Abstract
OBJECTIVE
To assess the efficacy of curcumin in delaying development of type 2 diabetes mellitus (T2DM) in the prediabetic population.
RESEARCH DESIGN AND METHODS
This randomized, double-blinded, placebo- controlled trial included subjects (n = 240) with criteria of prediabetes. All subjects were randomly assigned to receive either curcumin or placebo capsules for 9 months. To assess the T2DM progression after curcumin treatments and to determine the number of subjects progressing to T2DM, changes in β-cell functions (homeostasis model assessment [HOMA]-β, C-peptide, and proinsulin/insulin), insulin resistance (HOMA-IR), anti-inflammatory cytokine (adiponectin), and other parameters were monitored at the baseline and at 3-, 6-, and 9-month visits during the course of intervention.
RESULTS
After 9 months of treatment, 16.4% of subjects in the placebo group were diagnosed with T2DM, whereas none were diagnosed with T2DM in the curcumin-treated group. In addition, the curcumin-treated group showed a better overall function of β-cells, with higher HOMA-β (61.58 vs. 48.72; P < 0.01) and lower C-peptide (1.7 vs. 2.17; P < 0.05). The curcumin-treated group showed a lower level of HOMA-IR (3.22 vs. 4.04; P < 0.001) and higher adiponectin (22.46 vs. 18.45; P < 0.05) when compared with the placebo group.
CONCLUSIONS
A 9-month curcumin intervention in a prediabetic population significantly lowered the number of prediabetic individuals who eventually developed T2DM. In addition, the curcumin treatment appeared to improve overall function of β-cells, with very minor adverse effects. Therefore, this study demonstrated that the curcumin intervention in a prediabetic population may be beneficial.
The impacts of type 2 diabetes mellitus (T2DM) on global health care and economy are enormous (1). According to the World Health Organization, there are ∼311 million people worldwide who live with T2DM. This number continues to rise, especially in the newly developing and poorer countries in Asia and elsewhere. Because T2DM is currently incurable, a common treatment approach is to try to control the disease with lifelong use of antidiabetes drugs. Limiting the number of newly developed T2DM cases should be one of the better key strategies to restrict the global impacts of T2DM (2). In order to limit the number of new T2DM cases, the lifestyle of the prediabetic population has to be changed. However, this has been shown to be challenging (3). One of the alternative approaches to prevent development of T2DM is to intervene with the prediabetic population before disease progresses into fully developed T2DM (3). The intervention approach is appealing. It relies on timely identification of prediabetic individuals and provision of preventive treatment before the disease fully progresses. The intervention represents a chance for the diabetes-prone population to halt the disease progression and maintain a normal and healthy life. In recent years, several effective T2DM intervention regimens have been developed, with encouraging results (3–5). However, these regimens are not usually economically accessible, and they are not well-tolerated because of treatment-related toxicities (4,5). The focus now is to identify new effective therapeutic agents, with relatively low cost and low toxicity, that can be used regularly to control a progression of T2DM in the prediabetic population.
Curcumin is the principal curcuminoid found in turmeric (Curcuma longa Linn.), a popular spice in Asian cuisine. It is widely consumed and generally believed to be beneficial for human health (6). Curcumin extract from rhizomes of turmeric has been shown to contain anti-inflammation and antidiabetic properties (7–13). In addition, it could delay development of T2DM, improve β-cell functions, prevent β-cell death, and reduce insulin resistance in animals (8–16). This study aimed to determine the effectiveness of curcumin extract as an intervention agent to prevent T2DM development. We assessed T2DM progression and several indicative T2DM parameters in a large randomized, double-blinded, and placebo-controlled cohort. We found that curcumin extract effectively reduced the number of prediabetic individuals who progressed toward T2DM as well as improved functions of β-cells.
RESEARCH DESIGN AND METHODS
Study design and participants
This randomized, double-blinded, placebo-controlled trial was conducted at HRH Princess Maha Chakri Sirindhorn Medical Center of Srinakharinwirot University (Nakornnayok, Thailand). Two hundred forty patients were selected to participate in this study by inclusion and exclusion criteria (for a complete flow chart, see Supplementary Fig. 1). The subjects were enrolled in the 12-month–long study. We educated all subjects to perform in the same protocols for diet and exercise during a 3-month period after the enrollment (before the randomization). Standard lifestyle recommendations were provided for all subjects in written form. All of the subjects took part in a 20–30-min one-on-one workshop that emphasized the importance of a healthy lifestyle. Participants were encouraged to follow the Medical Nutrition Therapy and physical activity (17). To avoid any interference from other medications, during the recruitment process, we excluded all of patients who were taking any other medicines, as shown in the exclusion criteria. Only prediabetic subjects aged ≥35 years were included in this study. Prediabetes was diagnosed following the American Diabetes Association (ADA) practice guidelines. Subjects who fit into at least one of these three criteria were included: subjects with a fasting plasma glucose (FPG) between 100 and 124 mg/dL (indicating impaired fasting glucose), an oral glucose tolerance test (OGGT) plasma glucose at 2 h postglucose load (OGTT at 2 h) between 140 and 199 mg/dL (indicating impaired glucose tolerance), and a glycated hemoglobin (HbA1c) range from 5.7 to 6.4%. Diagnosis of prediabetes was confirmed by a second repeating test of all of the above-listed criteria on a different day.
Subjects diagnosed with diabetes according to the new ADA guidelines (18,19) were excluded from the study (subjects who are positive for any one of these following criteria: FPG level ≥126 mg/dL, OGTT at 2 h ≥200 mg/dL, and HbA1c ≥6.5%. The following subjects were also excluded from the study: subjects receiving oral hypoglycemic agents, antiplatelet drugs, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, fenofibrate, atorvastatin, rosuvastatin, and fluvastatin; subjects with serum creatinine ≥2.0 mg/dL or on dialysis; subjects with the liver enzyme alanine aminotransferase (ALT) ≥3 folds of upper limit of normal value range; subjects receiving other herbal medicines; subjects with secondary causes of hyperglycemia (receiving steroids or with pancreatic cancer); subjects with acute infections or chronic inflammatory diseases (cancer, rheumatoid arthritis); and subjects with gall bladder disease or history of cholecystectomy. This study (clinical trial reg. no. NCT01052025) was approved by the Ethic Committee of Faculty of Medicine, Srinakharinwirot University, Bangkok, Thailand (serial number SWUEC 9/2552) in accordance with the Declaration of Helsinki. Participants were informed and gave their consent before enrollment.
Randomization procedures
After steps of screening, consenting, and diet and lifestyle training, all subjects were randomly assigned to either the curcumin-treated group (intervention treatment condition) or placebo-treated group (control condition) using a fixed randomization scheme with assignment based on computer-generated random numbers performed by an independent researcher. The allocation scheme was sealed in opaque and consecutively numbered envelopes. Envelopes were opened sequentially by the independent person. The participants were informed that two types of interventions were being compared.
The intervention
All participants were instructed to take three capsules with blinded labels of either curcumin or placebo twice a day (total of six capsules per day) for 9 months continuously. Each curcumin capsule has curcuminoid content of 250 mg. Curcumin and identical placebo capsules were manufactured by the Government Pharmaceutical Organization of Thailand. Patients were asked to bring all capsules back at the follow-up visit at 3, 6, and 9 months for assessing their compliance. Numbers of capsules taken by the subjects were recorded (Supplementary Table 3).
Preparation of curcuminoids capsules
Dried rhizomes of turmeric (Curcuma longa Linn.) grown in Kanchanaburi province, Thailand, were ground into powder. The turmeric powder was extracted with ethanol and evaporated at low pressure to obtain ethanol extract in the form of semisolid containing oleoresin and curcuminoids. The oleoresin was removed to yield curcuminoid extract (total curcuminoids content between 75 and 85%). The peak ratio of curcumin:demethoxycurcumin and bisdemethoxycurcumin in the extract was determined by high-performance thin-layer chromatography. The extract (calculated for 250 mg of curcuminoids) was filled into capsules under the Good Manufacturing Procedures standard. Fingerprints of the extract and a detailed analysis of the chemical composition of the preparation in the extract are shown in Supplementary Fig. 2.
Study outcomes
The primary outcome was assessed by numbers of the subjects in the curcumin-treated or placebo-treated groups diagnosed with T2DM according to the ADA guidelines (18,19). Secondary outcomes were also measured as follows: changes of the β-cell functions (homeostasis model assessment [HOMA]-β, C-peptide, and proinsulin/insulin ratio), insulin resistance (IR) by HOMA-IR, obesity (body weight), abdominal obesity (waist circumference [WC]), and anti-inflammatory cytokine (adiponectin). Adverse effects of curcumin were determined by elevated creatinine ≥1.2 mg/dL and aspartate aminotransferase (AST)/ALT ≥3 times the upper limit of normal value range, and any symptoms of patient complaints were recorded (20). Other adverse effects related to peroxisome proliferator–activated receptor-γ in curcumin action were assessed, including bone mineral density (BMD), signs of edema, and coronary arterial disease (CAD) event.
Data collection and measurement methods
Measurements were made at baseline (before treatment) and at 3, 6, and 9 months after the intervention start. We recorded demographic data at the baseline; the researchers administered a questionnaire on medical history and medication, and measured body weight, height, and vital signs. The abdominal obesity/WC were measured by tape horizontally, midway between the inferior margin of the wrist and the superior border of the iliac crest (21). Histories of CAD and cerebrovascular disease were tracked from the subjects’ medical records. The diagnosis of CAD, based on the presence of angina symptoms and abnormalities in resting electrocardiogram, was also assessed at baseline and during each follow-up visit (at 3, 6, and 9 months). Hypertension was determined by history of high blood pressure (≥130/85 mmHg). Dyslipidemia was defined by any of the following: total cholesterol ≥200 mg/dL, triglycerides ≥150 mg/dL, LDL cholesterol ≥100 mg/dL, and/or HDL cholesterol ≤35 mg/dL or taking lipid-lowering drugs. BMD, a known fracture risk, was analyzed at the baseline and at 9 months after starting the intervention by dual X-ray absorptiometry (QDR 4500; Hologic) at the level of the lumbar spine region. OGTT at 2 h was performed in all subjects by taking 75 g oral glucose solution after overnight fasting; and then 2 h later, blood glucose level was measured. Blood was collected at 8:00 am from the antecubital vein while the subjects were in the recumbent position after an overnight fasting. Plasma samples for insulin, proinsulin, C-peptide, and adiponectin assays were frozen and stored at −80°C until the analysis of hormones were measured. FPG, HbA1c, total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride levels were measured according to the standard procedures. Plasma insulin, proinsulin, C-peptide, and adiponectin concentrations were determined using the radioimmunoassay kits from Millipore (St. Charles, MO) with a γ scintillation counter, which is calibrated for 125I measurement. HOMA-β, C-peptide, and the proinsulin/insulin ratio were measured for β-cell functions (22,23). HOMA-IR was calculated to assess change of IR (22,24).
Sample size
We estimated the size of the sample for this study based on data from the study by Nauck et al. (25). Calculations used an SD of 46.3. We needed to enroll at least 117 subjects in each treatment group to detect a difference of 17 in HOMA-β with 80% power at the 5% level of significance (26).
Statistical analysis
Demographic data at baseline were analyzed and presented as mean ± SEM for continuous variables and number with percent for categorical variables. Two-tailed Student t test and χ2 test were, respectively, used for continuous and categorical variables in comparisons between the two groups, using P < 0.05 for statistically significant difference. We used two-sided significance tests throughout. For analysis of outcome variables, values of mean ± SEM at 3, 6, and 9 months were presented for both groups. The analyses were performed on an intention-to-treat basis. Two-tailed Student t test was used to assess the statistical significant differences between means of the two groups at 3, 6, and 9 months, separately. Statistical analysis was performed using the Statistical Package for Social Sciences 11.5 software (SPSS Inc., Chicago, IL).
RESULTS
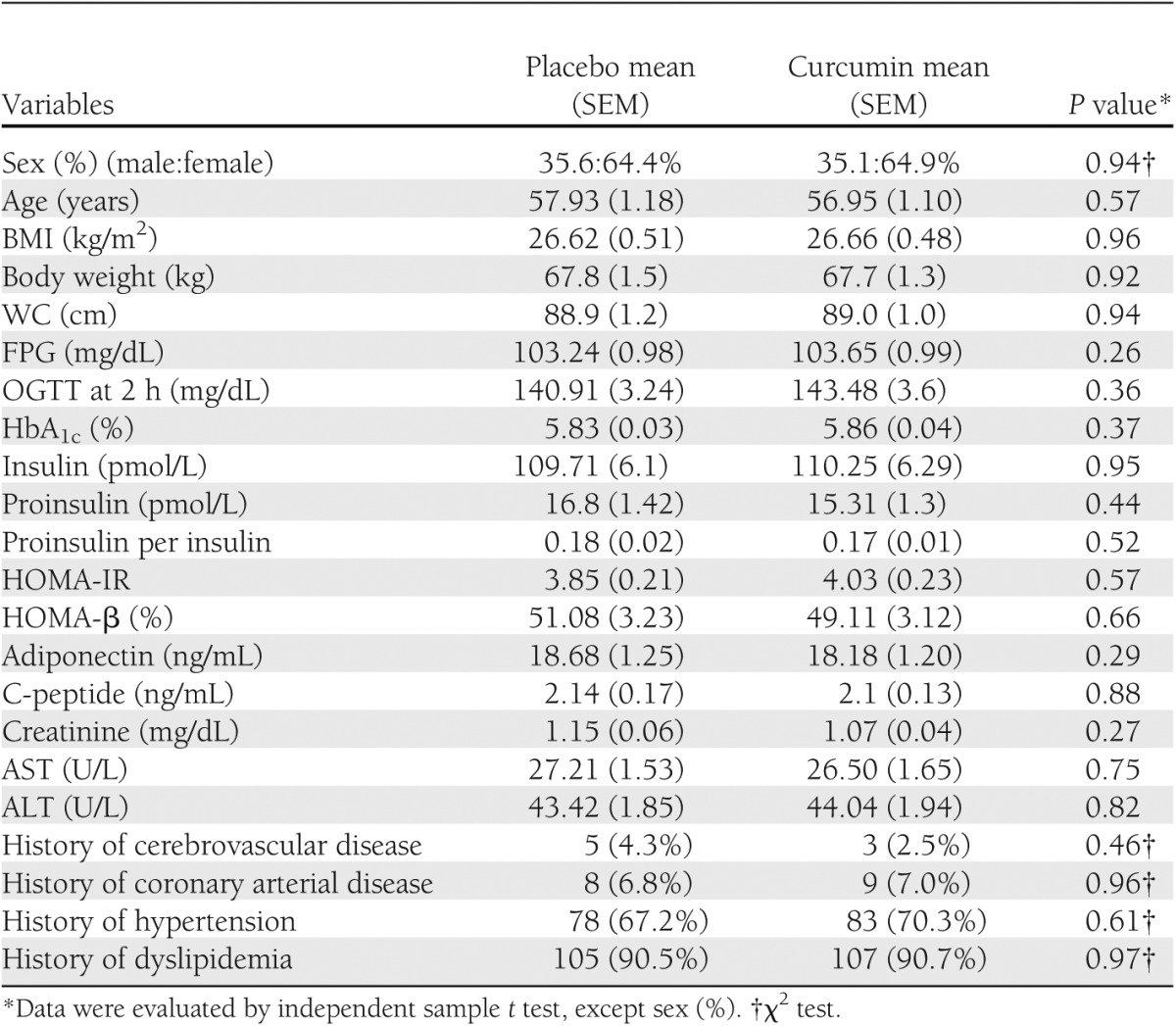
A flow chart of the trial is presented (Supplementary Fig. 1). A total of 240 subjects were initially enrolled in the study. The baseline characteristics of 237 subjects who were randomly allocated into the two groups are presented in Table 1. All parameters at the baseline between placebo-treated group and curcumin-treated group were not statistically different.
Table 1.
Baseline characteristics of subjects
Intervention outcomes
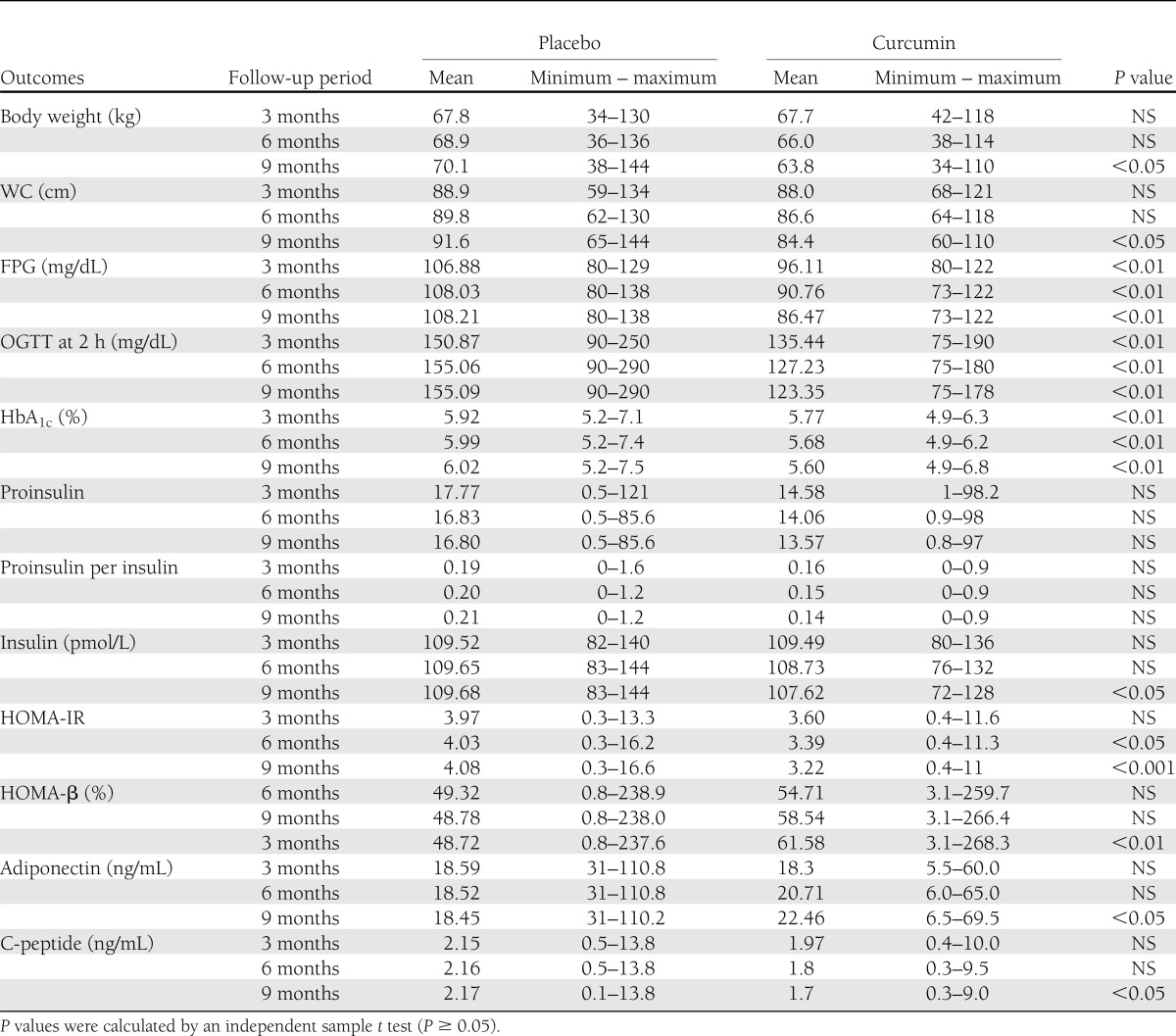
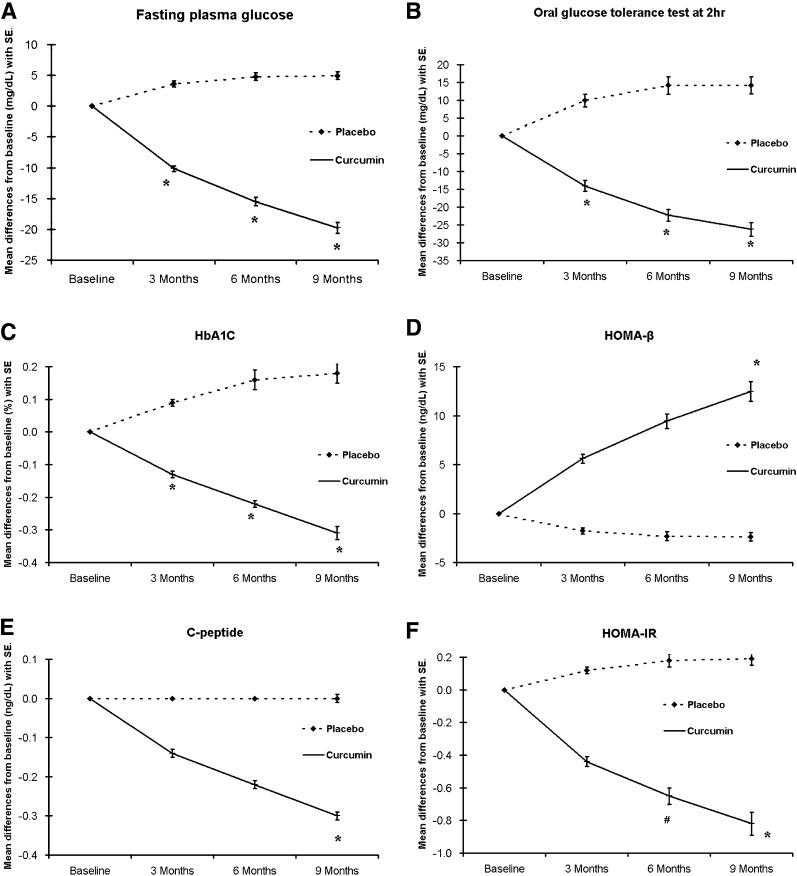
The means of diabetes-related blood chemistries used to assess the diabetic progression, such as HbA1c, FPG, and OGTT at 2 h were significantly lower in the curcumin-treated group when compared with the placebo group (P < 0.01) in all visits at 3, 6, and 9 months (Table 2). Differences from baselines for these three variables comparing the two groups are illustrated in the Fig. 1A–C.
Table 2.
Levels of blood chemistries indicating β-cell functions and obesity parameters
Figure 1.
Mean of parameters with SEM at baseline, 3, 6, and 9 months were compared between placebo- and curcumin-treated group. A: FPG. *P < 0.01. B: OGTT at 2 h. *P < 0.01. C: HbA1c. *P < 0.01. D: HOMA-β. *P < 0.01. E: C-peptide. *P < 0.05. F: HOMA-IR. *P < 0.001, #P < 0.05.
β-Cell function outcomes
HOMA-β, C-peptide, and proinsulin/insulin ratio were examined as outcomes related to β-cell functions (Table 2 and Fig. 1). Figure 1D shows that HOMA-β in the curcumin-treated group was increasingly elevated in all follow-up visits (at 3, 6, and 9 months) and became statistically significant at the final visit (9 months). Blood levels of C-peptide (Fig. 1E) were found to be significantly lower in curcumin-treated group when compared with those of placebo group. Although not significant, proinsulin/insulin ratio showed a lower trend in the curcumin-treated group (Table 2).
Insulin resistance and inflammatory cytokine outcomes
HOMA-IR level is a clinical representative of insulin resistance (22). HOMA-IR from both placebo and curcumin-treated groups was examined. The means of HOMA-IR of the curcumin-treated group were lower than those of placebo group at all follow-up visits (3, 6, and 9 months) (Fig. 1F). The differences were significant, particularly at the 6- and 9-month visits. Levels of adiponectin, an anti-inflammatory cytokine, in the placebo-treated group were virtually unchanged, whereas those of the curcumin-treated group were gradually elevated (at 3 and 6 months) and became significantly different from that of placebo-treated group at the final visit (9 months) (Table 2).
Diabetes prevention
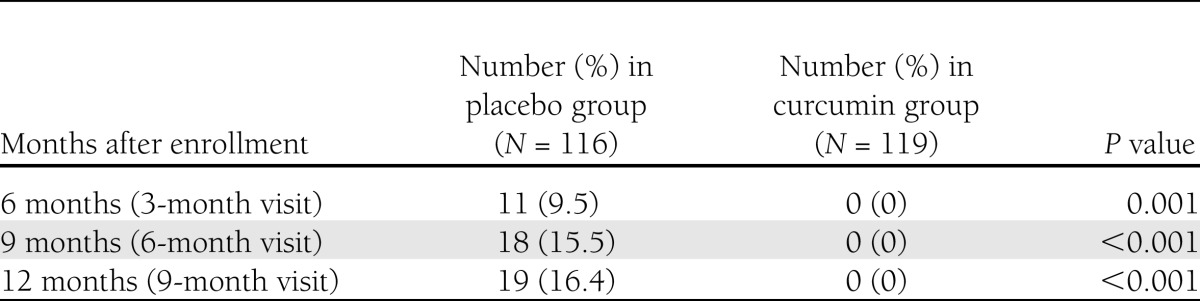
At 6, 9, and 12 months after the subjects were first identified with prediabetes conditions (at 3-, 6-, and 9-month visits), a number of subjects in the placebo-treated group developed T2DM: 11 subjects (9.5%) at 6 months, 18 (15.5%) at 9 months, and 19 (16.4%) at 12 months (Table 3 and Supplementary Table 1). However, none of the subjects in the curcumin-treated group developed T2DM (Table 3).
Table 3.
Number and percent of diabetic newly diagnosed subjects during following period
Adverse effects
To monitor possible adverse effects of curcumin intervention, we determined kidney and liver functions, BMD, body weights, and WCs (Supplementary Table 2 and Table 2). We found no significant differences in the means of AST, ALT, creatinine, and BMD between the curcumin-treated and placebo-treated group. In addition, during the course of our study, none of the subjects newly developed CAD or any signs of edema (data not shown). A few subjects from the curcumin-treated group reported minor symptoms such as itching (one subject), constipation (two subjects), and vertigo (one subjects). None in the curcumin-treated group showed hypoglycemia symptoms. Interestingly, at the last follow-up visit (9 months after intervention), we noticed a slight reduction of mean body weight and WC from the group of subjects treated with curcumin. We did not see such reductions in the placebo-treated group (Table 2).
Altogether, these results indicated that curcumin extract can be used for intervention, at least during a period of 9 months, without serious adverse effect.
At each follow-up visit, we counted numbers of remaining capsules brought to us by subjects. Numbers of the capsule consumed by subjects from both groups were very comparable (Supplementary Table 3). Therefore, the effects observed by us were not a result of different levels of compliance between two groups.
CONCLUSIONS
In an attempt to find safe, well-tolerated, and easily available intervention agents for the prediabetic population, we tested a potential candidate, ethanol-extracted curcumin, because of its known in vitro and in vivo antidiabetes activity (10–12,15,16). In this randomized, double-blinded, placebo-controlled clinical trial, we found that curcumin extract was able to substantially and significantly prevent T2DM development in the prediabetic population (0% of curcumin-treated subjects developed DM, whereas 16.4% of placebo-treated subjects developed DM). In addition, we found that curcumin intervention improved β-cell functions, indicated by an increased HOMA-β and reduced C-peptide. Meanwhile, although not statistically significant, curcumin intervention tended to decrease proinsulin/insulin ratio. These indicated that curcumin treatment may result in better β-cell function in the prediabetic population. HOMA-IR clinically represents IR. We found that in the curcumin-treated group, HOMA-IR was significantly lower when compared with that of the placebo group. From these results, we believe that curcumin intervention in the prediabetic population can prevent T2DM conversion and lower the IR level by maintaining healthy β-cell functions.
Adiponectin is an anti-inflammatory cytokine known to play a positive role in pathogenesis of T2DM (27,28). It has been shown that a higher adiponectin level is associated with a lower risk of T2DM (28). Our study showed that curcumin intervention significantly increases adiponectin levels. Curcumin has also been shown to reduce inflammation by downregulating other inflammatory cytokines, such as tumor necrosis factor-α, leptin, and resistin (7). In an in vivo diabetic mouse model, curcumin treatment significantly reduced macrophage infiltration of white adipose tissue and reversed many of the inflammatory derangements (8). Because inflammation is one of the main causes of β-cell degradation, we hypothesize that the anti-inflammation activity of curcumin is a key factor for the curcumin’s antidiabetic property.
Of note, we observed from our study the conversion rate of the placebo-treated group during a period of 12 months (from first screening to the end of study) to be 16.7%, which is significantly higher than that in a well-known American study (3). We reasoned that the high conversion rate may be specific to the ethnicity of the subjects. However, because of a lack of data on conversion rate in Thai prediabetes, we cannot directly verify our data. We then compared our result to a diabetes study conducted in a large Thai cohort by Aekplakorn et al. (29), the Electric Generating Authority of Thailand (EGAT) study. In this study, risk scores were developed from a Thai cohort of 2,677 individuals (29). The EGAT study identified a set of strong risk factors that accelerate the development of T2DM among the Thai population; these are old age, high BMI, high WC, hypertension, and history of diabetes in parent or sibling. We found that these factors also influenced our study (see the health parameter of the subject at the baseline in Table 1). Our subjects are mostly of old age, with high BMI (according to Asian standards), high WC, and some with hypertension and a history of diabetes in parent or sibling.
When we analyzed data based on the instruction from the EGAT study, we found that the prediabetic subjects from our study were assigned with high-risk scores (Supplementary Tables 4–6). When we followed the EGAT calculation, the estimated overall incidence rate (normalized to a period of 12 months) from our study would be 21.8% (within 12 months, an estimation of 21.8% of the prediabetic subjects would develop T2DM). We observed 16.4%, which is well within the estimation (not higher than expected).
Therefore, we believe that the high conversion rates found in the present study are a common characteristic of Thai prediabetes.
Several studies have shown that traditional Chinese herbs and dietary supplements may have potential antidiabetic activity (6,30–32). Although promising, most of these studies could not be easily interpreted, quite often because of inadequate study designs, such as lack of randomized control trials (30–32), small sample size (30–32), or lack of safety information (30–32). Our study was designed and set up specifically to overcome those previous problems. Our study showed that the curcumin extract can effectively prevent the prediabetic population from developing T2DM. Although we found that the results were quite remarkable, a longer trial may be required to see if the curcumin-treated prediabetic population will eventually develop T2DM.
We found that a 9-month treatment of curcumin was rather safe. We have not found any significant adverse effect caused by curcumin treatment when compared to the placebo treatment. Despite losing some body weight and WC, all of the subjects treated with curcumin appeared to be healthy. Because of its benefits and safety, we propose that curcumin extract may be used for an intervention therapy for the prediabetic population.
Acknowledgments
This study was supported by a grant from Thai Traditional Medical Knowledge Fund and the Department for Development of Thai Traditional and Alternative Medicine, Ministry of Public Health (to S.C.).
No potential conflicts of interest relevant to this article were reported.
S.C. designed the study, screened and examined all the recruited subjects, researched and analyzed data, and wrote and reviewed the manuscript. S.R. analyzed data and performed the statistical analysis. R.L. and C.P. provided trial advice. S.J. designed the study and wrote and reviewed the manuscript. S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the Thai Government Pharmaceutical Organization for the gift of the curcumin extract and placebo. The authors also thank all of the subjects for participating in this study and the team of the outpatient clinic at HRH Princess Maha Chakri Sirindhorn Medical Center of Srinakharinwirot University, Nakornnayok, Thailand.
Footnotes
Clinical trial reg. no. NCT01052025, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0116/-/DC1.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Hogan P, Dall T, Nikolov P, American Diabetes Association Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–932 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 2007;28:88–136 [DOI] [PubMed] [Google Scholar]
- 5.Hanley AJ, Zinman B, Sheridan P, Yusuf S, Gerstein HC, Diabetes Reduction Assessment With Ramipril and Rosiglitazone Medication (DREAM) Investigators Effect of Rosiglitazone and Ramipril on beta-cell function in people with impaired glucose tolerance or impaired fasting glucose: the DREAM trial. Diabetes Care 2010;33:608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochhar KP. Dietary spices in health and diseases (II). Indian J Physiol Pharmacol 2008;52:327–354 [PubMed] [Google Scholar]
- 7.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr 2010;30:173–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008;149:3549–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao W, Yu Z, Chiang Y, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE 2012;7:e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroda M, Mimaki Y, Nishiyama T, et al. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol Pharm Bull 2005;28:937–939 [DOI] [PubMed] [Google Scholar]
- 11.Nishiyama T, Mae T, Kishida H, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem 2005;53:959–963 [DOI] [PubMed] [Google Scholar]
- 12.Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal 2009;11:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob A, Wu R, Zhou M, Wang P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-gamma Activation. PPAR Res 2007;2007:89369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol 2008;155:702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo KI, Choi MS, Jung UJ, et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res 2008;52:995–1004 [DOI] [PubMed] [Google Scholar]
- 16.Jang EM, Choi MS, Jung UJ, et al. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism 2008;57:1576–1583 [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 19.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med 2003;9:161–168 [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome—a new worldwide definition. Lancet 2005;366:1059–1062 [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23.Tura A, Pacini G, Kautzky-Willer A, Ludvik B, Prager R, Thomaseth K. Basal and dynamic proinsulin-insulin relationship to assess beta-cell function during OGTT in metabolic disorders. Am J Physiol Endocrinol Metab 2003;285:E155–E162 [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 25.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007;9:194–205 [DOI] [PubMed] [Google Scholar]
- 26.Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials 1998;19:589–601 [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama H, Emoto M, Mori K, et al. Plasma adiponectin level is associated with insulin-stimulated nonoxidative glucose disposal. J Clin Endocrinol Metab 2006;91:290–294 [DOI] [PubMed] [Google Scholar]
- 28.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179–188 [DOI] [PubMed] [Google Scholar]
- 29.Aekplakorn W, Bunnag P, Woodward M, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care 2006;29:1872–1877 [DOI] [PubMed] [Google Scholar]
- 30.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol 2007;595:471–480 [DOI] [PubMed] [Google Scholar]
- 31.Collins M, McFarlane JR. An exploratory study into the effectiveness of a combination of traditional Chinese herbs in the management of type 2 diabetes. Diabetes Care 2006;29:945–946 [DOI] [PubMed] [Google Scholar]
- 32.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003;26:1277–1294 [DOI] [PubMed] [Google Scholar]