Abstract
OBJECTIVE
To determine the effect of statin use on progression of vascular calcification in type 2 diabetes (T2DM).
RESEARCH DESIGN AND METHODS
Progression of coronary artery calcification (CAC) and abdominal aortic artery calcification (AAC) was assessed according to the frequency of statin use in 197 participants with T2DM.
RESULTS
After adjustment for baseline CAC and other confounders, progression of CAC was significantly higher in more frequent statin users than in less frequent users (mean ± SE, 8.2 ± 0.5 mm3 vs. 4.2 ± 1.1 mm3; P < 0.01). AAC progression was in general not significantly increased with more frequent statin use; in a subgroup of participants initially not receiving statins, however, progression of both CAC and AAC was significantly increased in frequent statin users.
CONCLUSIONS
More frequent statin use is associated with accelerated CAC in T2DM patients with advanced atherosclerosis.
The role of statins in prevention of cardiovascular disease (CVD) in type 2 diabetes (T2DM) is well established. Despite the wide use of statins, however, calcific atherosclerosis is accelerated in T2DM and is associated with increased risk of CVD morbidity and mortality in this population (1). The purpose of the current study was to determine the effect of statin use on progression of vascular calcification in T2DM participants with advanced atherosclerosis.
RESEARCH DESIGN AND METHODS
The Risk Factors, Atherosclerosis, and Clinical Events in Diabetes (RACED) study is a seven-site substudy (1) of the Veterans Affairs Diabetes Trial (VADT) (2). The designs of both studies have been previously described (3–5). As part of this study, baseline and follow-up coronary artery calcification (CAC) and abdominal aortic artery calcification (AAC) volumetric scores were assessed by computed tomography, and change was estimated as the difference between square root transformation (SQRT) of the these scores (5,6). Multiple linear regression models of vascular calcium progression were adjusted for age, diabetes duration, ethnicity or race (non-Hispanic whites vs. others), current smoking, baseline CAC or AAC, CVD events (before and during the study), treatment assignment (intensive vs. standard), baseline and change in BMI, HbA1c, systolic and diastolic blood pressures, LDL, HDL, triglycerides, and time in the study. Sex was not included as one of the covariates because only 7% (n = 14) of the study population were women, and the results did not change appreciably with their exclusion from analyses.
RESULTS
The study included 197 participants with T2DM (mean duration, 12 ± 8 years), a mean age of 61 ± 9 years, and extensive atherosclerosis at baseline (median [25th–75th percentile] Agatston score; CAC, 258 [18–872]; AAC, 888 [159–3,831]). Follow-up calcium scans were completed on average 4.6 years later. Optimizing lipid levels was a critical part of the VADT (2); use of statins was therefore encouraged and carefully documented during the study. Participants underwent 14–28 VADT study visits, 3 months apart. At each visit, information regarding concomitant medications, including statin use, was updated. At the baseline examination, 61% (n = 121) of participants were taking statin agents (simvastatin, 70%; lovastatin, 25%; and atorvastatin, 5%). During the study, 82% of subjects (n = 161) reported frequent statin use in >50% of the visits, with a median use of 95% (25th–75th percentile, 85–100%). Eighteen percent (n = 36) reported statin use in ≤50% of the visits (median [25th–75th percentile], 14% [0–38%]).
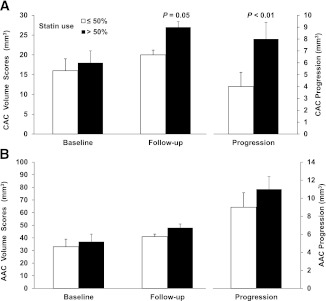
More frequent statin users had a higher prevalence of previous CVD than did others (41% vs. 19%; P = 0.01). There were no other significant differences between the groups at baseline (Supplementary Table 1). At the end of the study, more frequent statin users had lower total cholesterol (mean ± SD, 151 ± 34 vs. 167 ± 37 mg/dL; P = 0.01), LDL cholesterol (79 ± 28 vs. 91 ± 28 mg/dL; P = 0.01), and total cholesterol/HDL ratio (3.6 ± 1.1 vs. 4.1 ± 1.4 mg/dL; P = 0.05) compared with less frequent users. There were no significant differences in the incidence of CVD events during the study between categories of statin use (30% vs. 19%; P = 0.18); however, progression of CAC was significantly (P < 0.01) greater with more frequent statin use (Fig. 1), and adjustment for relevant covariates did not change the results. Because people with previous or incident CVD events may have accelerated progression of vascular calcification, we evaluated the effect of statins in the cohort after excluding these individuals. In these remaining participants (n = 105), those with frequent statin use had significantly greater CAC progression (7.1 ± 5.9 vs. 4.3 ± 5.4 mm3; P = 0.03). Progression of AAC was not significantly different in the whole group (Fig. 1); however, in those not receiving statins at the baseline examination (n = 76 with CAC and n = 73 with AAC scans, respectively), after adjustment for age and baseline calcium, progressions of both CAC and AAC were significantly higher in those who subsequently reported frequent statin use compared with less frequent users (CAC progression, 7.9 ± 0.8 vs. 3.5 ± 1.0 mm3; P < 0.01; AAC progression, 11.9 ± 1.3 vs. 7.6 ± 1.6 mm3; P = 0.04).
Figure 1.
Baseline, follow-up, and progression of CAC (A) and AAC (B) according to reported statin use. Volume scores at baseline and follow-up are SQRT values to stabilize interscan variability across the range of coronary calcium as reported by Hokanson et al. (6). Progression is the difference in SQRT volume scores of follow-up and baseline values. Data presented are means ± SE. White bars represent less frequent statin use (in ≤50% of the visits during the study); black bars represent frequent statin use (in >50% of the visits during the study).
CONCLUSIONS
In this cohort of T2DM patients with advanced atherosclerosis, we found that more frequent statin use was associated with accelerated progression of CAC. These findings are consistent with those reported in a previous study of T2DM participants without previous coronary artery disease (7). Results of this earlier study were believed to result from higher baseline CAC scores and insufficient lowering of LDL at follow-up in the statin users (7). In our study, however, there were no significant differences in baseline CAC or AAC according to statin use (Supplementary Table 1). In addition, at the end of the study more frequent statin users had significantly lower and nearly optimal LDL-cholesterol levels. Moreover, adjustment for baseline and on-trial risk factors, including LDL cholesterol and baseline CAC, did not explain the greater CAC progression in frequent statin users.
Randomized controlled trials in largely nondiabetic populations with no previous coronary artery disease demonstrated that, despite potent lipid-lowering effects, statin agents do not reduce the progression of CAC (8) or AAC (9). In fact, there was a trend toward progression of CAC with statin treatment in several studies (9–11). We now demonstrate that even in a setting of optimal lipid lowering and similar baseline CAC and AAC, statin agents promote calcification in T2DM subjects with advanced atherosclerosis. Because the variation in progression of AAC scores is greater, it is possible that the lack of significantly greater AAC progression with statin use is a sample size issue. Among those not initially on statins at baseline, however, the magnitude of AAC progression in those with subsequent frequent statin use was large enough to achieve statistical significance.
Statins have been implicated in calcification of vascular smooth muscle cells and mesenchymal cells (12,13). Statins also lower the lipid-rich core of atherosclerotic plaques and may enhance the density of calcification (14) as part of a healing process, potentially contributing to plaque stabilization and decreased CVD events. Alternatively, accelerated progression of calcified atherosclerosis in T2DM by statins may have the effect of lessening these medications’ overall benefit. Long-term follow-up in this cohort will help determine whether accelerated CAC and AAC progression in statin users is associated with more or fewer CVD events compared with statin users with less progression.
Acknowledgments
This work was supported by a grant from the Carl T. Hayden VA Medical Research Foundation. This work was supported in part by the Office of Research and Development, Medical Research Service and Cooperative Studies Program, U.S. Department of Veterans Affairs; by National Institutes of Health Grant R01-067690 (P.D.R.); by the Kronos Research Institute; and by a Clinical Research Award from the American Diabetes Association (P.D.R.).
No potential conflicts of interest relevant to this article were reported.
A.S. designed the study, researched the data, and wrote the manuscript. G.B. contributed to analysis and interpretation of the data and edited the manuscript. P.D.R. participated in critical review of the data and edited the manuscript. A.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in poster form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0464/-/DC1.
The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
References
- 1.Reaven PD, Sacks J, Investigators for the VADT Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia 2005;48:379–385 [DOI] [PubMed] [Google Scholar]
- 2.Abraira C, Duckworth W, McCarren M, et al. VA Cooperative Study of Glycemic Control and Complications in Diabetes Mellitus Type 2 Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications 2003;17:314–322 [DOI] [PubMed] [Google Scholar]
- 3.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 4.Reaven PD, Moritz TE, Schwenke DC, et al. Veterans Affairs Diabetes Trial Intensive glucose-lowering therapy reduces cardiovascular disease events in Veterans Affairs Diabetes Trial participants with lower calcified coronary atherosclerosis. Diabetes 2009;58:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saremi A, Moritz TE, Anderson RJ, et al. Veterans Affairs Diabetes Trial (VADT) Rates and determinants of coronary and abdominal aortic artery calcium progression in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2010;33:2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hokanson JE, MacKenzie T, Kinney G, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol 2004;182:1327–1332 [DOI] [PubMed] [Google Scholar]
- 7.Anand DV, Lim E, Darko D, et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol 2007;50:2218–2225 [DOI] [PubMed] [Google Scholar]
- 8.McEvoy JW, Blaha MJ, Defilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol 2010;56:1613–1622 [DOI] [PubMed] [Google Scholar]
- 9.Terry JG, Carr JJ, Kouba EO, et al. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the Coronary Artery Calcification Treatment with Zocor [CATZ] study). Am J Cardiol 2007;99:1714–1717 [DOI] [PubMed] [Google Scholar]
- 10.Houslay ES, Cowell SJ, Prescott RJ, et al. Scottish Aortic Stenosis and Lipid Lowering Therapy, Impact on Regression trial Investigators Progressive coronary calcification despite intensive lipid-lowering treatment: a randomised controlled trial. Heart 2006;92:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005;46:166–172 [DOI] [PubMed] [Google Scholar]
- 12.Trion A, Schutte-Bart C, Bax WH, Jukema JW, van der Laarse A. Modulation of calcification of vascular smooth muscle cells in culture by calcium antagonists, statins, and their combination. Mol Cell Biochem 2008;308:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kupcsik L, Meurya T, Flury M, Stoddart M, Alini M. Statin-induced calcification in human mesenchymal stem cells is cell death related. J Cell Mol Med 2009;13:4465–4473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovarnik T, Mintz GS, Skalicka H, et al. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J 2012;76:176–183 [DOI] [PubMed] [Google Scholar]