Abstract
OBJECTIVE
To estimate the health utility scores associated with type 2 diabetes, its treatments, complications, and comorbidities.
RESEARCH DESIGN AND METHODS
We analyzed health-related quality-of-life data, collected at baseline during Translating Research Into Action for Diabetes, a multicenter, prospective, observational study of diabetes care in managed care, for 7,327 individuals with type 2 diabetes. We measured quality-of-life using the EuroQol (EQ)-5D, a standardized instrument for which 1.00 indicates perfect health. We used multivariable regression to estimate the independent impact of demographic characteristics, diabetes treatments, complications, and comorbidities on health-related quality-of-life.
RESULTS
The mean EQ-5D–derived health utility score for those individuals with diabetes was 0.80. The modeled utility score for a nonobese, non–insulin-treated, non-Asian, non-Hispanic man with type 2 diabetes, with an annual household income of more than $40,000, and with no diabetes complications, risk factors for cardiovascular disease, or comorbidities, was 0.92. Being a woman, being obese, smoking, and having a lower household income were associated with lower utility scores. Arranging complications from least to most severe according to the reduction in health utility scores resulted in the following order: peripheral vascular disease, other heart diseases, transient ischemic attack, cerebral vascular accident, nonpainful diabetic neuropathy, congestive heart failure, dialysis, hemiplegia, painful neuropathy, and amputation.
CONCLUSIONS
Major diabetes complications and comorbidities are associated with decreased health-related quality-of-life. Utility estimates from our study can be used to assess the impact of diabetes on quality-of-life and conduct cost-utility analyses.
The health utility score reflects the level of physical, mental, and social functioning associated with a particular health state and the preference weight the general population gives to that health state (1). Conventionally, an optimal health state is assigned a score of 1.00 and death is assigned a score of 0.00. Health states that are less desirable than optimal health and more desirable than death are assigned a value between 1.00 and 0.00. Utility scores of individual health states are combined with survival times in each health state to calculate quality-adjusted life-years (QALYs), a health outcome measure that combines quality-of-life with length-of-life.
Diabetes progression simulation models are often used to assess the long-term cost-effectiveness of interventions for preventing diabetes and its complication. To estimate health outcomes of those interventions in QALYs, health utility scores associated with different patient characteristics, treatment modalities, and the different clinical states of each diabetes-related complication (retinopathy, nephropathy, neuropathy, health diseases, and stroke) are needed.
Health utility scores needed by the simulation model have been estimated using different standard health-related quality-of-life (HRQOL) instruments in different countries. Coffey et al. (2) used the Self-Administered Quality of Well Being index in the U.S. Studies from European countries (3–5) used the Euro-QoL (EQ)-5D with patients in the U.K., Norway, and eight European countries that participated in the Cost of Diabetes in Europe–Type 2 (CODE-2) study. Instruments vary in their underlying valuation systems and health state descriptions (6), and there is no evidence that one instrument is superior to the others. Health utility scores are sensitive to country settings because preference weights for health states can be affected by cultural norms and the availability of medical technologies (7,8). Johnson et al. (7,8) showed different preference values for the same health states, even among countries with the same language and similar cultures such as the U.S. and the U.K.
We estimated U.S. population-specific health utility scores for characteristics, treatments, and health states used commonly by diabetes progression simulation models. We improved on the previous health utility estimates in two important ways: First, we applied U.S. preference weights to the EQ-5D health state values. U.S. preference weights have been used to estimate health utility scores for diabetes patients compared with individuals without diabetes (9,10) or health utility scores for those at different levels of risk to develop diabetes (11). To our knowledge, no study has used U.S. preference weights to assess health utility scores associated with diabetes treatment modalities and complications.
Second, we derived our health utility scores from a large and diverse population of diabetic patients from across the U.S. This allowed us to estimate utility scores associated with more health states and to improve the accuracy and reliability of estimates for low-prevalence health states such as amputations. Because of our large study sample size, we were also able to estimate health utility scores across a broader spectrum of clinical severities for many diabetes-related complications than those that were previously available for U.S. diabetes patients.
RESEARCH DESIGN AND METHODS
The design of Translating Research Into Action for Diabetes (TRIAD) has been previously described (12). The primary objective of TRIAD was to determine how structural and organizational characteristics of health systems and health care provider groups influence the processes and outcomes of diabetes care. Six translational research centers collaborated with 10 health plans and 68 provider groups that served 180,000 diabetes patients. Health plans from California, Hawaii, Indiana, Michigan, New Jersey, New York, Pennsylvania, and Texas participated and included a racially and ethnically diverse membership. The institutional review boards at all translational research centers reviewed and approved the TRIAD study protocol, and all participants provided informed consent.
The TRIAD study population was a random sample of adult enrollees with diabetes from participating health plans. Patients were eligible to participate if they were aged ≥18 years, community dwelling, spoke English or Spanish, not pregnant, continuously enrolled in the health plan for at least 18 months, had diabetes for ≥1 year, and used services during their health plan enrollment. In addition, eligibility required at least two outpatient visits or one inpatient stay with a diagnostic code for diabetes (ICD-9 250.xx), laboratory tests or values suggesting diabetes (at least two HbA1c tests ordered or a diagnostic HbA1c or fasting blood glucose level), or a prescription for medications for diabetes (e.g., insulin or an oral antidiabetic agent). At the time of the survey, patients who met these criteria were included only if they confirmed that they had diabetes and received most of their diabetes care through the participating TRIAD health plan. Type 2 diabetes was defined as diabetes that was not currently treated with insulin or that was diagnosed after age 30 years, with or without current insulin treatment.
Data sources
Data were collected from patient surveys and record reviews. Patient surveys were completed between July 2000 and October 2001. Of the 13,086 individuals who were contacted and eligible, 11,927 (91%) completed the survey. Survey variables included age, sex, race/ethnicity, education, income, BMI, smoking, time since diagnosis of diabetes, treatment for diabetes, and HRQOL as measured by the EQ-5D.
Of the patients completing a survey, 73% consented to participate in the study and had records available for review. The final analytic sample included 7,327 people. The participants whose records were reviewed were similar to the overall study population (13). Centrally trained reviewers used standardized data collection forms to abstract each patient’s records of medical treatment and prescribed medications during the 12 months before the survey date; of these, 5% were abstracted by two abstractors. Inter-rater reliability (κ) ranged from 0.86 to 0.94 for the main quality measures derived from the medical record data. Variables that were obtained from the record review were diabetes-related micro- and macrovascular complications and comorbid conditions.
Health utility measure
We used the EQ-5D to derive health utility scores. The validity and reliability of the EQ-5D have been reported previously (10). We chose the EQ-5D over other health utility measures, such as the Short-Form Health Survey-6D, because of its simplicity, sufficient responsiveness to changes in health states, relatively wide range of utility scores generated by the instrument, and availability of preference weight for our study population (11,14). Briefly, the EQ-5D consists of two parts: five questions relating to distinct dimensions of a person’s functional capacity (mobility, self-care, usual activity, pain/discomfort, and anxiety/depression) and a visual analog scale. There are three responses for each domain of the questionnaire. Because the baseline survey was conducted primarily by phone, the visual analog scale of the EQ-5D was not implemented. The responses from the EQ-5D were combined with preference weights derived from a sample of the U.S. population to provide health utility scores (7).
Diabetes-related complications
We constructed categories within each type of complication based on clinically defined disease states and data availability and ordered the individual disease categories based on disease severity. The specific disease categories for each of the five diabetes-related complications include diabetic retinopathy, diabetic nephropathy, diabetic neuropathy, stroke, and cardiovascular disease (Table 2). Within diabetic retinopathy, individuals with laser treatment were considered to have “proliferative diabetic retinopathy.” Within cardiovascular disease, the category of “other coronary heart diseases” was defined as having indicators of coronary heart disease or coronary artery disease or undergoing procedures to treat coronary heart disease such as coronary angioplasty and coronary bypass. Peripheral vascular disease was categorized as “having” or “not having” the disease. A person had peripheral vascular disease if he or she had a record of peripheral vascular disease or had undergone procedures used to treat the disease such as peripheral vascular angioplasty or bypass.
Table 2.
Unadjusted EQ-5D health utility score by patient characteristics
Statistical analysis
Means and standard errors are reported for continuous variables (except age) and proportions for categoric variables. Tests for differences between categories in EQ-5D scores by patient level variables were tested using a χ2 test.
The EQ-5D utility scores were modeled by a multivariate linear regression model adjusting for demographic and clinical characteristics and for disease states. Missing values for independent variables were imputed five times using a multiple imputation method (15). The initial model was developed using all variables. When estimates of adjacent response categories for any diabetes-related complication in the model were not significantly different from each other or were inconsistent with the order of increasing severity, the two response categories were combined and the model was rerun. We repeated the process until the ordering of all variable coefficients increased in severity. Only those variables that had statistically significant estimated coefficients were kept in the final model.
The estimated coefficients of the indicator variables represent the penalty or deficit from optimal health associated with each variable. Subtracting penalty functions from the health utility scores for the healthy reference group created an additive model from which we derived the EQ-5D utility score for any combination of treatments, complications, and comorbidities. After combining some of the categoric variables, the reference group was defined as a nonobese man with type 2 diabetes who was not Asian or Hispanic, not using insulin treatment, and who had an annual household income of more than $40,000 with no diabetes-related complications, risk factors for cardiovascular disease, or comorbidities. All statistical analyses were performed using SAS 9.2 software (SAS Institute Inc., Cary, NC).
RESULTS
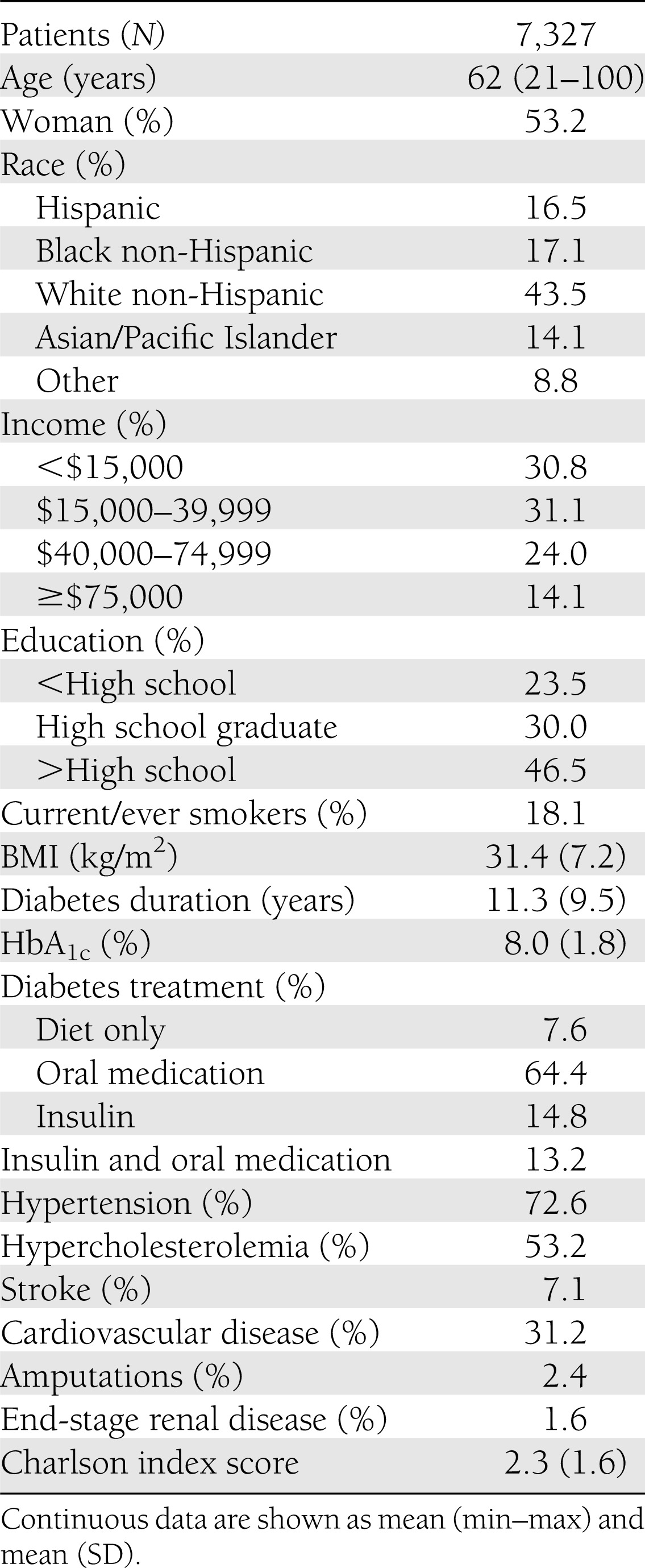
Table 1 presents the demographic characteristics of the study patients. In general, they reflect the characteristics of the population with type 2 diabetes in the U.S. Patients with type 2 diabetes tend to be older, to be more racially diverse, to have higher BMIs, and to have a higher proportion of individuals with low income and education levels compared with the general U.S. population (16). The average HbA1c level in the TRIAD population with type 2 diabetes (8.0%) was similar to that for the U.S. general population with type 2 diabetes during the same period (7.5%) (17). The mean time since a diagnosis of diabetes was 11.3 years. ore than 90% of patients were treated with oral antidiabetic agents, insulin monotherapy, or a combination of the two. About one-third of the study population had cardiovascular disease. The mean Charlson index, a weighted measure that incorporates 19 diseases to predict 10-year mortality risk according to severity of comorbidities, was 2.3. For reference, the predicted 10-year mortality risk is 48% for individuals with a Charlson index score of 2.0 (18).
Table 1.
Characteristics of study participants
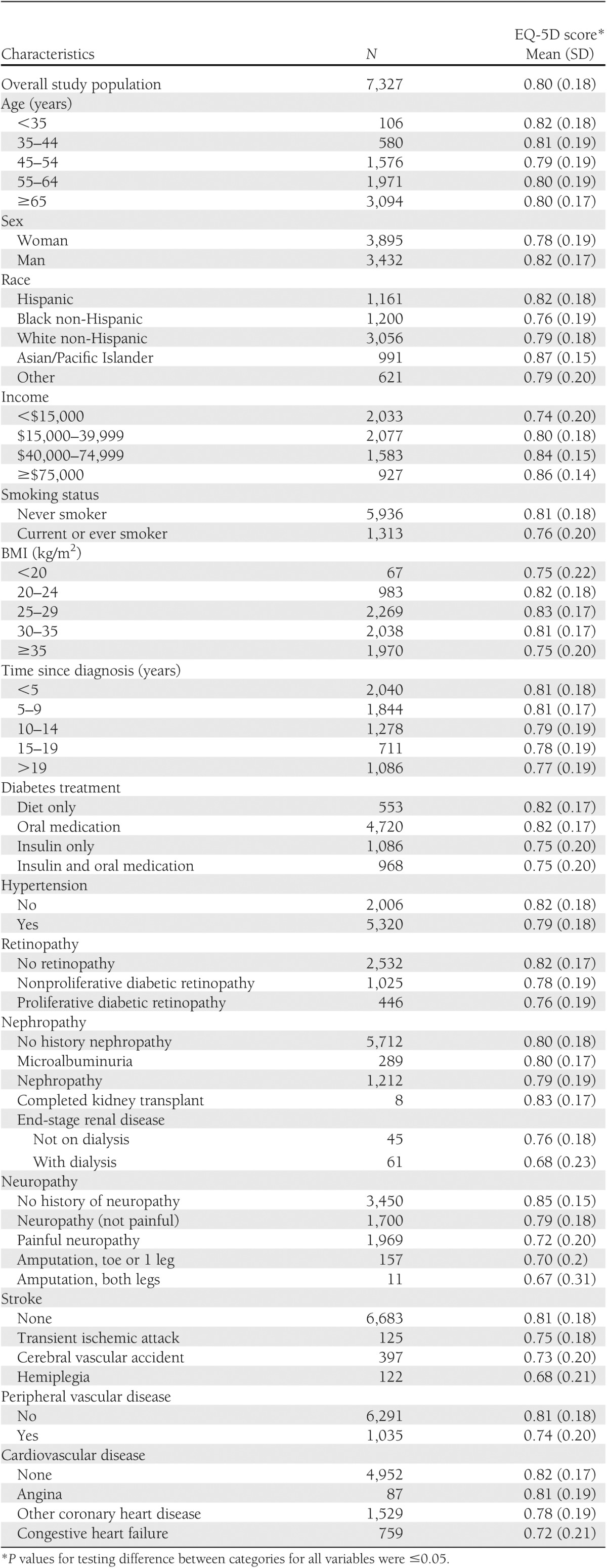
The mean EQ-5D score for study participants was 0.80. Table 2 presents the EQ-5D scores by demographic and clinical characteristics of the study population. The EQ-5D scores differed across subgroups by age, sex, race, level of education, and household income. The scores also differed by time since diagnosis of diabetes, BMI, smoking status, and diabetes treatment. In general, patients with diabetes-related complications or comorbidities had lower health utility scores than those without. Among those with the lowest EQ-5D scores were patients who required dialysis, who had hemiplegia, or who had experienced the amputation of both feet.
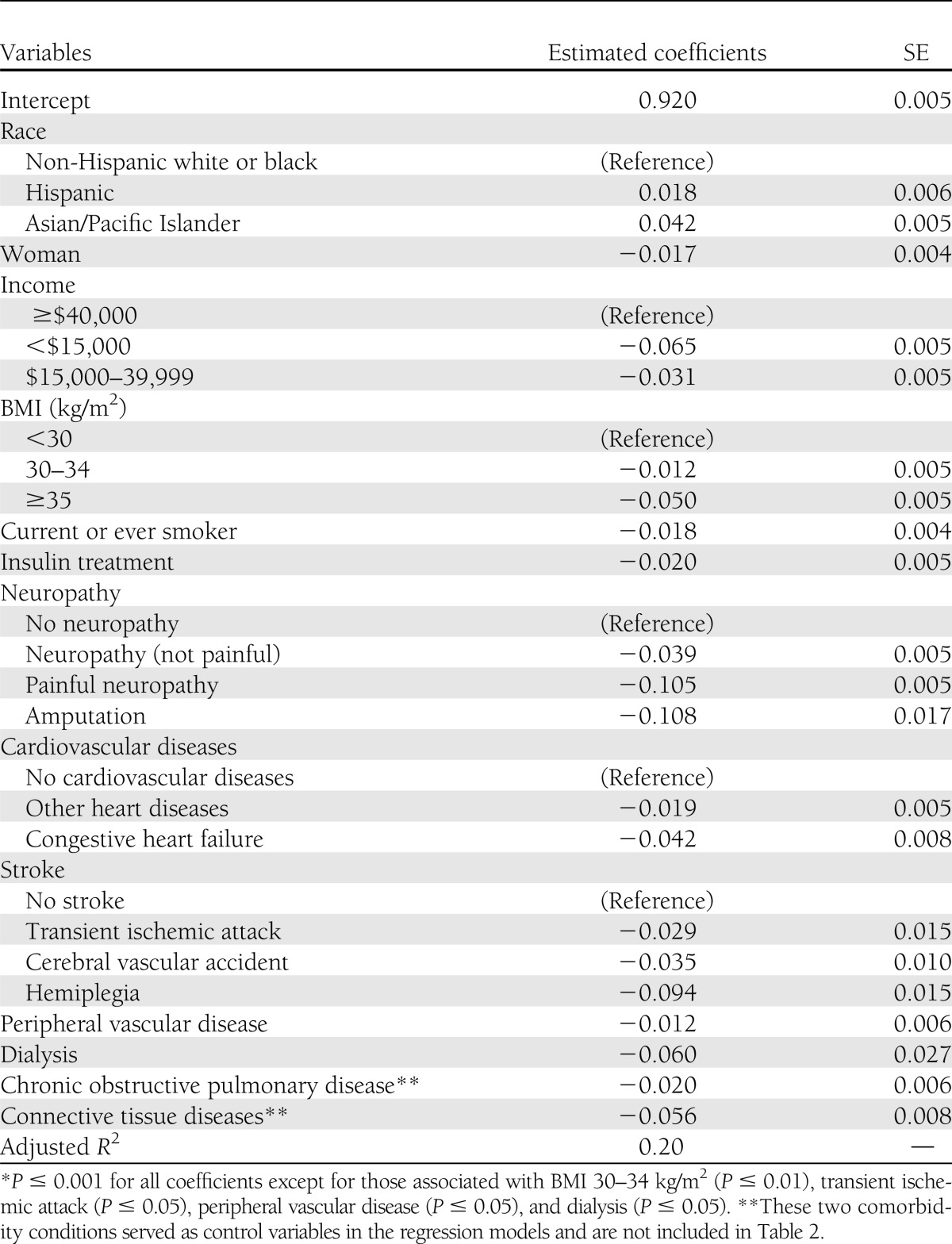
The penalty functions associated with each characteristic are presented in Table 3. The intercept value of 0.920 was the mean health utility score in the reference group. Being Asian or Hispanic was associated with a higher health utility score. Being a woman, being obese, smoking, using insulin, or having an annual household income of less than $40,000 were associated with a lower health utility score. The most severe microvascular complications, painful neuropathy and amputations, were associated with a decrement of more than 0.10 in the health utility score. Complications associated with a reduction of more than 0.03 in the health utility score were hemiplegia, nonpainful diabetic neuropathy, cerebral vascular accident, congestive heart failure, and dialysis. Other complications, such as other coronary heart disease, transient ischemic attack, and peripheral vascular disease, were associated with relatively small reductions in EQ-5D scores (<0.03). Nonproliferative and proliferative diabetic retinopathy did not independently reduce health utility scores and were dropped from the final model. Similarly, age, education, time since diagnosis of diabetes, and hypertension were not associated with significant independent reductions in health utility scores and were excluded from the final model.
Table 3.
Estimated coefficients for the multiple regression model
CONCLUSIONS
We derived health utility scores for adults with diabetes, its complications, and its comorbidities by using data collected in a large multicenter, prospective observational study of managed care patients from seven U.S. regions. As one might expect, diabetes-related complications were associated with lower utility score, and the magnitude of the impact varied by the complication. In increasing order of deficit, the complications were peripheral vascular disease, other heart diseases, transient ischemic attack, cerebral vascular accident, nonpainful diabetic neuropathy, congestive heart failure, dialysis, hemiplegia, painful neuropathy, and amputation. The variations in the effect of different diabetes complications on HRQOL were as much as 10-fold.
Insulin treatment was associated with a significant reduction in health utility scores. However, it is not clear whether the lower health utility scores associated with insulin treatment were because insulin treatment did not improve functional capacity or because insulin treatment served as a surrogate for greater diabetes severity. Those treated with insulin have been diagnosed with diabetes longer and have experienced more diabetes-related complications than those who are treated with diet or oral antidiabetic agents alone. In our study, insulin treatment was still associated with a lower utility score after adjusting duration of diabetes and diabetes-related complications, implying insulin treatment lowered health utility scores independently.
Studies that have assessed insulin use as part of overall quality-of-life have shown a decrease in quality-of-life as treatment moved from diet only to oral agents to insulin (19). However, studies comparing quality-of-life with specific treatment regimens and ongoing support strategies generally reported no decline, or even an improvement, in quality-of-life with insulin use (19). On the one hand, using insulin could lower quality-of-life directly because of inconvenience, adverse effects of insulin injections, and hypoglycemia, or indirectly by stigmatizing a patient as being unable to control his or her diabetes adequately (20). On the other hand, insulin use could also improve quality-of-life by leading to better glycemic control, which is positively associated with a high level of perceived quality-of-life (21).
Obesity, especially morbid obesity (BMI ≥35 kg/m2), was associated with a large reduction in health utility scores. Hlatky et al. (20) found a negative relationship between excess body weight and HRQOL among type 2 diabetic patients, even after adjusting for their demographic characteristics, diabetes duration, diabetes treatment mode, and presence of cardiovascular diseases and other comorbid conditions. Obesity impairs physical functioning, decreases energy levels, increases health distress, and decreases self-rated health.
Our model found a higher health utility score for Asians and Hispanics and a lower score for women and low-income individuals. These differences in health utility scores by race, sex, or income level are consistent with findings from previous studies (21). Causes for these differences are not well understood and need to be further studied. However, these results do not imply that a diabetes intervention is more cost-effective for Asians or Hispanics compared with other races, for men compared with women, or for those of lower income compared with those of middle income, because no interactions were found among any of the three variables for type of diabetes treatment or diabetes-related complications (data not shown).
Coffey et al. (2) estimated health utility scores associated with different diabetic treatments, complications, and comorbidities among individuals with type 2 diabetes. Their study used a self-administered version of the Quality of Well Being index to survey 1,257 people with type 2 diabetes who were patients at endocrinology, diabetes, and ophthalmology clinics at the University of Michigan Health System. The study estimated the mean health utility score for a diet-controlled nonobese diabetic man without any complications, comorbidities, or cardiovascular risk factors to be 0.687. We did not estimate the utility values for subjects with the same characteristics. The closest comparable utility value in our study was the mean utility value for our reference group, 0.920. The difference in these values is likely attributable to the HRQOL instruments used in the two studies. Health utility scores based on the EQ-5D tend to be higher than those from other HRQOL instruments. In fact, about a quarter of our subjects had a utility value of 1.00. EQ-5D cannot distinguish small differences in physical or mental health status in relatively healthy populations. This ceiling effect of EQ-5D has been documented in other studies (3,4).
The estimated range and level of penalty scores associated with individual complications between the Coffey et al. (2) study and ours were similar. They estimated that the penalty score, excluding blindness (we did not collect information on blindness), varied from 0.011 to 0.105, whereas our estimates varied from 0.012 to 0.108.
Clarke et al. (4) estimated health utility scores in 3,192 type 2 diabetic subjects who participated in the United Kingdom Prospective Diabetes Study (UKPDS) using the EQ-5D. They estimated that a man with type 2 diabetes and no complications would have a utility score of 0.850–0.962, depending on the specific regression model. Their estimated range of penalty scores for individual complications was much larger (0.055–0.280). Several factors could have contributed to the different results:
First, subjects in the UKPDS represented a relatively healthy and homogenous newly diagnosed type 2 cohort. Our population included type 2 diabetic patients with a wide range of treatments, complications, comorbidities, and duration of diagnosed diabetes.
Second, in calculating the utility score, we used the preference weights from the U.S. population, while Clarke and colleagues used preference weights derived from the U.K. population.
Finally, because of data availability, the variables included in the regression models were different. Microvascular complications were not included in their estimates. They also included fewer demographic characteristics and comorbidities. Having fewer variables in their regression model may have added weight to the variables they included.
Redekop et al. (22) assessed health utility scores using the EQ-5D in a sample of 1,136 Dutch type 2 diabetic subjects who participated in the CODE-2 study. Older age, obesity, woman, insulin therapy, and presence of complications were associated with lower health utility scores. They did not assess health utility scores for specific microvascular and macrovascular complications. Their relatively small study sample may have limited their ability to assess the effect of each individual complication on HRQOL.
Bagust and Beale (3) also used data collected in the CODE-2 study to estimate utility scores but combined the Dutch data used by Redekop et al. (22) with data from Belgium, Italy, Spain, and Sweden. Because of the much larger sample size of 4,641, this study was able to estimate EQ-5D utility scores associated with specific diabetes complications.
Except for coronary heart disease, our estimated penalty scores associated with individual complications were lower than those in the Bagust and Beale regression model (3). For example, the estimated penalty score for amputations was 0.108 in our study and 0.272 in the Bagust and Beale study. The estimated penalty score for coronary heart disease was 0.042 in our study and 0.028 in the Bagust and Beale study. The use of dissimilar preference weights in deriving the EQ-5D score and dissimilar demographic variables and definitions of complications may have contributed to differences in estimated health utility scores. The limited comparability of the estimated health utility scores between our study and the studies cited above might imply that penalty scores reported by different studies should be interpreted in the broad context of differences in study populations and quality-of-life instruments used and of the completeness and types of variables used in the regression models.
Glasziou et al. (14) estimated the health utility scores associated with diabetes and its complications for patients enrolled in a clinical trial in Australia. The health utility score for those who had no any diabetes-related complication was 0.88, a bit lower than the score in our study. Reduction in health utility scores by complications in decreasing order resulted in the following: stroke and/or transient ischemic attack, peripheral revascularization and/or amputation, unstable angina, myocardial infarction, coronary artery bypass graft, and eye disease. Differences in study population and definition of complications may have contributed to the different utility scores between the two studies.
Some limitations of our study should be noted. First, our EQ-5D data were collected in 2001. Health unity scores associated with diabetes and its complication can change due to improvements in physical, mental, and social functions of diabetes patients over time. Our study focused on assessing the relative decrement utility associated with each diabetes-related complication and its progression rather than the absolute level of health utility scores for those complications. Little evidence exists on the change in the relative function levels associated with different complications in the last 10 years. Thus, our estimates are very likely still applicable to the current diabetes population. Because our income level was measured in 2001 U.S. dollars, the income variable should be adjusted using an inflation calculator (23) if income is expressed in other years.
Second, our study population did not include uninsured diabetic patients. Patients with no health insurance tend to have a lower quality-of-life than those with health insurance (24). However, it is less clear that disutility associated with diabetes treatment mode and diabetes-related complications differ between the two groups.
In addition, our study population may not represent the entire managed care population with diabetes, and individuals in managed care might not be representative of the entire diabetic population; for example, poorer, sicker, or older individuals with type 2 diabetes may have been less likely to participate in the TRIAD survey.
Finally, our study was cross-sectional, and variation in responses could occur if HRQOL was measured at multiple points in time.
Cost-utility analysis, the type of economic evaluation most often used to assess interventions used for the prevention and control of type 2 diabetes, requires valid and accurate health utility estimates by patient characteristics, treatment modalities, and the different clinical states of each diabetes-related complication. We derived a set of such health utility estimates using data collected in a large multicenter diabetes study using U.S.-based preference weights. Our empirically derived health utility scores will allow researchers to calculate QALYs for studies involving individuals living in the U.S. with type 2 diabetes and representing a wide variety of demographic characteristics, treatments, complications, and comorbidities. The health utility scores provided should facilitate studies of the health burden of diabetes and the cost-utility analysis of alternative strategies for the prevention and treatment of diabetes in the U.S.
Acknowledgments
This study was jointly funded by Program Announcement No. 04005 from the Centers for Disease Control and Prevention, Division of Diabetes Translation, and the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support was provided by the Biostatistics and Economic Modeling Core of the Michigan Diabetes Research Training Center, Grant No. P60DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
P.Z. designed the study, performed statistical analyses, and wrote the manuscript. M.B.B. performed statistical analyses and reviewed and edited the manuscript. D.B. compiled the research database and reviewed and edited the manuscript. R.T.A. reviewed and edited the manuscript. R.L. performed statistical analyses and reviewed the manuscript. W.H.H. provided critical recommendations regarding study design and methodology, contributed to discussion, and reviewed and edited the manuscript. P.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all members of the TRIAD Study Group for collecting the data used in the study and the participation of their health plan partners and patients. The authors thank Bob Gerzoff for statistical assistance and Tony Pearson-Clarke for editorial assistance (both from Centers for Disease Control and Prevention).
Footnotes
The findings and conclusions in this report have not been formally disseminated by the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry and should not be construed to represent any agency determination or policy.
References
- 1.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York, Oxford University Press, 1996 [Google Scholar]
- 2.Coffey JT, Brandle M, Zhou H, et al. Valuing health-related quality of life in diabetes. Diabetes Care 2002;25:2238–2243 [DOI] [PubMed] [Google Scholar]
- 3.Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217–230 [DOI] [PubMed] [Google Scholar]
- 4.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340–349 [DOI] [PubMed] [Google Scholar]
- 5.Solli O, Stavem K, Kristiansen IS. Health-related quality of life in diabetes: the associations of complications with EQ-5D scores. Health Qual Life Outcomes 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sach TH, Barton GR, Jenkinson C, Doherty M, Avery AJ, Muir KR. Comparing cost-utility estimates: does the choice of EQ-5D or SF-6D matter? Med Care 2009;47:889–894 [DOI] [PubMed] [Google Scholar]
- 7.Johnson JA, Coons SJ, Ergo A, Szava-Kovats G. Valuation of EuroQOL (EQ-5D) health states in an adult US sample. Pharmacoeconomics 1998;13:421–433 [DOI] [PubMed] [Google Scholar]
- 8.Johnson JA, Luo N, Shaw JW, Kind P, Coons SJ. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care 2005;43:221–228 [DOI] [PubMed] [Google Scholar]
- 9.Bharmal M, Thomas J., 3rd Comparing the EQ-5D and the SF-6D descriptive systems to assess their ceiling effects in the US general population. Value Health 2006;9:262–271 [DOI] [PubMed] [Google Scholar]
- 10.Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care 2005;43:736–749 [DOI] [PubMed] [Google Scholar]
- 11.Grandy S, Fox KM. EQ-5D visual analog scale and utility index values in individuals with diabetes and at risk for diabetes: findings from the Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD). Health Qual Life Outcomes 2008;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TRIAD Study Group The Translating Research Into Action for Diabetes (TRIAD) study: a multicenter study of diabetes in managed care. Diabetes Care 2002;25:386–389 [DOI] [PubMed] [Google Scholar]
- 13.Kim C, Williamson DF, Mangione CM, et al. Translating Research Into Action for Diabetes (TRIAD) Study Managed care organization and the quality of diabetes care: the Translating Research Into Action for Diabetes (TRIAD) study. Diabetes Care 2004;27:1529–1534 [DOI] [PubMed] [Google Scholar]
- 14.Glasziou P, Alexander J, Beller E, Clarke P, ADVANCE Collaborative Group Which health-related quality of life score? A comparison of alternative utility measures in patients with type 2 diabetes in the ADVANCE trial. Health Qual Life Outcomes 2007;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlin JB, Galati JC, Royston P. A new framework for managing and analyzing multiply imputed data in Stata. Stata J 2008;8:49–67 [Google Scholar]
- 16.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care 1998;21(Suppl. 3):C11–C14 [DOI] [PubMed] [Google Scholar]
- 17.Hoerger TJ, Zhang P, Segel JE, Gregg EW, Narayan KM, Hicks KA. Improvements in risk factor control among persons with diabetes in the United States: evidence and implications for remaining life expectancy. Diabetes Res Clin Pract 2009;86:225–232 [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 19.Funnell MM. Quality of life and insulin therapy in type 2 diabetes mellitus. Insulin 2008;3:31–36 [Google Scholar]
- 20.Hlatky MA, Chung SC, Escobedo J, et al. BARI 2D Study Group The effect of obesity on quality of life in patients with diabetes and coronary artery disease. Am Heart J 2010;159:292–300 [DOI] [PubMed] [Google Scholar]
- 21.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev 1999;15:205–218 [DOI] [PubMed] [Google Scholar]
- 22.Redekop WK, Koopmanschap MA, Stolk RP, Rutten GE, Wolffenbuttel BH, Niessen LW. Health-related quality of life and treatment satisfaction in Dutch patients with type 2 diabetes. Diabetes Care 2002;25:458–463 [DOI] [PubMed] [Google Scholar]
- 23.US Inflation Calculator [article online], 2012. Available from http://www.usinflationcalculator.com/ Accessed 14 May 2012
- 24.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care 1997;20:562–567 [DOI] [PubMed] [Google Scholar]