Abstract
OBJECTIVE
Nonalcoholic fatty liver disease (NAFLD) coexists with insulin resistance (IR), but it is uncertain whether NAFLD and IR contribute independently to atherosclerosis. We tested whether fatty liver, IR, and metabolic syndrome (MetS) features (waist, glucose, triglyceride, HDL cholesterol [HDL-C], and blood pressure) were associated with a marker of atherosclerosis (coronary artery calcium [CAC] score >0), independently of cardiovascular risk factors and cardiovascular disease (CVD).
RESEARCH DESIGN AND METHODS
Data were analyzed from a South Korean occupational cohort of 10,153 people who all received ultrasound measurements of fatty liver and a cardiac computed tomography CAC score. IR was defined by homeostasis model assessment of IR (HOMA-IR) ≥75th percentile. Odds ratios (ORs) (95% CIs) for the presence of a CAC score >0 were estimated using logistic regression.
RESULTS
There were 915 people with a CAC score >0. MetS features were increased (glucose, blood pressure, triglyceride, and waist) or decreased (HDL-C) among people with a CAC score >0 (all comparisons against CAC score ≤0; P < 0.0001). Of subjects with a CAC score >0, 55% had fatty liver and 33.7% were insulin resistant. Fatty liver (OR 1.21 [95% CI 1.01–1.45]; P = 0.04) and HOMA-IR (1.10 [1.02–1.18]; P = 0.02) were associated with CAC score >0, independently of all MetS features, conventional cardiovascular risk factors, and prior evidence of CVD. The presence of IR and fatty liver combined was associated with CAC score >0 (1.53 [1.20–1.95]; P = 0.001).
CONCLUSIONS
Fatty liver and HOMA-IR are both associated with a CAC score >0 (independently of each other), features of MetS, conventional cardiovascular risk factors, and existing CVD.
Nonalcoholic fatty liver disease (NAFLD) often coexists with type 2 diabetes and insulin resistance (IR) but each factor may occur in the absence of the other factors. Several prospective studies have reported an increased incidence of cardiovascular events in people with NAFLD (1–12), but it is still unclear whether NAFLD is simply a risk marker that coexists with other recognized cardiovascular disease (CVD) risk factors, such as type 2 diabetes and central obesity, that are associated with IR or whether it is an independent cardiovascular risk factor (13,14). IR has been shown to be associated with coronary artery calcium (CAC) (15) as a marker of early atherosclerosis, but in this study, IR was not associated with CAC, after adjustment for metabolic syndrome (MetS) features, and the presence of NAFLD was not described in this cohort.
NAFLD is now recognized as a very common condition in people with type 2 diabetes and, if it were proven that NAFLD is an independent CVD risk factor, independently of type 2 diabetes and IR, a diagnosis of NAFLD could be used to identify subgroups of individuals who would receive particular benefit from intensive lifestyle modification and pharmacological treatment to decrease CVD risk (16,17). Moreover future treatments for NAFLD would need to test whether treating fatty liver specifically affects risk of CVD.
CAC scoring with cardiac computed tomography (CT) is a sensitive method to demonstrate the presence of early atherosclerosis, and the use of CAC scores may improve CV risk prediction in asymptomatic individuals (18). Thus, estimation of the CAC score provides a useful, noninvasive tool to investigate relationships between NAFLD, IR, and development of early coronary artery atherosclerosis in order to better understand the relationship between NAFLD, IR, and atherosclerosis.
Using data from a retrospective cohort study with measurements of fatty liver and CAC score, together with measurement of BMI, waist circumference, features of MetS, IR, conventional cardiovascular risk factors, and diabetes status in all subjects, we have investigated the relationship between fatty liver, IR, and a CAC score >0. We tested whether 1) fatty liver, IR, and conventional, easily measured features of MetS (waist, glucose, triglyceride, HDL cholesterol [HDL-C], and blood pressure) were associated with a CAC score >0 (as a marker of the presence of early atherosclerosis) and 2) any relationships were independent of conventional cardiovascular risk factors and existing vascular disease.
RESEARCH DESIGN AND METHODS
The study population consisted of individuals who had a comprehensive health examination in 2010 at Kangbuk Samsung Hospital. In South Korea, employees are required to participate in annual or biennial health examinations by the Industrial Safety and Health Law. Some subjects voluntarily pay for these health checks, and in other instances, the individual’s employer pays for the health check. Health checks include blood tests, anthropometry, and abdominal ultrasound examination, and, in some cases, the health check can include CAC scoring by CT. The CAC test is offered as part of the health check program and therefore there is no medical indication for performing the test. MetS (defined by the 2009 joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention criteria, with waist circumference thresholds that are specific for Asian populations, ≥90 cm for men and ≥80 cm for women) (19). Initially 14,976 participants underwent coronary CT scanning to establish a CAC score, and individuals were excluded from the current analyses if data were missing for the following variables: alcohol consumption (n = 151), smoking (n = 178), exercise (n = 146), BMI (n = 41), waist circumference (n = 42), hepatitis B surface antigen (HBsAg) (n = 132), and hepatitis C virus (HCV) Ab (n = 133). Individuals were also excluded if HBsAg testing was positive (n = 571), HCV Ab testing was positive (n = 34), or daily alcohol intake was >20 g. Data from 10,153 participants were analyzed. The study was approved by the institutional review board at Kangbuk Samsung Hospital, and the need for specific informed consent was waived by the review board because personal identifying information was not accessed.
Measurements and calculations
The health examination included a collection of full medical histories, physical examinations, and blood samples. Waist circumference was measured according to a standardized operating procedure. In brief, in the midaxilliary line, the midpoint between the lowest rib and the superior iliac crest was identified. At this point, a measuring tape (SECA 200, circumference measuring tape) was placed around the abdomen, ensuring that the tape was horizontal to the floor. A measurement was taken to the nearest 0.1 cm, at the end of a normal expiration. If the two readings varied by >1%, there was a computer-generated prompt to take a third reading. BMI was calculated as weight in kilograms divided by height in meters squared. Questionnaires were used to ascertain information regarding alcohol consumption (g/day), smoking (never, ex, current), and frequency of moderate activity each week. Moderate activity was defined as >30 min activity per day that induced slight breathlessness. The homeostasis model assessment of IR (HOMA-IR) index was calculated by the following equation: HOMA-IR = (fasting insulin [μIU/mL] × fasting glucose [mmol/L])/22.5. Since there are no population-specific thresholds to indicate IR in a Korean population, we stratified the populations using the 75th percentile to establish an IR group (HOMA-IR ≥75th percentile = IR), as described previously in this population (20); the European Group for the Study of Insulin Resistance has previously recommended that the top quartile of fasting insulin concentrations be used to define an insulin-resistant group in normal populations (21).
Blood samples for laboratory tests were collected after an overnight fast. Fasting plasma glucose, total cholesterol, triglyceride, LDL cholesterol (LDL-C), and HDL-C concentrations were measured using Bayer Reagent Packs on an automated chemistry analyzer (Advia 1650 Autoanalyzer; Bayer Diagnostics, Leverkusen, Germany). Abdominal ultrasonography (Logic Q700 MR; GE Healthcare, Milwaukee, WI) was performed in all subjects by experienced clinical radiologists, and fatty liver was diagnosed based on known standard criteria, including hepatorenal echo contrast, liver brightness, and vascular blurring using a 3.5-MHz probe (22).
CT scans were performed with a 64-slice MDCT scanner (Lightspeed VCT XTe-64 slice; GE Healthcare), and a standard scanning protocol was 32 × 0.625-mm section collimation, 400-ms rotation time, 120-kV tube voltage, and 31-mAS (310 mA × 0.1 s) tube current under electrocardiographic-gated dose modulation. The quantitative CAC score was calculated according to the method described by Agatston et al. (23). The presence of CAC was defined by a CAC score >0.
Statistical analysis
Statistical analysis of the data was performed using SPSS version 15.0 (SPSS, Point Richmond, CA). Continuous variables were expressed as mean ± SD for normally distributed variables or median (interquartile range) if not normally distributed. Comparisons between groups with CAC score = 0 and >0 were undertaken both with and without stratification by sex and quartiles of waist circumference. Categorical variables were expressed as percentages and compared between groups using the χ2 test. We used logistic regression analysis to determine the odds ratio (OR) of presence of coronary calcium associated with fatty liver, HOMA-IR (as a continuous variable), and IR (as a categorical variable, defined by identifying people in the top quartile of HOMA-IR).
Adjustments were also made for the following variables: age, sex, smoking status (never, ex, or current), frequency of moderate activity per week, alcohol consumption (units/day), and past history of cerebrovascular accident (CVA), coronary heart disease (CHD), diabetes, and hypertension diagnosed by questionnaire. P values <0.05 were considered to be statistically significant.
The association (OR ± 95% CIs) between fatty liver and CAC >0 score was investigated in various subgroups (men vs. women; younger vs. older according to median age [≤41 vs. >41 years]; low vs. high waist circumference [waist circumference thresholds of </≥90 cm for men, and </≥80 cm for women]; no MetS vs. MetS; and not insulin resistant vs. insulin resistant) in multivariable regression analyses.
RESULTS
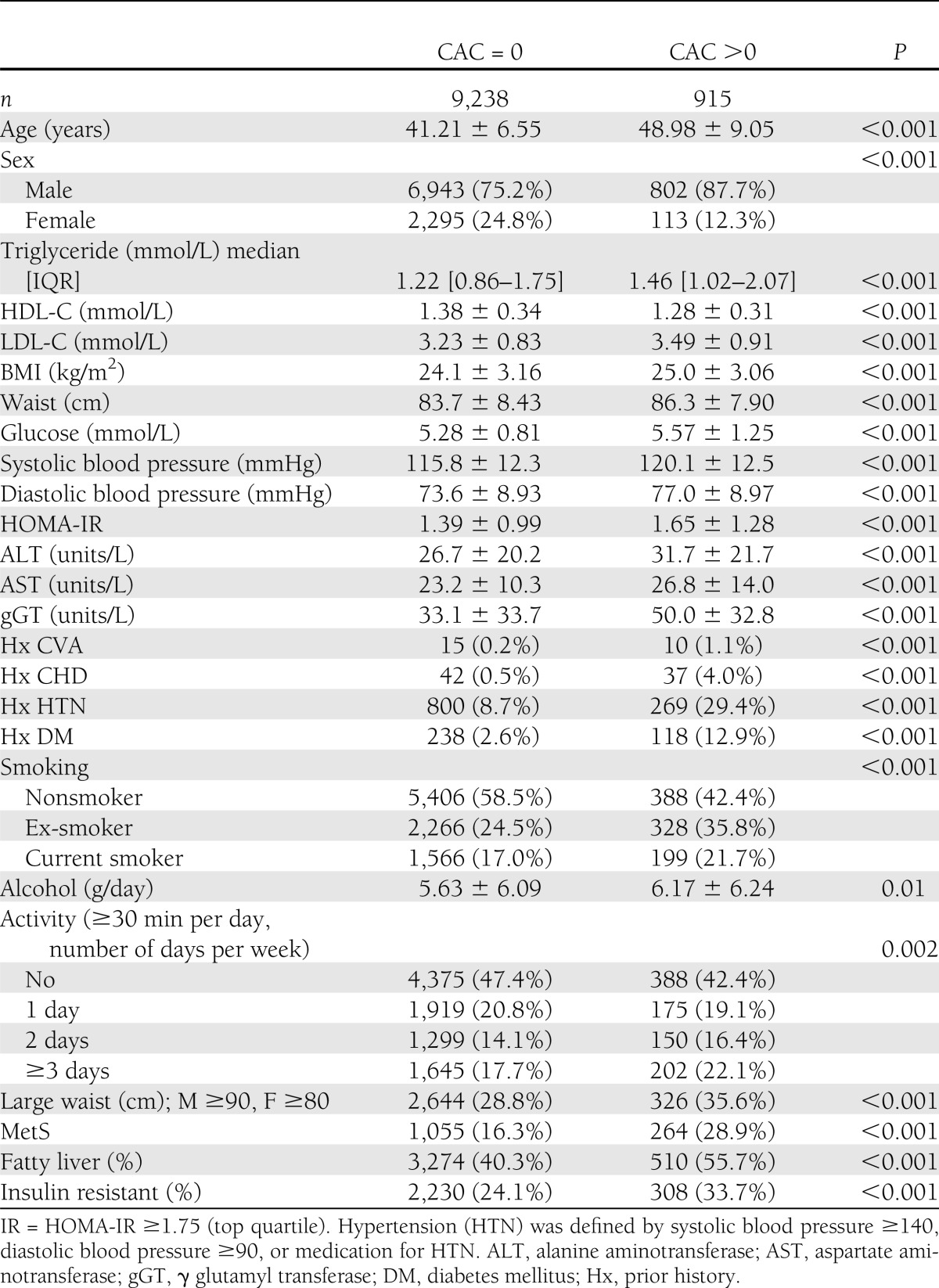
Table 1 shows the characteristics of the 915 people who had a CAC score >0 compared with those with a CAC score = 0. The proportion of people with fatty liver, diabetes, and MetS was higher in people with a CAC score >0. Levels of components of MetS (24) (glucose, blood pressure, triglyceride, and waist circumference) were higher, or lower (HDL-C), in people with a CAC score >0. HOMA-IR, and the proportion of people with IR, was increased in people with a CAC score >0.
Table 1.
Characteristics of 10,153 individuals according to CAC score = 0 and CAC score >0
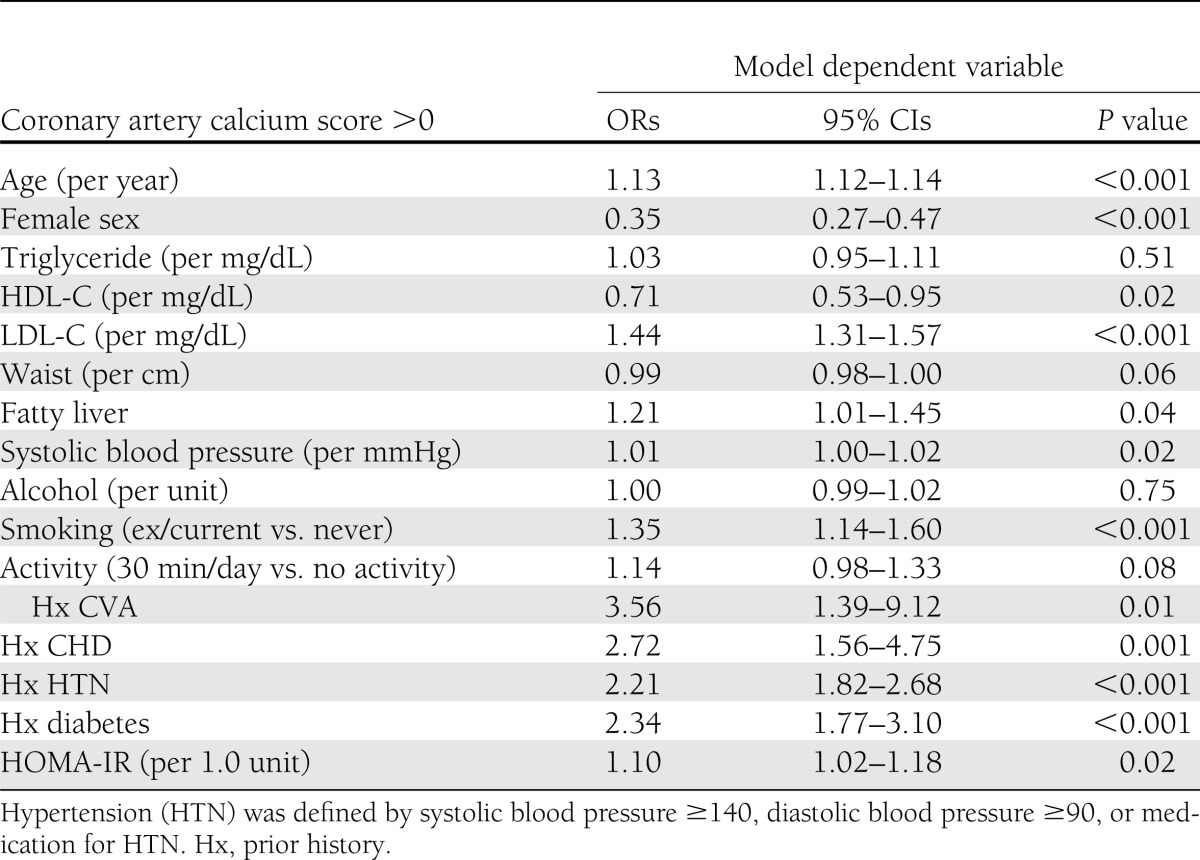
The results of multivariable regression models including all risk factors with CAC score >0 as the outcome are shown in Table 2. Diabetes, HOMA-IR (as a continuous variable), and fatty liver were all independently associated with CAC score >0 in addition to older age, smoking, increased LDL-C concentrations, and male sex. For MetS variables, triglyceride was not associated with CAC score >0, whereas significant associations were observed with HDL-C and systolic blood pressure, and there was a borderline significant association observed with waist circumference (P = 0.06).
Table 2.
Associations between fatty liver, cardiometabolic risk factors, and CAC score >0; derived from a multivariable logistic regression model containing all variables
Since fatty liver is strongly associated with IR, we identified people in the top quartile of HOMA-IR (≥1.75) to establish an IR group. The prevalence of CAC score >0 in four groups, 1) people with neither fatty liver nor IR, 2) people with fatty liver alone, 3) people with IR alone, and 4) people with both fatty liver and IR, is described in Table 3, which also demonstrates the prevalence of the combination of risk factors. Of all people with fatty liver, 44% were in the top quartile of HOMA-IR, and 74% of people in the top quartile of HOMA-IR had fatty liver.
Table 3.
Number and percentage of subjects with a CAC score >0% according to the presence of fatty liver and/or IR
To test the association between IR and CAC score >0, we repeated the regression model shown in Table 2, replacing HOMA-IR (as a continuous variable) with IR as a categorical variable defined as above. In this regression model, the relationships between both fatty liver and IR with CAC score >0 were similar compared with the data in Table 2: fatty liver, OR 1.20 (95% CI 1.00–1.44), P = 0.049; IR, 1.29 (1.07–1.55), P = 0.009. When fatty liver and HOMA-IR were omitted from this regression model, the goodness of fit of the multivariable model was improved significantly by the addition of fatty liver alone (χ2 = 5.9 on one degree of freedom; P = 0.015), IR alone (χ2 = 7.4 on one degree of freedom; P = 0.007), or both fatty liver and IR together (χ2 = 11.7 on two degrees of freedom; P = 0.009).
We further investigated the association between fatty liver and CAC >0 score in various subgroups (men vs. women; younger vs. older; low vs. high waist circumference; no MetS vs. MetS; and not insulin resistant vs. insulin resistant) (Fig. 1). There was no evidence of marked heterogeneity between strata, but there was limited power to detect interactions. However, there was a suggestion that the association between fatty liver and CAC >0 score may be stronger in women than men and in people without IR or MetS.
Figure 1.
Associations between fatty liver and CAC score >0 according to group. The association (OR ± 95% CIs) between fatty liver and CAC >0 score in various subgroups from stratified multivariable regression analyses including variables listed in Table 2.
In a further regression model, we investigated the effect of fatty liver alone, IR alone, and fatty liver and IR combined, including these groups and all other risk factors described in Table 3, in order to test the effect of combining fatty liver and IR on the OR for the association with CAC score >0. Fatty liver alone (OR 1.26 [95% CI 1.03–1.54]; P = 0.021) and IR alone (1.50 [1.09–2.08]; P = 0.013) were independently associated with CAC score >0. Importantly, the combination of fatty liver and IR showed a similar association with CAC score >0 (1.53 [1.20–1.95]; P = 0.001) to that observed with either factor alone.
Sensitivity analyses investigating the associations with higher levels of CAC showed similar point estimates but wider confidence intervals as a consequence of there being only 451 people with CAC >20 and 171 people with CAC >100 (for CAC >20: OR 1.221 [95% CI 0.955–1.560] for NAFLD and 1.094 (0.994–1.204) for IR; for CAC>100: 1.114 [0.751–1.651] for NAFLD and 1.201 [1.062–1.357] for IR).
CONCLUSIONS
Our results show for the first time that fatty liver, IR, and diabetes were all associated with a CAC score >0, independently of each other and MetS components. These results also show, importantly, that combining fatty liver and IR was not associated with a marked increase in OR for the association of both risk factors together with CAC >0 score. In contrast, we have shown in this cohort that combining fatty liver and IR was associated with a considerable increase in the OR for incident diabetes (20), suggesting that the relationship between fatty liver, IR, and diabetes is different from the relationship between fatty liver, IR, and CAC score as a marker of early atherosclerosis.
Our novel data were obtained from the retrospective analysis of data from a relatively healthy, young, occupational cohort, and the association between both fatty liver and HOMA-IR as a continuous variable (or IR as a categorical variable) and CAC score >0 persisted after adjustment for features of MetS and pre-existing vascular disease. These data show that the association between both fatty liver and HOMA-IR (or IR) and a CAC score >0 was independent of the presence of diabetes, emphasizing that diabetes is unlikely to mediate any relationship between NAFLD, or IR, and atherosclerosis. Sensitivity analyses investigating the associations with higher levels of CAC showed similar point estimates. Coefficients of variation for measurement of fatty liver and CAC within this cohort are not available, and it is possible that random misclassification bias may have underestimated the strength of the association.
To date, there are no similar studies that have investigated relationships between a marker of early development of coronary artery atherosclerosis and fatty liver, IR, and MetS features in large, predominantly healthy, middle-aged cohorts. Some previous studies have shown associations between increases in liver enzymes and the presence of CAC (25), or between liver fat, measured by ultrasound or CT, and CAC (26–28). In contrast, other investigators have failed to find independent associations between measures of liver fat and CAC (29,30). This study extends previous work by including people without diabetes and including assessment of the effects of both IR and fatty liver as a marker of NAFLD.
The strong associations between NAFLD and multiple complex metabolic and proinflammatory changes that have an effect on the vasculature (13,14) mean that it is very difficult to identify causality in assessing the relationship between NAFLD, IR, and CVD. It is plausible that a predisposition toward NAFLD could reflect the presence of particularly marked IR and that the development and progression of the liver disease per se (with increasing inflammation and fibrosis) could further worsen IR and thereby increase CVD risk. Some of the discrepant results between studies investigating relationships between NAFLD and CAC score could reflect varying severity of NAFLD both between subjects within studies and between studies. Nonalcoholic steatohepatitis (NASH) is a more severe form of NAFLD, and NASH is more strongly associated with CVD and IR than simple steatosis (10,16). The hepatic inflammation that occurs with NASH is marked by macrophage activation (31), and it is possible that vascular inflammation is also more marked with NASH (and increased IR), compared with simple steatosis. Consequently, it seems likely that altered liver fat metabolism, insulin sensitivity, and some component of the inflammatory process are the critical factors contributing to vascular disease in NAFLD (14). Thus, when considering the strength of the relationship between NAFLD and risk of CVD in individual patients, we suggest that the clinician needs to consider not just the severity of the liver disease but also markers of IR and inflammation.
There are a few limitations to our study. Fatty liver was assessed by liver ultrasound, and ultrasonography has limited sensitivity, being unable to detect liver fat infiltration that is <30% by liver weight. Ultrasonography was performed by experienced clinical radiologists who diagnosed fatty liver based on known standard clinical criteria that included hepatorenal echo contrast, liver brightness, and vascular blurring. We are therefore unable to include evidence of agreement between radiologists. However, in the presented analyses, we used the clinical definition of fatty liver as a dichotomous exposure variable. It is unlikely that fatty liver status would have been influenced by CAC score, and consequently any random misclassification bias of fatty liver status would bias our finding (showing the association between fatty liver and CAC score >0) toward the null. Although magnetic resonance spectroscopy may detect as little as 5% liver fat, undertaking this diagnostic test was not feasible in our large cohort. We are also unable to comment on NAFLD severity in this study because liver biopsy and histological assessment using the Kleiner score (32), which is the gold standard for assessing hepatic inflammation and fibrosis, were not performed. Consequently, we are unable to examine whether the more severe forms of NAFLD, such as NASH with fibrosis, are associated with higher CAC scores. There is no established definition of IR as a categorical variable, and the use of a particular centile or value defined from receiver operating curves provides an arbitrary, population-specific cut point. The validity of the cut point used for these analyses could be explored in other populations. Additionally, our study is limited to one ethnic group, and the distribution of risk factors and the association between NAFLD and CAC may differ by ethnic group.
In conclusion, we have shown that both fatty liver and IR (measured by continuous or categorical variables) are independently associated with the presence of CAC, a marker of preclinical atherosclerosis. These associations are independent of diabetes, MetS features, other conventional cardiovascular risk factors, and pre-existing CVD.
Acknowledgments
This study was partially supported by Samsung Biomedical Research Institute Grant SBRI C-B1-114-1. C.D.B. is supported in part by the Southampton National Institute for Health Research Biomedical Research Unit in Nutrition, Lifestyle, and Obesity.
No potential conflicts of interest relevant to this article were reported.
K.-C.S., S.H.W., H.J.K., and C.D.B. wrote the manuscript. All authors read and approved of the manuscript as written. K.C.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Clive Osmond (University of Southampton) for statistical advice.
References
- 1.Yun KE, Shin CY, Yoon YS, Park HS. Elevated alanine aminotransferase levels predict mortality from cardiovascular disease and diabetes in Koreans. Atherosclerosis 2009;205:533–537 [DOI] [PubMed] [Google Scholar]
- 2.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007;30:2119–2121 [DOI] [PubMed] [Google Scholar]
- 3.Söderberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010;51:595–602 [DOI] [PubMed] [Google Scholar]
- 4.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis 2007;191:391–396 [DOI] [PubMed] [Google Scholar]
- 5.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H, Vorarlberg Health Monitoring and Promotion Program Study Group Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005;112:2130–2137 [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Silventoinen K, Hu G, et al. Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28,838 middle-aged men and women. Eur Heart J 2006;27:2170–2176 [DOI] [PubMed] [Google Scholar]
- 7.Haring R, Wallaschofski H, Nauck M, Dörr M, Baumeister SE, Völzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology 2009;50:1403–1411 [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007;13:1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol 2007;27:2729–2735 [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–873 [DOI] [PubMed] [Google Scholar]
- 11.Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113–121 [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–1350 [DOI] [PubMed] [Google Scholar]
- 14.Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J 2012;33:1190–1200 [DOI] [PubMed] [Google Scholar]
- 15.Blaha MJ, DeFilippis AP, Rivera JJ, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2011;34:749–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond) 2009;116:539–564 [DOI] [PubMed] [Google Scholar]
- 17.Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab 2009;11:188–195 [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Achenbach S, Blumenthal RS, et al. American Heart Association Committee on Cardiovascular Imaging and Intervention. American Heart Association Council on Cardiovascular Radiology and Intervention. American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114:1761–1791 [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 20.Sung KC, Jeong WS, Wild SH, Byrne CD. Combined influence of insulin resistance, overweight/obesity, and fatty liver as risk factors for type 2 diabetes. Diabetes Care 2012;35:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balkau B, Charles MA, European Group for the Study of Insulin Resistance (EGIR) Comment on the provisional report from the WHO consultation. Diabet Med 1999;16:442–443 [DOI] [PubMed] [Google Scholar]
- 22.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832 [DOI] [PubMed] [Google Scholar]
- 24.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb 2005;12:295–300 [DOI] [PubMed] [Google Scholar]
- 25.Jung DH, Lee YJ, Ahn HY, Shim JY, Lee HR. Relationship of hepatic steatosis and alanine aminotransferase with coronary calcification. Clin Chem Lab Med 2010;48:1829–1834 [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Nien CK, Yang CC, Yeh YH. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci 2010;55:1752–1760 [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 23 January 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology 2010;254:393–400 [DOI] [PubMed] [Google Scholar]
- 29.Khashper A, Gaspar T, Azencot M, et al. Visceral abdominal adipose tissue and coronary atherosclerosis in asymptomatic diabetics. Int J Cardiol 2 June 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.McKimmie RL, Daniel KR, Carr JJ, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol 2008;103:3029–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tailleux A, Wouters K, Staels B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta 2012;1821:809–818 [DOI] [PubMed] [Google Scholar]
- 32.Kleiner DE, Brunt EM, Van Natta M, et al. Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321 [DOI] [PubMed] [Google Scholar]