Abstract
OBJECTIVE
Remogliflozin etabonate (RE), an inhibitor of the sodium-glucose transporter 2, improves glucose profiles in type 2 diabetes. This study assessed safety, tolerability, pharmacokinetics, and pharmacodynamics of RE in subjects with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Ten subjects managed with continuous subcutaneous insulin infusion were enrolled. In addition to basal insulin, subjects received five randomized treatments: placebo, prandial insulin, 50 mg RE, 150 mg RE, and mg RE 500.
RESULTS
Adverse events and incidence of hypoglycemia with RE did not differ from placebo and prandial insulin groups. RE significantly increased urine glucose excretion and reduced the rise in plasma glucose concentration after oral glucose. RE reduced incremental adjusted weighted mean glucose (0–4 h) values by 42–49 mg/dL and mean glucose (0–10 h) by 52–69 mg/dL.
CONCLUSIONS
RE can be safely administered with insulin in type 1 diabetes and reduces plasma glucose concentrations compared with placebo.
Remogliflozin etabonate (RE) is an oral prodrug of remogliflozin (1), a selective antagonist of the sodium-dependent glucose transporter 2 (SGLT2) located in renal proximal tubules (2–4). It lowers glucose concentrations in type 2 diabetes by inhibiting renal glucose reabsorption (5). Because this mechanism functions independently of insulin, RE could be an effective oral adjunct to insulin for treatment of type 1 diabetes. This clinical trial evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of RE administered to subjects with type 1 diabetes. This is the first report of administration of an SGLT2 inhibitor in this patient population.
RESEARCH DESIGN AND METHODS
This single-center, randomized, double-blinded, placebo-controlled trial enrolled 10 individuals with type 1 diabetes managed with continuous subcutaneous insulin infusion (Supplementary Table 1). Each subject participated in five treatment periods separated by 5–35 days. After an overnight fast, they continued basal insulin infusion (Novolin; Novo Nordisk, Princeton, NJ) and received randomized treatments as follows: 1) placebo insulin injection + RE placebo (placebo), 2) mealtime insulin injection + RE placebo (prandial insulin), 3) placebo insulin injection + 50 mg RE (RE 50 mg), 4) placebo insulin injection + 150 mg RE (RE 150 mg), and 5) placebo insulin injection + 500 mg RE (RE 500 mg).
Each individual received 75-g oral glucose and identical meals during all treatment periods. Frequent samples were obtained for measurement of plasma glucose and insulin concentrations. Urine samples were collected for 24 h to assess creatinine clearance and glucose excretion. Plasma samples were collected for the determination of RE, remogliflozin, and GSK279782 (active metabolite) concentrations. Baseline-adjusted weighted mean glucose (0–4 h) and (0–10 h) concentrations were calculated for all treatments, and comparisons were made by ANCOVA.
RESULTS
Frequency and severity of adverse events, including hypoglycemia, did not differ between treatments (Supplementary Table 2). No hypoglycemic episodes were severe or resulted in study discontinuation. Analyses of vital signs, electrocardiograms, and laboratory results did not indicate drug-related effects after RE administration.
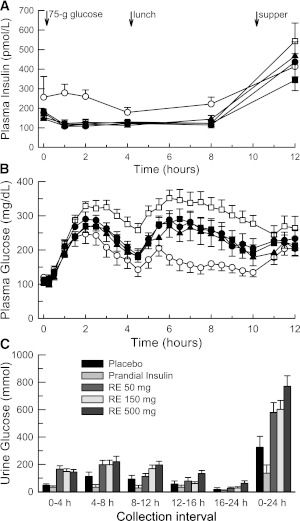
Figure 1 shows mean insulin and glucose concentrations during each treatment period. Serum insulin concentrations were elevated in the prandial insulin period when subjects received individual prescribed insulin boluses ranging from 5 to 12.5 units (0.098 ± 0.023 units/kg). When mealtime boluses were withheld in the placebo period, mean (±SD) plasma glucose concentrations reached a maximum of 330 ± 40 mg/dL. Glucose concentrations were attenuated during other treatment periods, reaching 247 ± 86 mg/dL with prandial insulin compared with 290 ± 67, 274 ± 56, and 270 ± 58 mg/dL for RE 50 mg, RE 150 mg, and RE 500 mg treatments, respectively.
Figure 1.
Mean (± SEM) 12-h serum insulin (A), 12-h plasma glucose (B), and urine glucose (C) profiles observed for each treatment period. After the overnight fast, basal insulin infusion was continued, and each subject received the following five treatments in random order: 1) placebo insulin injection + RE placebo (□; placebo), 2) mealtime insulin injection + RE placebo (○; prandial insulin), 3) placebo insulin injection + RE 50 mg (●; RE 50 mg), 4) placebo insulin injection + RE 150 mg (▲; RE 150 mg), and 5) placebo insulin injection + RE 500 mg (■; RE 500 mg). Relative to RE dosing, the morning glucose challenge, lunch, and supper were scheduled at 0.25, 4.25, and 10.25 h, respectively. All subjects received randomized insulin or placebo injections 15 min before the glucose challenge and lunch plus their regularly prescribed bolus of rapid-acting insulin 15 min before supper.
Baseline-adjusted weighted mean glucose (0–4 and 0–10 h) concentrations for RE treatment periods differed from placebo and prandial insulin periods (Supplementary Table 3). Compared with placebo, mean glucose (0–4 h) values changed by −49, −42, and −43 mg/dL in the RE 50 mg, RE 150 mg, and RE 500 mg periods, respectively. Mean glucose (0–10 h) values changed by −65, −69, and −62 mg/dL in the RE 50 mg, RE 150 mg, and RE 500 mg periods, respectively. Improved mean glucose (0–4 h) concentrations resulting from SGLT2 inhibition represented 55 to 65% of the observed effect of an insulin bolus. Glucose reductions were sustained through the lunch meal, confirming that effects applied to both oral glucose and mixed meals.
Single doses of RE resulted in a trend toward more negative 24-h fluid balance than placebo or prandial insulin treatments (Supplementary Table 4). Increased fractional urine glucose excretion and total 24-h urine glucose excretion (Fig. 1) during RE treatment periods were associated with higher urine volumes.
After administration of RE tablets to subjects with type 1 diabetes, the prodrug was absorbed and converted to the active entity, remogliflozin, followed by the formation of a second active metabolite, GSK279782. Pharmacokinetic parameters in this population were within the range of values reported for normal volunteers and subjects with type 2 diabetes (5).
CONCLUSIONS
This clinical trial was the first to explore the feasibility of administering an SGLT2 inhibitor with insulin to patients with type 1 diabetes. Single oral doses of up to 500 mg of RE were generally safe and well tolerated. All RE regimens significantly decreased glucose concentrations relative to placebo after a glucose load, and glucose improvements were sustained for 10 h, although no dose response was observed over the dose range studied. Drug administration augmented urine glucose excretion relative to placebo and prandial insulin treatment periods.
These data build upon previous results from rodent models of insulin deficiency induced by streptozotocin (6). They address concerns that RE could worsen hypoglycemia when administered with insulin in type 1 diabetes. Two factors favor a low hypoglycemia potential with SGLT2 inhibition as follows: 1) urine glucose excretion during SGLT2 inhibition decreases as plasma glucose concentrations fall into the hypoglycemic range; and 2) blunting peak postprandial glucose excursions could simplify prandial insulin dose adjustments. Urine glucose excretion stimulated by RE could potentially limit weight gain in these patients. Type 2 diabetes trials with SGLT2 inhibitors have demonstrated ≈2-kg weight loss linked to a change in fat mass (7,8). Weight loss is a perceived problem in type 1 diabetes, but intensive treatment strategies result in weight gain for many patients. Indeed, fears of hypoglycemia and weight gain are associated with worsening compliance with treatment (9).
Additional treatments for type 1 diabetes are needed to improve outcomes. Enrolled subjects were treated with continuous insulin infusion pumps in specialist clinics with access to all medications and treatment tools indicated for use in type 1 diabetes, yet 50% had HbA1c levels >7%, and 70% were overweight or obese. Although this trial included only single doses of RE and did not combine prandial dosing with rapid-acting insulin, it provides important guidance for adjusting insulin that will enable safe conduct of longer-term trials combining SGLT2 inhibitors with insulin. RE was administered safely in combination with insulin in type 1 diabetes and had effects on postprandial glucose profiles and urine glucose excretion that could improve the balance between mealtime insulin boluses and food intake to enhance glucose control without increasing hypoglycemia or weight.
Acknowledgments
This study was funded by GlaxoSmithKline, plc.
R.O.-S., P.K.M., J.Y., E.K.H., D.J.N., and R.L.D. are employees of GlaxoSmithKline, plc. No other potential conflicts of interest relevant to this article were reported.
S.M. participated in the design and conduct of the study, collection of data, data analysis, and writing of the manuscript and approved the final manuscript. D.A.A. and A.A.M. participated in the conduct of the study and collection of data and approved the final manuscript. R.O.-S. and P.K.M. participated in the design of the study, data analysis, and writing of the manuscript and approved the final manuscript. J.Y. participated in the design of the statistical analysis plan for the study and data and writing of the manuscript and approved the final manuscript. E.K.H. and D.J.N. participated in the design of the study, data analysis, and writing of the manuscript and approved the final manuscript. R.R.H. participated in the design and conduct of the study, collection of data, data analysis, and writing of the manuscript and approved the final manuscript. R.L.D. participated in the design and conduct of the study, data analysis, and writing of the final manuscript and approved the final manuscript. R.L.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the clinical research staff at the Center for Metabolic Research (http://www.vacmr.org), Veterans Administration San Diego Healthcare System, for the excellent clinical care and analytical assistance with this study. The authors also thank Chuck James and Glenn Smith of GlaxoSmithKline, plc, for their excellent analytical work for the drug metabolism, distribution, and kinetics, and to Jenny Leong for scientific support and Yufei Du and Peixin Sun of GlaxoSmithKline, plc, for assistance with statistical analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0508/-/DC1.
Clinical trial reg. no. NCT00575159, clinicaltrials.gov.
References
- 1.Fujimori Y, Katsuno K, Ojima K, et al. Sergliflozin etabonate, a selective SGLT2 inhibitor, improves glycemic control in streptozotocin-induced diabetic rats and Zucker fatty rats. Eur J Pharmacol 2009;609:148–154 [DOI] [PubMed] [Google Scholar]
- 2.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 1994;93:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells RG, Pajor AM, Kanai Y, Turk E, Wright EM, Hediger MA. Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am J Physiol 1992;263:F459–F465 [DOI] [PubMed] [Google Scholar]
- 4.You G, Lee WS, Barros EJ, et al. Molecular characteristics of Na(+)-coupled glucose transporters in adult and embryonic rat kidney. J Biol Chem 1995;270:29365–29371 [DOI] [PubMed] [Google Scholar]
- 5.Dobbins RL, O’Connor-Semmes R, Kapur A, et al. Remogliflozin etabonate, a selective inhibitor of the sodium-dependent transporter 2 reduces serum glucose in type 2 diabetes mellitus patients. Diabetes Obes Metab 2012;14:15–22 [DOI] [PubMed] [Google Scholar]
- 6.Luippold G, Klein T, Mark M, Grempler R. Empagliflozin, a novel potent and selective SGLT-2 inhibitor, improves glycaemic control alone and in combination with insulin in streptozotocin-induced diabetic rats, a model of type 1 diabetes mellitus. Diabetes Obes Metab 2012;14:601–607 [DOI] [PubMed] [Google Scholar]
- 7.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–1031 [DOI] [PubMed] [Google Scholar]
- 9.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab 2007;9:799–812 [DOI] [PubMed] [Google Scholar]