95.3 ± 0.4 (P for trend = NS). Geometric mean values of insulin and HOMA-IR varied across PD quartiles in a dose-responsive fashion (Fig. 1). In multivariable linear regression analysis, geometric mean HOMA-IR levels increased by 1.04 for every 1 mm of mean PD increase (P = 0.007), and this finding was similar when restricting the analysis to nonobese participants (regression coefficient = 1.05; P = 0.006).
Figure 1.
The association between PD quartiles and geometric mean insulin (A) and geometric mean HOMA-IR (B) values. Adjusted for age, sex, race/ethnicity, education level, smoking status, activity level, BMI, total caloric intake, systolic blood pressure, total cholesterol-to-HDL cholesterol ratio, and triglycerides. Men and women (n = 3,616) 20–85 years of age enrolled in the continuous NHANES 1999–2004. (A high-quality color representation of this figure is available in the online issue.)
Mean AL was not associated with glucose, insulin, or insulin resistance. Mean HOMA-IR values across quartiles of AL were 2.00 ± 0.06, 2.03 ± 0.06, 1.94 ± 0.06, and 1.90 ± 0.07 (P for linear trend = 0.53); glucose and insulin data are not shown.
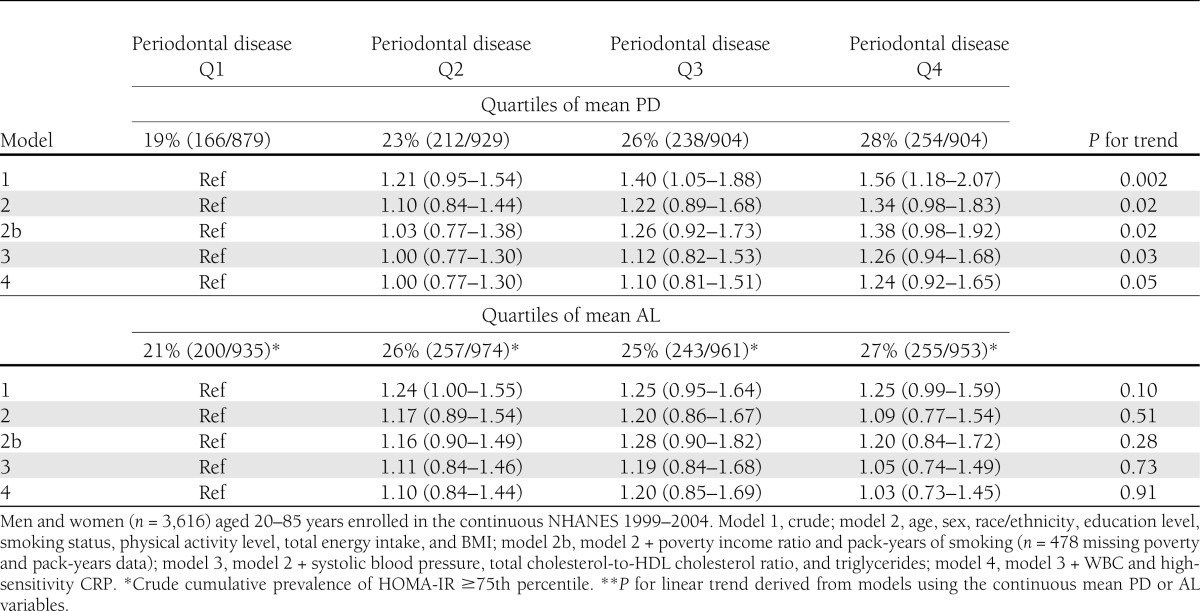
In fully adjusted logistic regression models, a 1-mm increase in continuous PD was associated with an increased risk of HOMA-IR ≥75th percentile: RR 1.24 (95% CI 1.03–1.48); P for trend = 0.03. Findings were consistent when considering risk for HOMA-IR ≥75th percentile across quartiles of PD (Table 2). Mean AL was not associated with elevated HOMA-IR risk (Table 2).
Table 2.
Cumulative prevalence RRs (95% CI) for HOMA-IR ≥75th percentile by mean periodontal PD and AL categories
Relative to participants with no/mild periodontitis, HOMA-IR risk was not increased among participants with moderate periodontitis but was increased among participants with severe periodontitis: RRs for moderate and severe periodontitis 0.85 (0.61–1.19) and 2.30 (1.27–4.15).
Results were consistent among age-subgroups; the RRs for fourth versus first quartile of PD among participants aged 20–39 (n = 1,732), 40–59 (n = 1106), or ≥60 (n = 778) years were 1.25 (95% CI 0.79–1.96), 1.34 (0.75–2.38), and 1.39 (0.80–2.42). Similarly, findings were consistent among nonobese participants as well as never smokers; the respective RRs comparing participants in the fourth versus first PD quartile were 1.21 (0.77–2.31) (among nonobese participants) and 1.32 (0.88–1.99) (among never smokers).
Inflammatory mediation
In linear regression analyses, there was evidence that the association between PD and HOMA-IR was mediated by WBC. Mean PD was positively associated with both WBC and HOMA-IR. Further, WBC was positively associated with HOMA-IR after adjustment for PD (Table 1). It was estimated that 6% of the total association between mean PD and HOMA-IR was mediated by WBC (P < 0.05) (Table 1). There was no evidence that CRP mediated the association between mean PD and HOMA-IR (Table 1).
Inflammatory interaction (effect modification)
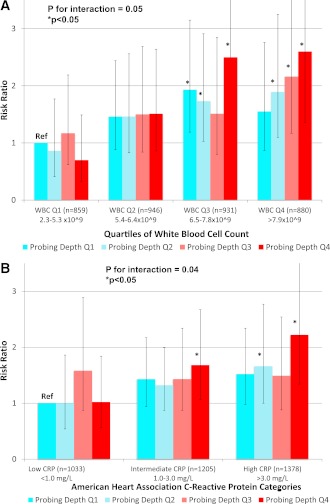
PD was only associated with HOMA-IR in the presence of elevated systemic inflammation. For example, participants with both fourth-quartile PD and WBC values (vs. first-quartile PD and WBC) realized a 160% increase in the risk of HOMA-IR ≥75th percentile: RR 2.60 (95% CI 1.36–4.97); P for interaction = 0.05 (Fig. 2). Similarly, the RR comparing participants with fourth-quartile PD and CRP >3.0 mg/L versus first-quartile PD and CRP <1.0 mg/L was 2.22 (1.34–3.68); P for interaction = 0.04 (Fig. 2). These findings are generalizable to a sizable proportion of U.S. adults; after applying NHANES sampling weights, 16% of participants had both mean PD values ≥50th percentile and a CRP value >3.0 mg/L, corresponding to 20,519,829 diabetes-free adults. Similarly, 24% (31,278,079) of U.S. adults had both PD ≥50th and WBC ≥50th percentile.
Figure 2.
Cumulative prevalence RRs (95% CI) for HOMA-IR ≥75th percentile across increasing levels of periodontal PD and either WBC count defined in quartiles (A) or CRP defined via AHA categories (B) (19). Adjusted for age, sex, race/ethnicity, education level, smoking status, activity level, BMI, systolic blood pressure, total cholesterol-to-HDL cholesterol ratio, and triglycerides. Men and women (n = 3,616) aged 20–85 years enrolled in the continuous NHANES 1999–2000.
CONCLUSIONS
We have found periodontal PD, but not AL, to be positively associated with increased risk of elevated fasting insulin and insulin resistance in a dose-responsive fashion. In the full sample, the risk of elevated HOMA-IR increased by ∼30% across PD quartiles. There was only weak evidence for mediation by systemic inflammation but much stronger support for the hypothesis of a synergistic interaction between elevated systemic inflammation and periodontal status, such that periodontal status was only associated with insulin resistance among participants with WBC ≥6.5 × 109 cells/L or CRP ≥1.0 mg/L. All findings remained after comprehensive adjustment for demographics, health-related behaviors, systemic inflammation, smoking status, and adiposity.
The current data support recently published findings that periodontal infection might be a risk factor for the development of T2DM. Previously published prospective data from the Study of Health in Pomerania (SHIP) demonstrated that baseline periodontal status predicted 5-year progression of A1C (5). These SHIP data extended a previous cross-sectional report of elevated A1C levels among participants with periodontitis (20) as well as an earlier publication from NHANES I reporting increased levels of baseline periodontal disease to predict incident diabetes during two decades of follow-up (4). Although a recent study had equivocal findings for incident diabetes in a Japanese population (21), the mean follow-up time was only 6 years, which limited the number of incident cases and minimized power to detect the association observed. Collectively, these earlier studies were limited by an inability to address the potential role of insulin resistance as a mechanistic explanation of the aforementioned A1C change and incident diabetes findings.
Chronic inflammation is a plausible biological mechanism linking infections and insulin resistance. Animal models have shown that inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), can induce a state of insulin resistance (22), possibly as a consequence of TNF-α’s ability to interrupt serine phosphorylation of insulin receptor substrate-1 (23), and epidemiological data in humans have repeatedly shown inflammation to be an independent risk factor for both insulin resistance (8) and T2DM (9,10,24). There are many potential exogenous inflammatory stimuli that might trigger inflammatory responses, some of which also have been linked to insulin resistance and diabetes development, such as air pollution (25) and organic pollutants (26). Accordingly, periodontal infection has been repeatedly demonstrated to be associated with elevated levels of systemic inflammation (27), and periodontal therapy has been shown to result in changes in systemic monocytic gene expression (28) as well as decreases in systemic inflammation (29,30) and insulin resistance (31). Therefore, it is plausible that insulin resistance might be reduced via appropriate anti-infective/anti-inflammatory periodontal therapy.
Despite strong biological plausibility, our current findings provide only weak support for inflammatory mediation. This might be due to the fact that our inflammatory construct was limited in this analysis and importantly did not include TNF-α, which likely mischaracterizes any true causal inflammatory intermediates. Alternatively, these data do strongly support the possibility of synergy between periodontal infection and systemic inflammation, and this is unlikely to be a chance finding, as the results are statistically significant and based on a priori hypotheses generated from previously published data (5). Although the biological mechanisms that might underpin the observed interaction are not immediately obvious, it is possible that our crude inflammatory markers (WBC and CRP) are simply surrogates of an underlying genetic susceptibility to infection-induced insulin resistance. Unfortunately, more advanced methods that could address both mediation and interaction concurrently (i.e., exposure-mediator interactions) in a complex sampling design such as NHANES are not readily available. Nevertheless, as these data are cross-sectional, results from such analyses are unlikely to add meaningful value from the standpoint of causal inference. Longitudinal studies that can more precisely investigate the interplay between microbial exposures, inflammatory response, and insulin resistance are necessary.
The fact that PD, and not AL, was associated with HOMA-IR is notable because it suggests that clinical indicators of current infection and/or inflammation are more relevant when studying cross-sectional associations between periodontal infection and insulin resistance. As previously discussed, evidence of irreversible, historical oral infection (e.g., AL) might be more informative when studying insidious and/or irreversible outcomes presumed to be partly caused by chronic infectious exposure. Alternatively, ephemeral measures, such as PD, which are closely associated with the presence of current periodontal inflammation in response to potentially pathogenic periodontal microbiota (32,33), might be more precise for outcomes that are acute and/or reversible (34). The strong association between severe periodontitis (defined according to recommendations from the CDC/AAP working group) (16) and insulin resistance is consistent with this line of thinking because it not only requires clinical evidence of historical infection (i.e., periodontal sites with high AL) but the definition also incorporates a measure of current disease (i.e., periodontal sites with deep PD). In contrast, moderate periodontitis, which does not require clinical signs of current disease, was unrelated to insulin resistance. The low prevalence of severe periodontitis in these data precluded exploration of inflammatory interactions.
The specificity of insulin resistance findings to PD measures (as opposed to AL) is also notable because it minimizes potential confounding. Factors such as age and smoking are generally stronger risk factors for AL and radiographic bone loss but weaker risk factors for PD (35), and the current data support this notion (see results). Nevertheless, in these data, even crude associations between AL and insulin resistance were weak and not dose responsive.
The finding that increased levels of periodontal disease are associated with increased fasting insulin resistance is meaningful for the prediction of future T2DM development. Elevations in insulin resistance have been repeatedly shown to be strong predictors of incident diabetes (36,37), and the HOMA-IR method has been validated for large epidemiological studies for subjects of various ethnicities and a wide range of glucose tolerance (38).
Our exposure was based on clinically assessed measures of periodontal disease because these measures are manifestations of host response to adverse microbial exposures in dysbiotic periodontal biofilms. Previous studies have included direct assessments of oral (39) or gut (40) microbial exposures to study either cardiovascular or obesity risk; similar approaches can provide more precise characterizations of infection–insulin resistance associations in future studies. Consequently, our current findings may be attenuated due to a lack of comprehensive information on microbial exposures, both oral and otherwise. The fact that these data are cross-sectional is also a limitation, as we cannot infer temporality.
We have found clinical measures of periodontal infection to be associated with elevated insulin resistance in a nationally representative, population-based sample of diabetes-free adult men and women. The findings remained after comprehensive multivariable adjustment and strongly suggest a synergistic interaction between oral infection and inflammatory response. Future research that can incorporate direct assessments of exposure to periodontal bacterial species and more comprehensive assessments of inflammation during longitudinal follow-up is necessary for more direct causal inference.
Acknowledgments
This research was supported by National Institutes of Health grants R00-DE-018739 (to R.T.D.) and R01-DE-13094 (to M.D.). Additional funding support was provided by a Calderone Research Award from the Mailman School of Public Health, Columbia University (to R.T.D.); a Pilot and Feasibility Award to R.T.D. from the Diabetes and Endocrinology Research Center, College of Physicians and Surgeons, Columbia University (DK-63608); a Chair in Chronic Disease, École des Hautes Études en Santé Publique, Paris, France (to M.D.); and a Mayo Chair Endowment, School of Public Health, University of Minnesota (to D.R.J.).
No potential conflicts of interest relevant to this article were reported.
R.T.D. obtained and analyzed the data and wrote the manuscript. A.S. analyzed the data and wrote the manuscript. P.N.P., M.R., W.T.F., and M.D. wrote, reviewed, and edited the manuscript. D.R.J. analyzed the data and wrote, reviewed, and edited the manuscript. R.T.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Sharon Schwartz (Columbia University) for her thoughtful review of the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0072/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol 2001;6:99–112 [DOI] [PubMed] [Google Scholar]
- 3.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 2011;7:738–748 [DOI] [PubMed] [Google Scholar]
- 4.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care 2008;31:1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the Study of Health in Pomerania (SHIP). Diabetes Care 2010;33:1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yki-Järvinen H, Sammalkorpi K, Koivisto VA, Nikkilä EA. Severity, duration, and mechanisms of insulin resistance during acute infections. J Clin Endocrinol Metab 1989;69:317–323 [DOI] [PubMed] [Google Scholar]
- 7.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol 2005;76(Suppl.):2075–2084 [DOI] [PubMed] [Google Scholar]
- 8.Park K, Steffes M, Lee DH, Himes JH, Jacobs DR., Jr Association of inflammation with worsening HOMA-insulin resistance. Diabetologia 2009;52:2337–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334 [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004;53:693–700 [DOI] [PubMed] [Google Scholar]
- 11.Dye BA, Barker LK, Selwitz RH, et al. Overview and quality assurance for the National Health and Nutrition Examination Survey (NHANES) oral health component, 1999-2002. Community Dent Oral Epidemiol 2007;35:140–151 [DOI] [PubMed] [Google Scholar]
- 12.Dye BA, Nowjack-Raymer R, Barker LK, et al. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003-04. J Public Health Dent 2008;68:218–226 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention National Center for Health Statistics. National Health and Nutrition Examination Survey 1999–2001 [Internet]. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab10am_met_plasma_glucose.pdf Accessed 10 December 2011.
- 14.Centers for Disease Control and Prevention National Center for Health Statistics. National Health and Nutrition Examination Survey 1999–2001 [Internet]. Available from http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab10am_met_insulin.pdf Accessed 10 December 2011.
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 16.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol 2007;78(Suppl.):1387–1399 [DOI] [PubMed] [Google Scholar]
- 17.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol 2010;171:618–623 [DOI] [PubMed] [Google Scholar]
- 18.MacKinnon DP. Introduction to Statistical Mediation Analysis New York, Lawrence Erlbaum Associates, 2008. [Google Scholar]
- 19.Pearson TA, Mensah GA, Alexander RW, et al. Centers for Disease Control and Prevention. American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511 [DOI] [PubMed] [Google Scholar]
- 20.Wolff RE, Wolff LF, Michalowicz BS. A pilot study of glycosylated hemoglobin levels in periodontitis cases and healthy controls. J Periodontol 2009;80:1057–1061 [DOI] [PubMed] [Google Scholar]
- 21.Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study. J Dent Res 2011;90:41–46 [DOI] [PubMed] [Google Scholar]
- 22.Ling PR, Bistrian BR, Mendez B, Istfan NW. Effects of systemic infusions of endotoxin, tumor necrosis factor, and interleukin-1 on glucose metabolism in the rat: relationship to endogenous glucose production and peripheral tissue glucose uptake. Metabolism 1994;43:279–284 [DOI] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996;271:665–668 [DOI] [PubMed] [Google Scholar]
- 24.Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol 2003;23:650–655 [DOI] [PubMed] [Google Scholar]
- 25.Krämer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect 2010;118:1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR., Jr Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2007;30:622–628 [DOI] [PubMed] [Google Scholar]
- 27.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities study. Arch Intern Med 2003;163:1172–1179 [DOI] [PubMed] [Google Scholar]
- 28.Papapanou PN, Sedaghatfar MH, Demmer RT, et al. Periodontal therapy alters gene expression of peripheral blood monocytes. J Clin Periodontol 2007;34:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res 2004;83:156–160 [DOI] [PubMed] [Google Scholar]
- 30.Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007;356:911–920 [DOI] [PubMed] [Google Scholar]
- 31.Sun WL, Chen LL, Zhang SZ, Wu YM, Ren YZ, Qin GM. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med 2011;50:1569–1574 [DOI] [PubMed] [Google Scholar]
- 32.Demmer RT, Papapanou PN, Jacobs DR, Jr, Desvarieux M. Bleeding on probing differentially relates to bacterial profiles: the Oral Infections and Vascular Disease Epidemiology Study. J Clin Periodontol 2008;35:479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demmer RT, Papapanou PN, Jacobs DR, Jr, Desvarieux M. Evaluating clinical periodontal measures as surrogates for bacterial exposure: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). BMC Med Res Methodol 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demmer RT, Kocher T, Schwahn C, Völzke H, Jacobs DR, Jr, Desvarieux M. Refining exposure definitions for studies of periodontal disease and systemic disease associations. Community Dent Oral Epidemiol 2008;36:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol 1994;65:260–267 [DOI] [PubMed] [Google Scholar]
- 36.Hanley AJ, Williams K, Gonzalez C, et al. San Antonio Heart Study. Mexico City Diabetes Study. Insulin Resistance Atherosclerosis Study Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes 2003;52:463–469 [DOI] [PubMed] [Google Scholar]
- 37.Lorenzo C, Wagenknecht LE, D’Agostino RB, Jr, Rewers MJ, Karter AJ, Haffner SM. Insulin resistance, beta-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care 2010;33:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 39.Desvarieux M, Demmer RT, Jacobs DR, Jr, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens 2010;28:1413–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031 [DOI] [PubMed] [Google Scholar]