Abstract
OBJECTIVE
Dendritic cells (DCs) are largely responsible for the activation and fine-tuning of T-cell responses. Altered numbers of blood DCs have been reported in type 1 diabetes (T1D). We aimed at characterizing the less well-known phenotypic properties of DCs in T1D.
RESEARCH DESIGN AND METHODS
In a case-control setting, samples from a total of 90 children were studied by flow cytometry or by quantitative real-time PCR (qPCR).
RESULTS
We found decreased numbers of myeloid DCs (mDCs) (8.97 vs. 13.4 cells/μL, P = 0.009, n = 31) and plasmacytoid DCs (pDCs) (9.47 vs. 14.6 cells/μL, P = 0.018, n = 30) in recent-onset T1D. Using a panel of antibodies against functionally important DC markers, we detected a decreased expression of CC chemokine receptor 2 (CCR2) on mDCs (percentage above negative control, P = 0.002, n = 29) and pDCs (median intensity, P = 0.003, n = 30) from T1D patients. In an independent series of children, the reduced expression of CCR2 was confirmed by qPCR in isolated mDCs (P = 0.043, n = 20). Serum concentrations of CCR2 ligands monocyte chemotactic protein-1 and -3 did not differ between the groups. A trend for an enhanced responsiveness of the nuclear factor-κB pathway (P = 0.063, n = 39) was seen in mDCs from children with β-cell autoantibodies, which is possibly related to the reduced CCR2 expression, since CCR2 on mDCs was downregulated by nuclear factor-κB–activating agents.
CONCLUSIONS
Given the role of CCR2 in DC chemotaxis and in DC-elicited Th1 differentiation, our results may indicate a functionally important DC abnormality in T1D affecting the initiation and quality of immune responses.
Type 1 diabetes (T1D) is considered an autoimmune disease resulting from T-cell–mediated immune reactivity against the insulin-producing pancreatic β-cells (1). Dendritic cells (DCs) are professional antigen-presenting cells mainly responsible for the activation of naive T cells, and they also critically influence the characteristics of the resulting T-cell responses. In addition to their role in the activation of autoreactive and regulatory T cells in the context of β-cell autoimmunity (2), DCs are, by their very nature, pivotal for the initiation of antimicrobial immune responses. This is of interest in T1D, where microbial stimuli have been linked to the development of the disease (3,4). Furthermore, the strongest genetic risk determinants for T1D are the highly polymorphic HLA class II alleles that are expressed by antigen-presenting cells, e.g., DCs.
DCs can be classified in two broad categories both in mice and humans, namely myeloid (mDCs) and plasmacytoid (pDCs) DCs. These express diverse, and largely exclusive, sets of pathogen-recognition receptors and are therefore differentially activated by various microbial agents (5). Functional differences between the DC subtypes exist but are not entirely clear. However, evidence has accumulated to suggest that pDCs are, at least to some extent, inclined toward induction of T-cell–mediated immune tolerance (6).
In the animal models of T1D, the nonobese diabetic (NOD) mouse and the Biobreeding (BB) rat, the genetic background leads not only to a spontaneous development of autoimmune diabetes at an early age but also to altered DC function (7,8). In human T1D, controversy exists whether numerical disturbances of circulating DCs are associated with the disease (9–15). Moreover, the current knowledge of the phenotypic properties of DCs in T1D is limited. Among the most interesting functional DC abnormalities, implicated both in the NOD model and in human T1D, is the dysregulated or enhanced nuclear factor (NF)-κB pathway (8,15,16). However, its function has not been assessed in the prediabetes phase preceding the diagnosis of clinical disease.
To explore DC alterations in T1D, we screened a panel of phenotypic markers on peripheral blood mDCs and pDCs from children with newly diagnosed T1D and control subjects. The markers were selected to cover the aspects of microbe recognition, chemotaxis, adhesion, antigen presentation, costimulation, and immunoregulation. Findings were then validated in an independent series of patients and control children. Finally, we studied the activation of the NF-κB pathway in DCs from children with β-cell autoimmunity.
RESEARCH DESIGN AND METHODS
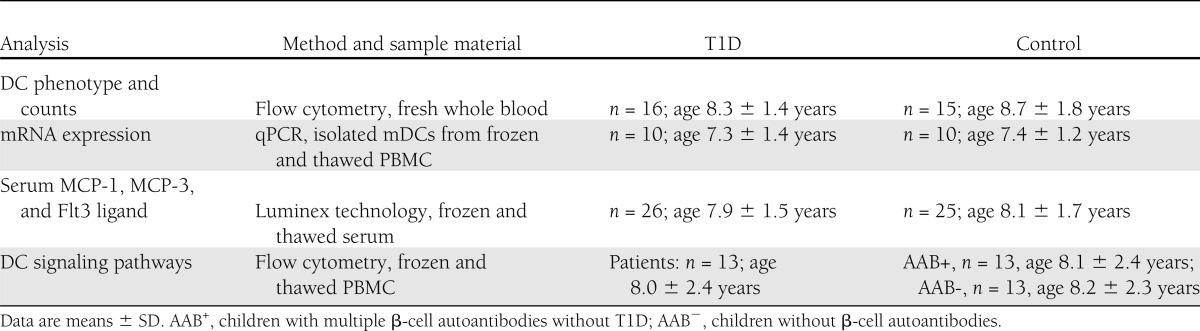
The study groups and sample materials are characterized in Table 1, while details of the individual children are provided in Supplementary Table 1. Sixteen children aged 5–9 years with newly diagnosed T1D were enrolled to DC enumeration and phenotype screening, and the blood sample was generally drawn within 4–7 days of diagnosis. Fifteen age-matched (±2 years) siblings of unrelated T1D patients served as control subjects. These control siblings tested negative for biochemically defined autoantibodies (insulin autoantibody, GAD antibody, IA-2 antibody, and zinc transporter 8 antibody [ZnT8A]). Quantitative real-time PCR (qPCR) measurements were performed in mDC samples from 10 newly diagnosed patients and 10 age- and sex-matched, autoantibody-negative siblings of unrelated patients. Phosphorylation of NF-κB and signal transducers and activators of transcription (STAT) proteins STAT1 and STAT3 was studied in 13 newly diagnosed patients (age 3–11 years) and in age-, sex-, and HLA genotype–matched siblings of unrelated T1D patients both with β-cell autoimmunity (at least two autoantibodies, n = 13) and without signs of autoimmunity (n = 13). Informed consent was obtained from guardians, and assent was asked for from children ≥10 years of age. For functional experiments with isolated mDCs, blood donor buffy coats (obtained from the Finnish Red Cross) were used. The study protocol was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa.
Table 1.
Study subjects and methods
Sample preparation for DC phenotyping and counting
All monoclonal antibodies (mAbs) (specified in Supplementary Table 2) were titrated for optimal performance. Negative-control mAbs comprised isotype-matched clones of irrelevant specificities. The following markers were screened: CD86, CD80, CD83, CD40, HLA-DR, intracellular adhesion molecule-1, leukocyte function-associated antigen-3 (LFA-3), L-selectin, α4-integrin, β7-integrin, dendritic cell–specific ICAM-3–grabbing nonintegrin (DC-sign), toll-like receptor (TLR)2, TLR4, CC chemokine receptor (CCR)2, CCR5, CCR7, CXC chemokine receptor (CXCR)3, CXCR4, CD38, and CD200R. mAbs were added to heparinized venous blood, and the samples were incubated for 20 min at room temperature. FACS Lysing solution (BD Biosciences, San Jose, CA) was added according to the manufacturer’s instructions, and cells were washed with washing buffer consisting of 5% FCS in PBS, supplemented with 0.02% (w/v) sodium azide. Samples were then suspended in 1% (w/v) paraformaldehyde (PFA) in PBS and stored at 4°C overnight before analysis. For isolated DCs, the erythrocyte-lysing step and PFA were omitted, and samples were analyzed on the same day.
Sample preparation for phosphoepitope measurements
Peripheral blood mononuclear cells (PBMCs) were thawed, suspended in RPMI-1640 medium (Gibco/Invitrogen, Carlsbad, CA) containing 10% heat-inactivated human AB serum (Innovative Research, Novi, MI), and kept in a CO2 incubator at 37°C for 2 h before addition of premixed cocktails of mAbs against cell-surface markers (Supplementary Table 2). After 10 min, the following recombinant cytokines (purchased from Peprotech, Rocky Hill, NJ) were added for 10 min to induce phosphorylation of NF-κB p65, STAT1, and STAT3: interleukin (IL)-1β (at a final concentration of 20 ng/mL), interferon-γ (IFN-γ) (103 IU/mL), and IL-10 (100 ng/mL), respectively. Cells were fixed with 1.75% (w/v) PFA for 10 min at room temperature and then permeabilized with 80% (vol/vol) methanol for 10 min (on ice). After washing with washing buffer containing 10% FCS, mAbs recognizing the serine- or tyrosine-phosphorylated forms of NF-κB p65 (pS529), STAT1 (pY701), and STAT3 (pY705) were added for 30 min. Cells were then washed twice with washing buffer and stored overnight in 1% (w/v) PFA in PBS at 4°C before the analysis.
Flow cytometry
DC enumeration and surface phenotyping was performed with FACSCalibur instrument (BD Biosciences). The gating strategy is shown in Supplementary Fig. 1. For pDC counting, BDCA2+CD123+ staining was used for gating. Cell doublets were excluded based on light scatter, except for DC enumeration. The specificity of mDC and pDC identification was confirmed by HLA-DR+CD11c+ or HLA-DR+CD123+ staining, respectively. The absolute DC numbers were calculated by multiplying the relative counts by the absolute total leukocyte count determined in a routine hematology laboratory. Cell-surface molecule expression was quantified as median fluorescence intensity (MFI) in arbitrary units (AU) after subtraction of the negative-control antibody intensity. In addition, we determined the percentage of DCs with an intensity above the negative-control antibody level.
Phosphorylated signaling molecules were measured using FACSAria instrument (BD Biosciences). CD11c and CD123 were used here as additional markers for the gating of mDC and pDCs, respectively. CD14+ monocytes were identified by their bright CD14 staining. Cytokine-induced phosphorylation was measured as a fold increase of MFI over the unstimulated sample. Intensities were calibrated to a set of particles containing known amounts of fluorescein isothiocyanate (Spherotech, Libertyville, IL). Cell viability was assessed with an amine-reactive violet fluorescent dye (Invitrogen). FlowJo software (Tree Star, Ashland, OR) was used to analyze flow cytometry data.
Isolation of mDCs
CD1c (BDCA-1)+ Dendritic Cell Isolation kit (Miltenyi Biotec, Gladbach, Germany) was used according to the manufacturer’s instructions, with one LD and two consequent MS columns. Cell viability was assessed by 7-AAD staining (eBioscience, San Diego, CA). mDC purity of ∼95% or higher was routinely achieved, as determined by flow cytometry.
Culture of mDCs
Twenty-five thousand CD1c+ mDCs per well were plated in 200 μL RPMI-1640 medium containing 10% heat-inactivated human AB serum and 50 μmol/L β-mercaptoethanol on U-bottom 96-well plates (Nunc, Roskilde, Denmark). For the maturation of mDCs, 100 ng/mL Escherichia coli LPS (Difco, Detroit, MI), 1 μg/mL TLR7/8 ligand CL097 (InvivoGen, San Diego, CA), or recombinant human IL-1β (R&D Systems, Minneapolis, MN) at 20 ng/mL was added. In separate experiments, mDCs were treated with 10 nmol/L recombinant human monocyte chemotactic protein (MCP)-1 or MCP-3 (both from R&D Systems) and/or 1 μmol/L CCR2b inhibitor RS102895 (Sigma-Aldrich, St. Louis, MO). Cells were kept in an incubator for 2 h or overnight.
qPCR analyses
Extraction of mRNA and qPCR analyses using Stepone plus real-time PCR systems sequence detector (Applied Biosystems, Foster City, CA) was performed as previously described (17). CCR2 transcripts were measured with an assay (Hs00356601_m1) recognizing both splice variants (CCR2A and CCR2B). Other gene products measured were MCP-1 (hs00234140_m1), DC-sign (Hs01588349_m1), and CD200R (Hs00708558_s1). Ribosomal 18S RNA (Hs99999901_s1) was used as an endogenous control, and target gene expression was analyzed by the 2−ΔΔCt method. Results are expressed as relative units (RU).
Cytokine/chemokine measurements
The concentrations of MCP-1, MCP-3, and Fms-related tyrosine kinase 3 ligand (Flt-3L) were analyzed using components from Milliplex MAG Human cytokine/chemokine kit (Millipore Corporation, Billerica, MA). Analyses were performed with Magpix system and xPonent software (Luminex Corporation, Austin, TX) and with Prism 5 software (GraphPad Software, La Jolla, CA) using five-parameter logistic curve-fitting method.
HLA genotyping
HLA typing of major T1D risk DR-DQ haplotypes was performed with a PCR-based lanthanide-labeled hybridization method using time-resolved fluorometry for detection (18).
Diabetes-associated autoantibodies
Islet cell antibodies were analyzed with indirect immunofluorescence on human group 0 donor pancreas, while insulin autoantibody, GAD antibody, IA-2 antibody, and ZnT8A were quantified with specific radiobinding assays as previously described (18).
Statistical analyses
Independent samples in two groups were analyzed by Mann-Whitney U test or by unpaired t test (with or without Welch correction), when appropriate, and multiple groups were compared with Kruskal-Wallis test. However, because of heterogeneous distribution patterns of IL-1β–induced NF-κB responses, data were converted to binomial variables with an upper limit for a “normal response” set at the 90th percentile in the control group, and Fisher exact test was used for the determination of statistical significance. For dependent samples, paired t test or repeated-measures ANOVA, followed by Dunnett posttest, was applied. Alternatively, we used Wilcoxon signed-rank test or Friedman test with Dunn posttest for nonnormal data. Spearman rank correlation was used to evaluate the correlation between two parameters. The significance level was set to P < 0.01 to account for multiple testing regarding the vast array of DC surface molecules screened by flow cytometry. In the remaining analyses, P < 0.05 was considered statistically significant. Two-tailed tests were used, except in the confirmatory qPCR analyses (one-tailed tests). Data were analyzed with Prism 5 software.
RESULTS
Numbers of mDCs and pDCs
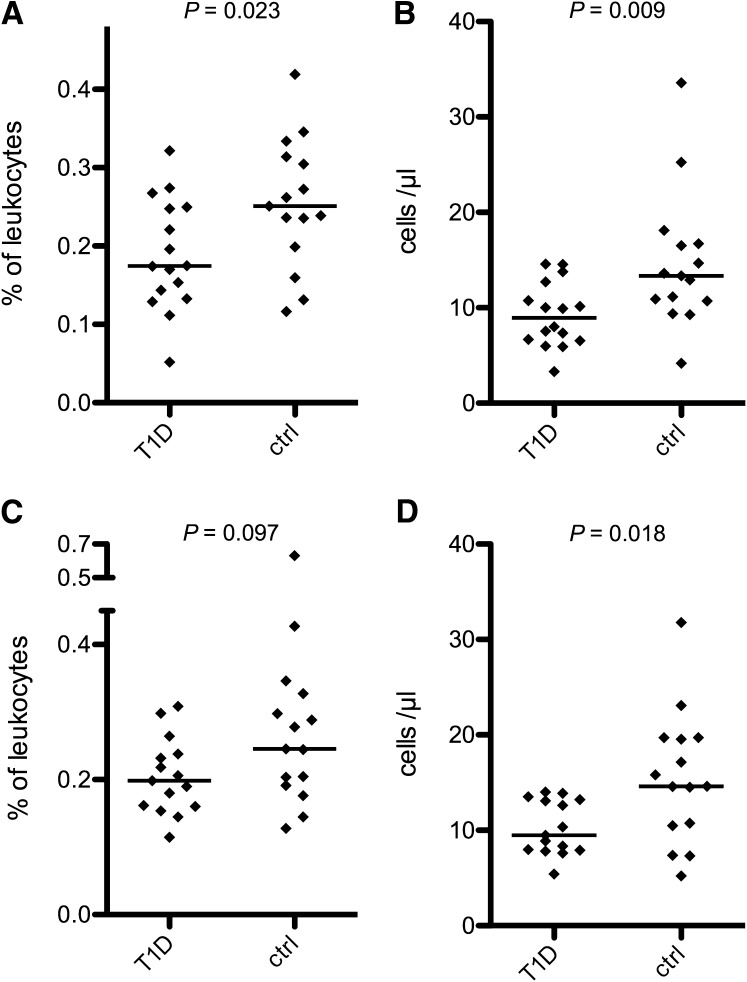
The proportion of CD1c+ mDCs of total leukocytes was decreased in T1D patients (median 0.175 vs. 0.251%, P = 0.023 [Fig. 1A]), and a similar trend was also seen for pDCs (0.198 vs. 0.245%, P = 0.097 [Fig. 1C]). The absolute counts for both mDCs (8.97 vs. 13.4 cells/μL, P = 0.009 [Fig. 1B]) and pDCs (9.47 vs. 14.6 cells/μL, P = 0.018 [Fig. 1D]) were lower in patients than in control subjects. DC numbers did not correlate with plasma glucose levels or with blood pH at diagnosis (data not shown). We observed no difference in the relative proportions of DC subtypes (median mDC-to-pDC ratio 1.04 vs. 0.915, P = 0.868). Total leukocyte counts and blood hemoglobin concentrations did not differ between the patients and control subjects (data not shown). We also looked for a possible relationship between the HLA class II risk genotype and the reduced DC numbers. The absolute mDC and pDC counts were lower in patients with the DQB1*0302/x genotype (n = 9/16; “x” indicates a neutral haplotype) in comparison with other patients (P = 0.031 and P = 0.021, respectively), whereas no difference was seen in control subjects (n = 6/15).
Figure 1.
Blood DC counts are decreased in T1D. The numbers of circulating DCs were determined by flow cytometry. The relative (A) and absolute (B) counts of mDCs were lower in recent-onset T1D patients compared with the healthy children. The relative pDC counts also tended to be lower in patients (C), and the absolute pDC counts were significantly decreased (D). ctrl, control. Horizontal lines indicate median levels.
Because of the decreased DC counts in T1D, the concentrations of DC growth factor Flt-3L in sera were measured. No differences were observed between the groups, but the concentrations were below the detection limit in the vast majority of individuals (data not shown).
CCR2 expression on peripheral blood DCs
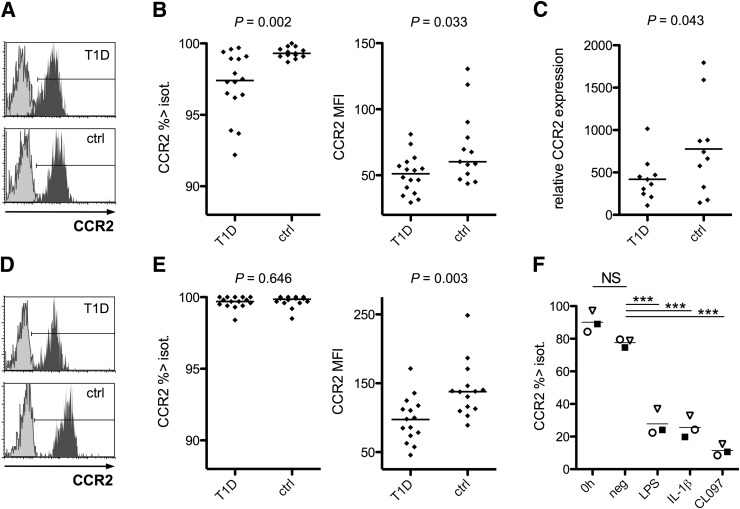
We screened with flow cytometry 20 markers for DC function on circulating mDCs and pDCs (Supplementary Fig. 2). A decreased expression of chemokine receptor CCR2 was detected on both mDCs (median MFI 51.1 vs. 60.2 AU, P = 0.033; % > negative control mAb level 97.4 vs. 99.3, P = 0.002 [Fig. 2B]) and pDCs (MFI 97.9 vs. 138 AU, P = 0.003; 99.7 vs. 99.9%, NS [Fig. 2E]) in recent-onset T1D. The reduction in CCR2 expression was confirmed in isolated mDCs from an independent series of patients and control subjects by qPCR analysis (mean expression of transcripts 419 vs. 777 RU, P = 0.043 [Fig. 2C]). Plasma glucose levels at diagnosis did not correlate with CCR2 expression as measured by flow cytometry or by qPCR (data not shown), and blood pH was not associated with the findings either (data not shown). Altered CCR2 expression was not related to particular HLA risk genotypes.
Figure 2.
CCR2 expression on blood DCs is reduced in T1D. mDCs were gated for flow-cytometric analysis as demonstrated in Supplementary Fig. 1. A representative example of CCR2 staining in patient and control (ctrl) samples is shown. Light-colored histograms indicate isotype (isot.)-control antibody levels (A). The percentage of mDCs with CCR2 expression above the isotype-control antibody-based cutoff level was decreased in T1D patients, and the MFI values for CCR2 expression were also somewhat lower in patients’ mDCs. Horizontal lines indicate median levels (B). mDCs were isolated from an independent series of recent-onset patients and control subjects for qPCR analyses. The expression of CCR2 mRNA transcripts in mDCs was decreased in patients, confirming the flow-cytometric finding. Horizontal lines indicate the means (C). CCR2 expression on pDCs, as assessed by flow cytometry, is shown in a representative patient and control sample (D). The percentage of pDCs expressing CCR2 above the isotype-control level did not differ between the groups. However, expression was significantly lower in patients, as indicated by the decreased MFI values (E). mDCs were isolated from general population blood donor buffy coats to purities >97% and stimulated with the indicated stimuli or left untreated. Overnight incubation with LPS, IL-1β, or CL097 induced a clear decrease in CCR2 expression, as measured by flow cytometry, demonstrating that CCR2 expression on human mDCs is downregulated by various DC-maturing stimuli. Individual donors are indicated with different symbols. ***P < 0.001 (F). neg, negative.
Using flow cytometry, we also detected a significantly decreased expression of the immunoregulatory CD200R molecule (median MFI 1.48 vs. 2.49 AU, P = 0.005; 15.3 vs. 25.2%, P = 0.004) on pDCs (Supplementary Fig. 2). However, the staining intensity of CD200R on pDCs was low even in healthy children, and CD200R mRNA transcripts were not readily detectable by qPCR in enriched pDC fractions from diabetic or control children (data not shown). On mDCs, the expression of DC-sign (CD209) was higher in T1D, approaching statistical significance (8.45 vs. 5.86%, P = 0.013) (Supplementary Fig. 2). In DC-sign mRNA transcript levels, no difference was found by qPCR, however (n = 7 patients and 7 control subjects; data not shown).
The general phenotype of circulating mDCs and pDCs in both T1D patients and control children was typical for an immature DC, with high levels of HLA-DR but negative or low-level expression of CD80, CD83, and CD40 (Supplementary Fig. 2). Moderate CD86 expression was, however, seen in mDCs, with somewhat higher levels in patients (P = 0.077) (Supplementary Fig. 2), whereas CD40-positive mDCs tended to be more frequent in control subjects (P = 0.046) (Supplementary Fig. 2).
Because increased serum concentrations of the CCR2 ligand MCP-1 (CCL2) have been reported in established T1D (19) and exogenous MCP-1 has been shown to inhibit CCR2 expression in a human myeloid cell line in vitro (20), we measured the serum concentrations of MCP-1 and MCP-3 (CCL7) using Luminex technology. The levels of MCP-1 and MCP-3 did not differ significantly between patients and control children (median 451 vs. 380 pg/mL, NS, and 8.35 vs. 16.6 pg/mL, NS, respectively, Supplementary Fig. 3A and B). To rule out an enhanced autocrine MCP-1 production in DCs, we quantified MCP-1 mRNA transcripts in purified mDCs by qPCR. The expression of MCP-1 in mDCs from diabetic and control children was similar (mean expression of transcripts 152 vs. 148 RU, NS [Supplementary Fig. 3C]).
In vitro modulation of CCR2 expression in mDCs
To elucidate the expression of CCR2 in different stages of human mDCs, we stimulated isolated mDCs with various agents known to induce mDC maturation. As shown in Fig. 2F, activation of mDCs through TLR4 (LPS), IL-1 receptor (IL-1β), or TLR8 (TLR7/8 ligand CL097) resulted in a pronounced downregulation of CCR2. Exogenous MCP-1 or MCP-3 did not downregulate CCR2 transcripts in human mDCs in vitro (Supplementary Fig. 3D and E).
Activation of NF-κB pathway
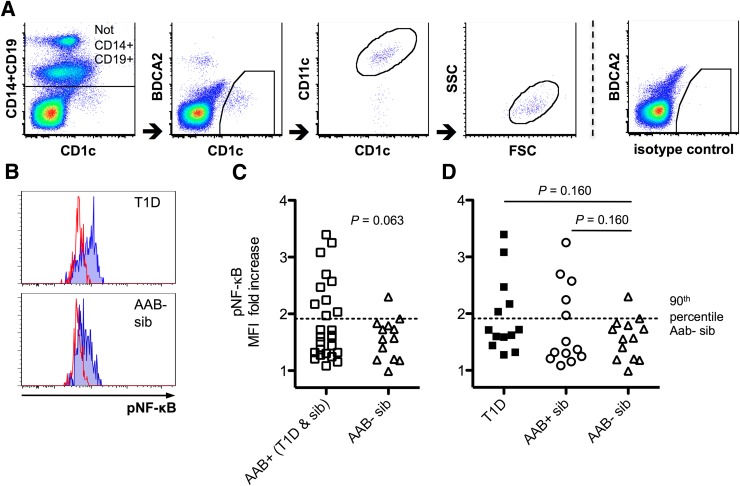
As CCR2 expression was decreased in recent-onset T1D patients and was also downregulated by NF-κB–inducing stimuli, we asked whether alterations in the levels of phosphorylated NF-κB p65 could be detected in DCs and monocytes from children with newly diagnosed diabetes and from high-risk children with multiple diabetes-associated autoantibodies. IL-1β–induced NF-κB responses were generally higher in mDCs than in monocytes (median MFI fold increase in autoantibody-negative control children 1.57 vs. 1.29, respectively; P < 0.001), and pDCs did not show detectable IL-1β–induced NF-κB responses in this setting (data not shown). Upon activation by IL-1β, children with T1D and/or β-cell autoimmunity exhibited a trend for an enhanced NF-κB response in mDCs (P = 0.063) (Fig. 3C). We did not detect differences in monocytes.
Figure 3.
The responsiveness of NF-κB pathway in children with multiple diabetes-associated autoantibodies. mDCs were identified in frozen and thawed PBMC samples by multicolor flow cytometry (A). A representative example of high- and low-level NF-κB phosphorylation (pNF-κB) response to IL-1β stimulation is shown. Unstained histograms represent unstimulated samples (B). NF-κB responsiveness was more pronounced among children with β-cell autoimmunity, approaching statistical significance (C). NF-κB responses were somewhat enhanced both in recent-onset T1D patients and in autoantibody-positive children without T1D compared alone with autoantibody-negative control children, although statistical significance was not achieved (D). AAB+, children with multiple β-cell autoantibodies without T1D; AAB−, children without β-cell autoantibodies; sib, sibling; FSC, forward scatter; SSC, side scatter.
In addition, we measured the levels of phosphorylated STAT1 and STAT3 proteins, known to affect the maturation and activation status of DCs, both spontaneously and after activation with IFN-γ and IL-10, respectively. The STAT1 response to IFN-γ stimulation was considerably stronger in mDCs and monocytes than in pDCs (median MFI fold increase in autoantibody-negative control children 4.80 and 6.50 vs. 1.82, respectively; P = 0.018 and P < 0.001, respectively), and the IL-10–induced STAT3 response was significantly higher in mDCs compared with pDCs and monocytes (2.88 vs. 2.39 and 2.22, respectively; P < 0.001 and P = 0.032, respectively). No significant differences in STAT1 and STAT3 signaling or in the spontaneous phosphoepitope staining intensities for NF-κB, STAT1, or STAT3 (with negative control mAb MFI subtracted) were detected between the various groups of children studied (data not shown).
CONCLUSIONS
We found a reduction in both mDC and pDC numbers in children with newly diagnosed T1D, which is in accordance with a previous study comprising a large number of pediatric patients (12). The conflicting results in earlier reports regarding numerical abnormalities of blood DC subtypes in T1D (9–15) may, at least partly, be due to differences in disease duration and age of the patients studied. Reduction in DC numbers could reflect poor DC production, given that decreased yield and maturation of DCs from monocyte precursors have also been reported in T1D and in individuals at risk (21–23). It is, however, not clear at present whether the decrease in DC counts would constitute a functionally significant defect.
In the phenotype analyses, we observed a reduced expression of CCR2 at the protein level on mDCs and pDCs in children with T1D, which was confirmed in an independent series of patients and control subjects at the mRNA level in mDCs. It has been suggested that upon DC maturation, the expression of CCR2, along with CCR3, CCR5, and CCR6, is downregulated and CCR7 expression upregulated, a concept sometimes referred to as the chemokine receptor “switch paradigm” (24). This would facilitate the migration of maturing DCs from peripheral tissues to lymph nodes. In mice, CCR2 expression is downregulated during DC activation in vitro (25). Our results here confirm the downregulation of CCR2 upon in vitro activation using human mDCs, which, to our knowledge, has not previously been demonstrated. Despite altered CCR2 expression, no clear evidence for an increased DC maturation was observed in T1D. Although CD86 expression on mDCs was somewhat elevated, CD40 expression tended to be lower in patients, and CD80 expression was at the isotype-control level.
Human blood mDCs migrate in response to CCR2 ligands MCP-1 and MCP-3 (24,26). Because pancreatic islets secrete MCP-1, especially in the presence of inflammatory cytokines (27) or when infected by enteroviruses (28), the reduction in CCR2 expression and DC counts could have been due to CCR2-mediated translocation from circulation to tissues, e.g., pancreas. We could not, however, demonstrate in diabetic children the absence of circulating DCs with the highest CCR2 expression; rather, we demonstrated an overall decrease in CCR2 levels. Moreover, blood pDCs do not show considerable migratory responses to CCR2 ligands (24). Thus, infiltration of CCR2-high DCs from the peripheral circulation to the inflamed islets does not readily explain our findings. On the other hand, it has been reported that DC migration in response to MCP-1 is severely impaired in NOD mice (29), which suggests that the decreased CCR2 expression could have functional relevance to cell migration in the context of autoimmune diabetes. MCP-1 may inhibit CCR2 expression (20), and increased MCP-1 production has been reported in circulating or adhered monocytes from T1D patients at the mRNA or protein level, respectively (30–32). However, we did not detect MCP-1 upregulation in mDCs or elevated levels in sera, and CCR2 expression was not downregulated by MCP-1 in cultured mDCs. Importantly, no correlation between plasma glucose and CCR2 expression was observed in patients, suggesting that the decreased CCR2 levels were not secondary to hyperglycemia. Because of the relatively small number of children in our study, a future study with a larger sample size is nevertheless needed.
CCR2 expression on DCs, or on DC precursors, is indispensable for proper Th1 responses (33,34) and for the clearance of at least some intracellular pathogens in mice (33). Since certain microbes, enteroviruses in particular, seem to be associated with T1D (3), it is tempting to speculate that the reduction in CCR2 expression may affect not only DC migratory properties but also microbial clearance. In support of this view, Lohmann et al. (35) have reported decreased IFN-γ responses and reduced expression of Th1 chemokine receptors on CD4+ lymphocytes in T1D. Furthermore, patients with T1D exhibited decreased Coxsackie virus B4–specific Th1 responses in our earlier study (36). Of note, the attenuated induction of Th1 cells may eventually contribute to the T1D-associated Th17 immunity (17), as IFN-γ exerts a potent downregulatory effect on Th17 differentiation (37). The recent demonstration of a role of CCR2 on DCs for central tolerance against blood-borne antigens (38) is also of interest in relation to our findings.
Intriguingly, the CCR2 gene is located in the T1D-associated 3p21.31 area, together with CCR5, which is considered a candidate gene for T1D (39). Based on the tight linkage in the region, it is, however, currently uncertain whether the culprit is CCR5, CCR2, or both. Notably, we did not detect any differences in CCR5 expression on DCs between patients and control subjects.
To further evaluate the alterations of DCs in T1D, we studied the cytokine-induced phosphorylation of NF-κB, STAT1, and STAT3 in DCs. We found a trend for an enhanced NF-κB response to IL-1β in mDCs from children with β-cell autoimmunity. The NF-κB pathway is a key mediator of signals promoting DC activation and maturation, acting downstream of, for example, TLRs and the IL-1 receptor. Accordingly, the enhanced responsiveness of NF-κB pathway may be related to the reduced CCR2 expression on DCs in T1D. Dysregulation of NF-κB signaling in human T1D has previously been demonstrated in monocyte-derived DCs (16). More recently, an enhanced LPS-induced NF-κB phosphorylation response was shown in primary blood mDCs from T1D patients (15). Given its central role in DC activation, dysregulation of NF-κB may interfere with the delicate balance between tolerance and immunity.
Taken together, our results imply a modest DC defect in T1D, a phenomenon first suggested by Drexhage and colleagues in 1995 (21). Although the cause for CCR2 downregulation is currently unknown, genetic mechanisms are somewhat implicated (40). DC alterations, in association with an HLA risk genotype for T1D, may contribute to the development of β-cell autoimmunity via dysregulation of tolerance and microbial immune responses. Studies on DCs during the prediabetes phase are warranted.
Acknowledgments
This study was funded in part by Finska Läkaresällskapet, Sigrid Juselius Foundation, Foundation for Pediatric Research, and The Diabetes Research Foundation.
No potential conflicts of interest relevant to this article were reported.
J.K.N. designed the study, performed experiments, analyzed data, wrote the first draft of the manuscript, and contributed to the critical review and revision of the manuscript. J.V. designed the study and contributed to the critical review and revision of the manuscript. H.M.S. was involved in the qPCR analyses and contributed to the critical review and revision of the manuscript. N.E. took part in the Luminex analyses and contributed to the critical review and revision of the manuscript. T.H. was responsible for the autoantibody analyses and contributed to the critical review and revision of the manuscript. J.I. was responsible for the HLA genotyping and contributed to the critical review and revision of the manuscript. M.K. designed the study, was responsible for the autoantibody analyses, and contributed to the critical review and revision of the manuscript. O.V. designed the study, wrote the first draft of the manuscript, and contributed to the critical review and revision of the manuscript. O.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Sinikka Tsupari, University of Helsinki and Helsinki University Central Hospital, Anneli Suomela, National Institute for Health and Welfare, and Camilla Virta, National Institute for Health and Welfare, for excellent technical assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2460/-/DC1.
References
- 1.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med 1987;166:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 2010;10:501–513 [DOI] [PubMed] [Google Scholar]
- 3.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008;455:1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 2001;194:863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolic T, Welzen-Coppens JM, Leenen PJ, Drexhage HA, Versnel MA. Plasmacytoid dendritic cells in autoimmune diabetes - potential tools for immunotherapy. Immunobiology 2009;214:791–799 [DOI] [PubMed] [Google Scholar]
- 7.Delemarre FG, Simons PJ, de Heer HJ, Drexhage HA. Signs of immaturity of splenic dendritic cells from the autoimmune prone biobreeding rat: consequences for the in vitro expansion of regulator and effector T cells. J Immunol 1999;162:1795–1801 [PubMed] [Google Scholar]
- 8.Poligone B, Weaver DJ, Jr, Sen P, Baldwin AS, Jr, Tisch R. Elevated NF-kappaB activation in nonobese diabetic mouse dendritic cells results in enhanced APC function. J Immunol 2002;168:188–196 [DOI] [PubMed] [Google Scholar]
- 9.Peng R, Li Y, Brezner K, Litherland S, Clare-Salzler MJ. Abnormal peripheral blood dendritic cell populations in type 1 diabetes. Ann N Y Acad Sci 2003;1005:222–225 [DOI] [PubMed] [Google Scholar]
- 10.Summers KL, Behme MT, Mahon JL, Singh B. Characterization of dendritic cells in humans with type 1 diabetes. Ann N Y Acad Sci 2003;1005:226–229 [DOI] [PubMed] [Google Scholar]
- 11.Summers KL, Marleau AM, Mahon JL, McManus R, Hramiak I, Singh B. Reduced IFN-alpha secretion by blood dendritic cells in human diabetes. Clin Immunol 2006;121:81–89 [DOI] [PubMed] [Google Scholar]
- 12.Vuckovic S, Withers G, Harris M, et al. Decreased blood dendritic cell counts in type 1 diabetic children. Clin Immunol 2007;123:281–288 [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Makala LH, Jin Y, et al. Type 1 diabetes patients have significantly lower frequency of plasmacytoid dendritic cells in the peripheral blood. Clin Immunol 2008;129:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen JS, Pang K, Skowera A, et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes 2009;58:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyers AJ, Shah RR, Gottlieb PA, Zipris D. Altered Toll-like receptor signaling pathways in human type 1 diabetes. J Mol Med (Berl) 2010;88:1221–1231 [DOI] [PubMed] [Google Scholar]
- 16.Mollah ZU, Pai S, Moore C, et al. Abnormal NF-kappa B function characterizes human type 1 diabetes dendritic cells and monocytes. J Immunol 2008;180:3166–3175 [DOI] [PubMed] [Google Scholar]
- 17.Honkanen J, Nieminen JK, Gao R, et al. IL-17 immunity in human type 1 diabetes. J Immunol 2010;185:1959–1967 [DOI] [PubMed] [Google Scholar]
- 18.Knip M, Virtanen SM, Seppä K, et al. Finnish TRIGR Study Group Dietary intervention in infancy and later signs of beta-cell autoimmunity. N Engl J Med 2010;363:1900–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyici S, Erturk E, Budak F, et al. Serum monocyte chemoattractant protein-1 and monocyte adhesion molecules in type 1 diabetic patients with nephropathy. Arch Med Res 2006;37:998–1003 [DOI] [PubMed] [Google Scholar]
- 20.Tangirala RK, Murao K, Quehenberger O. Regulation of expression of the human monocyte chemotactic protein-1 receptor (hCCR2) by cytokines. J Biol Chem 1997;272:8050–8056 [DOI] [PubMed] [Google Scholar]
- 21.Jansen A, van Hagen M, Drexhage HA. Defective maturation and function of antigen-presenting cells in type 1 diabetes. Lancet 1995;345:491–492 [DOI] [PubMed] [Google Scholar]
- 22.Skarsvik S, Tiittanen M, Lindström A, Casas R, Ludvigsson J, Vaarala O. Poor in vitro maturation and pro-inflammatory cytokine response of dendritic cells in children at genetic risk of type 1 diabetes. Scand J Immunol 2004;60:647–652 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Honeyman MC, Harrison LC. Impaired yield, phenotype, and function of monocyte-derived dendritic cells in humans at risk for insulin-dependent diabetes. J Immunol 1998;161:2629–2635 [PubMed] [Google Scholar]
- 24.Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol 2001;167:1862–1866 [DOI] [PubMed] [Google Scholar]
- 25.Vecchi A, Massimiliano L, Ramponi S, et al. Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J Leukoc Biol 1999;66:489–494 [DOI] [PubMed] [Google Scholar]
- 26.Vanbervliet B, Homey B, Durand I, et al. Sequential involvement of CCR2 and CCR6 ligands for immature dendritic cell recruitment: possible role at inflamed epithelial surfaces. Eur J Immunol 2002;32:231–242 [DOI] [PubMed] [Google Scholar]
- 27.Piemonti L, Leone BE, Nano R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes 2002;51:55–65 [DOI] [PubMed] [Google Scholar]
- 28.Olsson A, Johansson U, Korsgren O, Frisk G. Inflammatory gene expression in Coxsackievirus B-4-infected human islets of Langerhans. Biochem Biophys Res Commun 2005;330:571–576 [DOI] [PubMed] [Google Scholar]
- 29.Bouma G, Nikolic T, Coppens JM, et al. NOD mice have a severely impaired ability to recruit leukocytes into sites of inflammation. Eur J Immunol 2005;35:225–235 [DOI] [PubMed] [Google Scholar]
- 30.Padmos RC, Schloot NC, Beyan H, et al. LADA Consortium Distinct monocyte gene-expression profiles in autoimmune diabetes. Diabetes 2008;57:2768–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyan H, Drexhage RC, van der Heul Nieuwenhuijsen L, et al. Monocyte gene-expression profiles associated with childhood-onset type 1 diabetes and disease risk: a study of identical twins. Diabetes 2010;59:1751–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouma G, Coppens JM, Lam-Tse WK, et al. An increased MRP8/14 expression and adhesion, but a decreased migration towards proinflammatory chemokines of type 1 diabetes monocytes. Clin Exp Immunol 2005;141:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato N, Ahuja SK, Quinones M, et al. CC chemokine receptor (CCR)2 is required for langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, b cell outgrowth, and sustained neutrophilic inflammation. J Exp Med 2000;192:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jimenez F, Quinones MP, Martinez HG, et al. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J Immunol 2010;184:5571–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohmann T, Laue S, Nietzschmann U, et al. Reduced expression of Th1-associated chemokine receptors on peripheral blood lymphocytes at diagnosis of type 1 diabetes. Diabetes 2002;51:2474–2480 [DOI] [PubMed] [Google Scholar]
- 36.Skarsvik S, Puranen J, Honkanen J, et al. Decreased in vitro type 1 immune response against coxsackie virus B4 in children with type 1 diabetes. Diabetes 2006;55:996–1003 [DOI] [PubMed] [Google Scholar]
- 37.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–1132 [DOI] [PubMed] [Google Scholar]
- 38.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol 2009;183:3053–3063 [DOI] [PubMed] [Google Scholar]
- 39.Barrett JC, Clayton DG, Concannon P, et al. Type 1 Diabetes Genetics Consortium Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 2009;41:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.T1DBase. Available fromhttp://www.t1dbase.org