Abstract
OBJECTIVE
A key milestone in progress towards providing an efficacious and safe closed-loop artificial pancreas system for outpatient use is the development of fully automated, portable devices with fault detection capabilities to ensure patient safety. The ability to remotely monitor the operation of the closed-loop system would facilitate future physician-supervised home studies.
RESEARCH DESIGN AND METHODS
This study was designed to investigate the efficacy and safety of a fully automated, portable, closed-loop system. The Medtronic Portable Glucose Control System (PGCS) consists of two subcutaneous glucose sensors, a control algorithm based on proportional-integral-derivative with insulin feedback operating from a BlackBerry Storm smartphone platform, Bluetooth radiofrequency translator, and an off-the-shelf Medtronic Paradigm Veo insulin pump. Participants with type 1 diabetes using insulin pump therapy underwent two consecutive nights of in-clinic, overnight, closed-loop control after a baseline open-loop assessment.
RESULTS
Eight participants attended for 16 overnight studies. The PGCS maintained mean overnight plasma glucose levels of 6.4 ± 1.7 mmol/L (115 ± 31 mg/dL). The proportion of time with venous plasma glucose <3.9, between 3.9 and 8 (70 and 144 mg/dL), and >8 mmol/L was 7, 78, and 15%, respectively. The proportion of time the sensor glucose values were maintained between 3.9 and 8 mmol/L was greater for closed-loop than open-loop (84.5 vs. 46.7%; P < 0.0001), and time spent <3.3 mmol/L was also reduced (0.9 vs. 3%; P < 0.0001).
CONCLUSIONS
These results suggest that the PGCS, an automated closed-loop device, is safe and effective in achieving overnight glucose control in patients with type 1 diabetes.
Intensive management of type 1 diabetes is necessary to achieve near-normal glucose levels to obtain A1C values associated with a reduced risk of microvascular and macrovascular complications. Large-scale studies have revealed that in some patients, such efforts are associated with an increased risk of severe hypoglycemia (1). The effects of intensive management on the incidence of severe hypoglycemia may be even greater in children and adolescents (2), particularly in the setting of diminished counterregulatory hormone responses (3,4). Despite the development of insulin analogs and increasing use of insulin pump therapy, approximation of physiologic insulin delivery has not been achievable by most. Presently, children with an A1C <7% spend approximately one-quarter of each 24-h period with glucose levels >11.1 mmol/L (200 mg/dL) (3). Even with use of sensor-augmented pump therapy, the epitome of technology currently available to patients, one-third or less patients achieve an A1C target <7% (5,6), and the incidence of severe hypoglycemia is not reduced.
Currently, there are two principal approaches to β-cell replacement therapy. Islet-cell transplantation has demonstrated promising results in recovery of hypoglycemia awareness and reduction in episodes of hypoglycemia (7). Unfortunately, there are risks associated with immunosuppressive therapy (8), and currently, <75% of patients are insulin-independent 4 years after transplant (7). The second and, arguably, more promising therapeutic approach to β-cell replacement is a closed-loop artificial pancreas incorporating a continuous glucose sensor, insulin pump, and control algorithm.
Commercially available insulin pumps and glucose sensors are considered sufficiently accurate for use in a closed-loop system (9,10). Despite the delays inherent in absorption and action of insulin delivered subcutaneously, previous studies have demonstrated superiority of such systems over standard pump therapy (11–18). Automation of insulin delivery is not a novel concept (11,12); however, the closed-loop system in many reports was not fully automated. In some studies, sensor glucose was entered manually every 5 to 15 min (15–17) or changes to the pump delivery rate were made manually by a physician or research nurse (13–17). Furthermore, insulin delivery in studies published to date was based on a control algorithm contained in a desktop or laptop computer (11–18), implying that the system was not readily portable or practical in an ambulatory setting. A key milestone in progress toward making a closed-loop artificial pancreas system available for outpatient use is the development of fully automated, portable devices with fault detection capabilities to ensure safety. An additional desirable feature of these devices is the ability to remotely monitor the operation of the closed-loop system via data transmitted over a wireless network, facilitating future physician-supervised home studies.
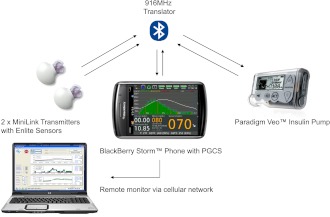
The Medtronic Portable Glucose Control System (PGCS) is a portable, automated, closed-loop device consisting of a BlackBerry Storm smartphone (Research in Motion, Waterloo, ON, Canada), an unmodified Medtronic Paradigm Veo insulin pump, two MiniLink REAL-Time Transmitters (Medtronic Minimed, Northridge, CA) modified to transmit at 1-min rather than 5-min intervals, two Enlite glucose sensors (Medtronic Minimed), and a Medtronic custom-built radiofrequency translator, as illustrated in Fig. 1.
Figure 1.
The components of the Medtronic PGCS.
In this study, we describe the safety and efficacy of the PGCS, an automated closed-loop device, focusing on overnight glucose control in adolescents and young adults with type 1 diabetes.
RESEARCH DESIGN AND METHODS
This study was designed to determine the efficacy and safety of an automated, portable, overnight closed-loop system based on fingerstick meter glucose calibration. The primary end point was the percentage of time spent in euglycemia with overnight closed-loop control (range 3.9–8 mmol/L [70–144 mg/dL]). The secondary end points were 1) comparing overnight efficacy of the portable closed-loop system versus open-loop control, as measured by sensor glucose values, and 2) evaluating the length of time under closed-loop control without investigator intervention.
Participants fulfilling the following criteria were recruited: age 12–25 years, diagnosis of type 1 diabetes for >1 year, A1C <8.5%, and insulin pump therapy use for at least 6 months. Written informed consent was obtained from subjects aged ≥18 years and written parental consent and subject assent for subjects aged <18 years. The study was approved by the human research ethics committee of Princess Margaret Hospital for Children.
Study procedures
Baseline open-loop assessment.
At the initial outpatient visit, participants underwent insertion of a subcutaneous glucose sensor and iPro2, Professional Continuous Glucose Monitoring device (Medtronic Minimed) to be worn for 6 days to establish baseline overnight glycemic levels. Participants were also given a calibrated glucometer, OneTouch Verio (LifeScan, Inc., Milpitas, CA) for the study duration. The iPro2 was returned and uploaded at the end of the 6-day period. Glucose levels from the glucometer were merged with iPro2 sensor data to obtain a retrospective 6-day continuous glucose tracing. Participants were asked to proceed with their routine diabetes care.
Overnight in-clinic closed-loop studies.
During the week after the baseline open-loop assessment, participants were admitted to the clinical research facility at 1700 h for the overnight closed-loop studies. Two subcutaneous glucose sensors were inserted into sites chosen by the participant, and an intravenous catheter was placed in an antecubital vein for blood sampling. The participant’s pump was disconnected, and the study pump (Paradigm Veo; Medtronic Minimed) containing the subject’s usual analog insulin, insulin aspart (NovoRapid; Novo Nordisk, Bagsvaerd, Denmark), or insulin lispro (Humalog; Eli Lilly, Indianapolis, IN), was attached. An evening meal was consumed at 1800 h. An insulin bolus was delivered by the participant, based on his or her usual insulin-to-carbohydrate ratio and insulin sensitivity factor. Participants were switched from open-loop to closed-loop control at 2100 h, and this was continued until 0700 h the following morning. Participants reverted to open-loop control from 0700 to 2100 h, followed by a second consecutive night of overnight closed-loop control.
Venous plasma glucose was measured every 30 min on both nights during overnight closed-loop control using the YSI 2300 STAT (Yellow Springs Instruments, Yellow Springs, OH). If the plasma glucose was <2.8 mmol/L (50 mg/dL) on two occasions 15 min apart or the plasma glucose was >15 mmol/L (270 mg/dL) for 1 h, closed-loop control was stopped and the system reverted to open-loop control. Blood samples for plasma insulin determination were collected hourly during overnight closed-loop control. Samples were assayed using a one-step chemiluminescent noncompetitive immunoassay (ARCHITECT; Abbott Diagnostics, Abbott Park, IL) with coefficients of variation of <2% for means of 17, 46, and 130 mU/L.
System considerations
Raw sensor data were transmitted to the BlackBerry by way of the radiofrequency (RF) translator module that translates the Medtronic proprietary 916-MHz RF protocol to the Bluetooth communication protocol, and vice versa. After sensor data are processed, the BlackBerry calculates pump strokes based on the closed-loop control algorithm and sends pump delivery commands over the Bluetooth interface to the translator. The translator forwards the same commands to the Veo pump over the translator’s 916-MHz interface. Insulin is delivered as a series of microboluses in multiples of 0.025 units every min.
The algorithm calculates the average of two sensor signals to determine insulin delivery. The deviation of sensor signal is continuously evaluated to ensure that both sensors track within predetermined parameters. If the sensor deviation falls outside these parameters, the fault detection will be triggered to request another blood glucose level to be entered for calibration.
Remote monitoring of the subject was accomplished by packaging real-time data into compressed files and sending them to a remote monitoring station over the wireless cellular communication network. During this study, experiments were remotely monitored in real-time in Perth, Western Australia, and in Northridge, California. Historical data were also available daily directly from BlackBerry downloads and remotely in archived form.
Control algorithm
Specifics of the control algorithm have been described in detail (12,19). Insulin delivery was modeled on the multiphasic insulin response of the β-cell and consisted of three principal components—proportional, integral, and derivative (PID)—as used in other studies (11,12), with a modification to include feedback of a model-predicted insulin profile (19). Insulin feedback (IFB) allows for more physiologic replication of a functioning β-cell, which is widely believed to reduce insulin secretion as plasma insulin levels increase (20). Further details regarding the PID algorithm with IFB are available in the Supplementary Data.
The PGCS used fault detection settings regarding 1) correlation of sensor signals, 2) deviation of sensor glucose (SG1 and SG2) from the mean (SG), 3) mutual difference in rate of glucose change (direction of trend), 4) loss of sensor signal, RF, or Bluetooth connectivity, and 5) insulin delivery limits. A system of triggers was developed to allow appropriate temporal tolerance for the faults to clear. The system required a minimum of one calibration every 12 h and could request a calibration in the event of scenario 1, 2, or 3 above. If a calibration is not entered within 15 min of the request, or both sensor signals or the Bluetooth connection are lost for 15 min, the system automatically reverts to open-loop mode. As the system reverts to open-loop mode, the pump rate is set to deliver no insulin for 2 h. This is a temporary basal rate setting before it reverts to the preprogrammed basal rate after 2 h. This will ensure that a hypoglycemic patient will not receive further basal insulin for 2 h.
Statistics
Depending on distribution, data are expressed as mean ± SD or as median and range or interquartile range (25th–75th centile), where appropriate. Comparisons between overnight closed-loop and open-loop control were made using a Mann-Whitney U test for nonparametric data and a Student t test for data conforming to a normal distribution. Distribution of sensor glucose values was compared using χ2 tests. Calculations used StatsDirect 2.7.2 software (StatsDirect Ltd, Cheshire, U.K.).
RESULTS
Eight subjects (four males) underwent 145 h of closed-loop control over 16 nights. Median age was 14.8 years (range 12.6–24), duration of diabetes was 6.9 years (range 3.8–21.5), duration of pump therapy was 3.8 years (range 2.6–10.8), and BMI was 21 kg/m2 (range 18–30). Mean A1C was 7.3 ± 1.1%, and total daily dose of insulin was 0.9 ± 0.1 units/kg. All participants were negative for C-peptide.
Sensor performance
Interstitial glucose values tracked venous plasma glucose levels effectively during overnight closed-loop control. Point accuracy of the sensor, expressed as median (interquartile range) and mean ± SD relative absolute difference from the venous plasma glucose were 7.5% (3.3–18.5) and 12.3 ± 12.7%, respectively, similar to previously published studies (9,10,12,15) and in keeping with the published accuracy of this sensor (21).
Glycemic control
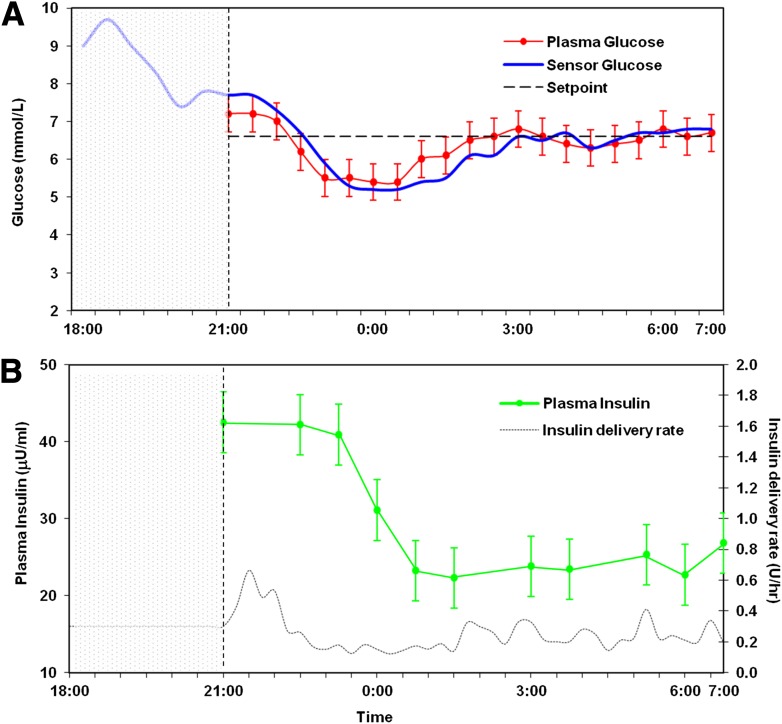
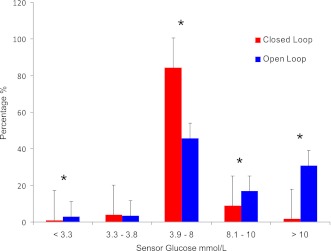
Mean plasma glucose during overnight closed-loop control was 6.4 ± 1.7 mmol/L (115 ± 31 mg/dL). The proportion of time with venous plasma glucose <3.9, between 3.9 and 8 (70 and 144 mg/dL), and >8 mmol/L was 7, 78, and 15% respectively. Time in target during closed-loop operation (3.9–8.0 mmol/L) was significantly higher after midnight than before midnight (85 vs. 66%; P = 0.001). Plasma glucose <3.3 mmol/L occurred in 3 of 16 study nights and within the first 3.5 h. Plasma glucose readings <3.9 mmol/L were significantly more common in the first 3 h of closed-loop control than thereafter (13.9 vs. 4%; P = 0.005). Sensor and venous plasma glucose levels during closed-loop control are shown in Fig. 2A and insulin delivery rates and hourly plasma insulin in Fig. 2B. Glycemic control during closed-loop insulin delivery was superior to prestudy home overnight open-loop control (Fig. 3).
Figure 2.
Summary of the overnight closed-loop experiment in the eight participants. The open-loop period is represented by the shaded area. An evening meal and prandial bolus were administered at 1800, and the closed-loop mode commenced at 2100. A: Sensor glucose (blue) closely tracks plasma glucose (red). B: Pump delivery rates are represented as a black dashed line, and hourly plasma insulin measurements are represented in green.
Figure 3.
Comparison of overnight closed-loop (red) with prestudy open-loop (blue) control.*P < 0.0001.
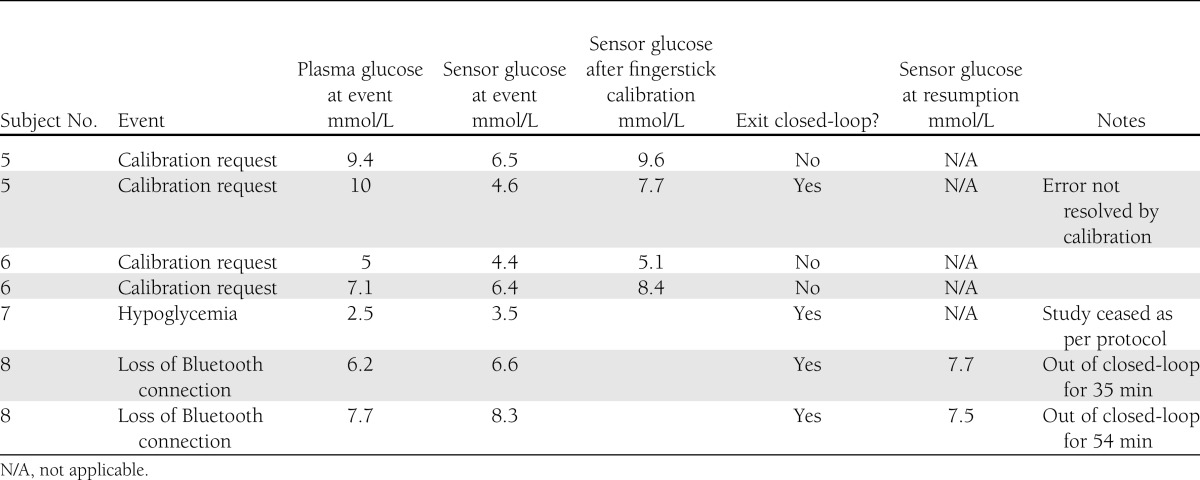
Investigator intervention
Investigator intervention was required on 7 of 16 nights (Table 1). No intervention was required for the first four subjects. Sensor fault detection requested fingerstick calibration on both closed-loop nights for two subjects. These subjects had the greatest relative-absolute difference between sensor glucose and plasma glucose, and between them accounted for 46% of plasma glucose levels out of target range for the entire study. The sensors were inserted in the left and right abdomen in subject 5 and in the left and right buttocks in subject 6. These subjects had similar BMIs, 22 and 26 kg/m2, respectively; thus, patient phenotype did not explain the difference between sensor and plasma glucose.
Table 1.
Investigator intervention during automated, portable, closed-loop control
For one further subject (2 nights), the Bluetooth link was lost for >15 min and needed to be re-established. The loss of Bluetooth connectivity was attributed to the smartphone Bluetooth interface. One patient (age 24 years, weight 94 kg, total daily dose 105 units) required rescue carbohydrates for plasma glucose 2.5 mmol/L (sensor 3.5 mmol/L). He commenced closed-loop 3 h after an evening meal where he gave himself a preprandial bolus of 22.7 units but did not finish the meal. The plasma glucose was 3.8 mmol/L (sensor glucose 4.7 mmol/L) at commencement of closed-loop control. His usual basal rate was 2.7 units/h, and during closed-loop, he received only 0.67 units of insulin over 3 h before the study night was terminated, as per protocol.
CONCLUSIONS
This study demonstrates the feasibility and safety features of a portable, automated, closed-loop system for overnight glucose control in adolescents and young adults with type 1 diabetes. This represents a further step toward making such technology available to patients in the outpatient setting. Monitoring of system operation from a remote location using data transmission over a wireless network is an achievable goal and is an additional safety feature, paving the way for physician-supervised home studies. Automated fault detection requested fingerstick calibration of the system when sensor correlation was below a specified threshold or sensor glucose values deviated significantly from the mean. Further study is necessary to determine the optimum fault detection settings to maximize patient safety yet minimize disruption to the operation of the closed-loop system.
Initial commercially available fully closed-loop systems will likely focus on overnight glucose control to minimize nocturnal hypoglycemia. The effects of intensive management of type 1 diabetes on the incidence of hypoglycemia are well documented. In the Diabetes Control and Complications Trial (1), 55% of severe hypoglycemia occurred during sleep, and in the pediatric population, 75% of episodes of severe hypoglycemia occurred overnight (22). Although arousal thresholds are not impaired by hypoglycemia (23), 71% of low-glucose alarms are not responded to during sleep (24). An overnight closed-loop system that automatically suspends insulin delivery in response to impending hypoglycemia would minimize exposure to prolonged hypoglycemia and adverse outcomes such as seizures (25). In our study, although plasma glucose <3.3 mmol/L (60 mg/dL) occurred in 3 of 16 study nights, the sensor readings indicated that hypoglycemia was significantly less common during closed-loop compared with open-loop therapy. Episodes of hypoglycemia during closed-loop therapy occurred during the first 3.5 h and likely related to “insulin on board” from earlier open-loop therapy. Development of a “start-up algorithm” is necessary to smooth the transition between open-loop and closed-loop sessions and account for insulin delivered before initiation of the closed-loop program to further reduce exposure to hypoglycemia.
The other main concern regarding nocturnal hypoglycemia pertains to the use of glucose sensors that may overread the true plasma glucose value, leading to an overestimation of insulin requirements. In this series of experiments, as previously (12), the overnight glucose target was set at 6.6 mmol/L (120 mg/dL) to provide a margin of error. The median error of currently available real-time continuous glucose monitors is between 10 and 16% when plasma glucose levels are between 70 and 180 mg/dL (9,10,26,27). A sensor error overreading the plasma glucose by 33% would drive the true glucose to only 90 mg/dL. Furthermore, the Veo calibration algorithm was recently compared with that in the Paradigm REAL-time (PRT) sensor-augmented pump (28). Sensor error reduced significantly in the 40–120 mg/dL range, and hypoglycemia detection was improved from 55% in the PRT to 82% in the Veo, while retaining accuracy at higher glucose levels.
This study represents progress toward the development of an automated, portable, closed-loop system for outpatient use. The associated remote monitoring capabilities will facilitate future physician-supervised home studies. Further research is needed to optimize automated fault detection settings and to ensure current mechanisms are effective. In the current study, fault detection settings successfully identified sensor failure. Despite the limitations of current transcutaneous sensors, this portable glucose control system reduced exposure to hypoglycemia and hyperglycemia in adolescents and young adults with type 1 diabetes. Future developments of the system will include a smartphone with more robust Bluetooth connectivity. The combination sensor-infusion set requiring only one insertion site is currently undergoing clinical trials and will also be incorporated into this system to minimize insertion sites for the patient.
Acknowledgments
Closed-loop systems, insulin pumps, and glucose sensors were provided by Medtronic Diabetes via an unrestricted grant. T.T.L. is supported by Juvenile Diabetes Research Foundation post-doctoral fellowship.
D.B.K., N.K., M.C., G.S., M.N.K., and A.R. are employees of Medtronic Minimed and are Medtronic shareholders. T.T.L. received travel reimbursement from Medtronic. T.W.J. received honoraria for scientific lectures and travel reimbursement from Medtronic, sanofi-aventis, Eli Lilly, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
M.J.O. researched data, contributed to discussion, and wrote the manuscript. A.J.R. researched data and reviewed the manuscript. D.B.K., N.K., A.R., E.A.D., T.W.J. contributed to study design and discussion and reviewed the manuscript. M.C. and G.S. researched data and contributed to study design and discussion. M.N.K. researched data, contributed to discussion, and reviewed the manuscript. T.T.L. researched data, contributed to study design and discussion, and reviewed the manuscript. T.W.J. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Johnson & Johnson Australia for provision of glucometers for this study and the participants and families for taking part in this study.
Parts of this work were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0761/-/DC1.
References
- 1.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 2.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care 2004;27:2293–2298 [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Research in Children Network (DirecNet) Study Group, Buckingham B, Beck RW, Tamborlane WV, et al. Continuous glucose monitoring in children with type 1 diabetes. J Pediatr 2007;151:388–393, 393.e1-2.
- 4.Ly TT, Gallego PH, Davis EA, Jones TW. Impaired awareness of hypoglycemia in a population-based sample of children and adolescents with type 1 diabetes. Diabetes Care 2009;32:1802–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 6.Hermanides J, Nørgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med 2011;28:1158–1167 [DOI] [PubMed] [Google Scholar]
- 7.Alejandro R, Barton FB, Hering BJ, Wease S, Collaborative Islet Transplant Registry Investigators 2008 Update from the Collaborative Islet Transplant Registry. Transplantation 2008;86:1783–1788 [DOI] [PubMed] [Google Scholar]
- 8.Azzi J, Geara AS, El-Sayegh S, Abdi R. Immunological aspects of pancreatic islet cell transplantation. Expert Rev Clin Immunol 2010;6:111–124 [DOI] [PubMed] [Google Scholar]
- 9.Tansey MJ, Beck RW, Buckingham BA, et al. Diabetes Research in Children Network (DirecNet) Study Group Accuracy of the modified Continuous Glucose Monitoring System (CGMS) sensor in an outpatient setting: results from a diabetes research in children network (DirecNet) study. Diabetes Technol Ther 2005;7:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Research In Children Network (DirecNet) Study Group. Buckingham BA, Kollman C, Beck R, et al. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther 2006;8:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 12.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 13.Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Tech 2009;3:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruttomesso D, Farret A, Costa S, et al. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: preliminary studies in Padova and Montpellier. J Diabetes Sci Tech 2009;3:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 16.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care 2010;33:1072–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed-loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elleri D, Allen JM, Nodale M, et al. Automated overnight closed-loop glucose control in young children with type 1 diabetes. Diabetes Technol Ther 2011;13:419–424 [DOI] [PubMed] [Google Scholar]
- 19.Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab 2011;96:1402–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argoud GM, Schade DS, Eaton RP. Insulin suppresses its own secretion in vivo. Diabetes 1987;36:959–962 [DOI] [PubMed] [Google Scholar]
- 21.Keenan DB, Mastrototaro JJ, Zisser H, et al. Accuracy of the Enlite 6-day glucose sensor with guardian and Veo calibration algorithms. Diabetes Technol Ther 2012;14:225–231 [DOI] [PubMed] [Google Scholar]
- 22.Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care 1997;20:22–25 [DOI] [PubMed] [Google Scholar]
- 23.Ly TT, Jones TW, Griffiths A, et al. Hypoglycemia does not change the threshold for arousal from sleep in adolescents with type 1 diabetes. Diabetes Technol Ther 2012;14:101–104 [DOI] [PubMed] [Google Scholar]
- 24.Buckingham B, Block J, Burdick J, et al. Diabetes Research in Children Network Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther 2005;7:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buckingham B, Wilson DM, Lecher T, Hanas R, Kaiserman K, Cameron F. Duration of nocturnal hypoglycemia before seizures. Diabetes Care 2008;31:2110–2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care 2006;29:44–50 [DOI] [PubMed] [Google Scholar]
- 27.Wilson DM, Beck RW, Tamborlane WV, et al. DirecNet Study Group The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care 2007;30:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keenan DB, Cartaya R, Mastrototaro JJ. Accuracy of a new real-time continuous glucose monitoring algorithm. J Diabetes Sci Tech 2010;4:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]