Abstract
OBJECTIVE
To investigate if there is a reduced risk of type 1 diabetes in children breastfed or exclusively breastfed by performing a pooled analysis with adjustment for recognized confounders.
RESEARCH DESIGN AND METHODS
Relevant studies were identified from literature searches using MEDLINE, Web of Science, and EMBASE. Authors of relevant studies were asked to provide individual participant data or conduct prespecified analyses. Meta-analysis techniques were used to combine odds ratios (ORs) and investigate heterogeneity between studies.
RESULTS
Data were available from 43 studies including 9,874 patients with type 1 diabetes. Overall, there was a reduction in the risk of diabetes after exclusive breast-feeding for >2 weeks (20 studies; OR = 0.75, 95% CI 0.64–0.88), the association after exclusive breast-feeding for >3 months was weaker (30 studies; OR = 0.87, 95% CI 0.75–1.00), and no association was observed after (nonexclusive) breast-feeding for >2 weeks (28 studies; OR = 0.93, 95% CI 0.81–1.07) or >3 months (29 studies; OR = 0.88, 95% CI 0.78–1.00). These associations were all subject to marked heterogeneity (I2 = 58, 76, 54, and 68%, respectively). In studies with lower risk of bias, the reduced risk after exclusive breast-feeding for >2 weeks remained (12 studies; OR = 0.86, 95% CI 0.75–0.99), and heterogeneity was reduced (I2 = 0%). Adjustments for potential confounders altered these estimates very little.
CONCLUSIONS
The pooled analysis suggests weak protective associations between exclusive breast-feeding and type 1 diabetes risk. However, these findings are difficult to interpret because of the marked variation in effect and possible biases (particularly recall bias) inherent in the included studies.
Childhood type 1 diabetes is caused by the autoimmune destruction of the pancreatic β-cells. The increases in type 1 diabetes incidence in recent decades (1) and the concordance rate of <50% in monozygotic twins (2) suggests a role for environmental factors in the development of diabetes. The proportional greater relative increases in incidence in children <5 years old (1) indicates that environmental exposures in early life, such as infant feeding, could play an important role.
In 1994, a meta-analysis (3) concluded that breast-feeding for a short period reduced the risk of type 1 diabetes by 40%, but another meta-analysis in 1996 (4), largely containing the same studies, concluded that the weak reduction in diabetes risk after breast-feeding was small and may have methodological explanations. However, both these meta-analyses had limitations. The associations with type 1 diabetes (extracted from published studies) were based upon categorized durations of breast-feeding, which differed between studies. Furthermore, in these meta-analyses, it was not possible to adjust the association between breast-feeding and type 1 diabetes consistently for potential confounders (factors such as maternal age, birth weight, and maternal diabetes that were associated with both diabetes risk and the propensity to breast-feed). Also, studies that recorded breast-feeding results but did not report them in sufficient detail were excluded, potentially exaggerating any breast-feeding association through reporting bias. These weaknesses were largely because both were literature-based meta-analyses (based only upon reported associations in published studies) rather than collaborative meta-analyses or pooled analyses (using individual participant data from included studies after contacting authors of articles containing relevant data) (5). Since the publication of these two meta-analyses, many additional observational studies have investigated breast-feeding and type 1 diabetes, allowing a new meta-analysis to be conducted on an independent group of more recent studies.
The aim of this study was to assess the evidence of an association between duration of breast-feeding (and exclusive breast-feeding) and type 1 diabetes using collaborative meta-analysis techniques from observational studies published since 1996. Importantly, this pooled analysis includes individual participant data, allowing consistent categorization of breast-feeding across studies and adjustment for potential confounders such as maternal age, birth weight, and maternal diabetes and will also include studies investigating breast-feeding but not reporting detailed results.
RESEARCH DESIGN AND METHODS
Literature search
The main literature search was conducted using MEDLINE through Ovid Online. The search was adapted from a previous study (6) and comprised search terms for infant feeding consisting of text words (including breast fed, breast feed, breast feeds, breast feeding, breast milk, bottle fed, bottle feed, bottle feeds, bottle feeding, infant feed, infant feeds, infant feeding, infant nutrition, formula fed, formula feed, formula feeds, formula feeding, infant diet, infant diets, dried milk, and early nutrition) or MEDLINE subject heading key words (including breast feeding, bottle feeding, milk, human, infant food, and infant formula) and terms for type 1 diabetes including the text words (IDDM or [diabetes and type 1]) or the MEDLINE subject heading key word “diabetes mellitus, type 1.” Similar searches were conducted on Web of Science and EMBASE. The searches were limited to studies on humans published after January 1996 [to avoid overlap with two previous meta-analyses (3,4)] up to 1 May 2011. No language restrictions were applied. Titles and then abstracts were screened independently by two investigators (C.R.C. and C.C.P.) to establish if the studies were likely to provide relevant data based on the following inclusion criteria: 1) they identified a group with type 1 diabetes and a group without type 1 diabetes, and 2) they recorded breast-feeding in these groups. Studies were excluded if they contained <20 patients with diabetes or if they were family-based (because the association between infant feeding and type 1 diabetes may be different in individuals with a strong genetic susceptibility). The corresponding author of each included article was asked if they were aware of any additional studies.
The corresponding author of each study was contacted to provide individual participant data to allow breast-feeding to be consistently categorized across studies, standardize adjustment of the association with breast-feeding for potential confounders (including maternal diabetes, birth weight, gestational age, maternal age, birth order, Caesarean section, and socioeconomic status), and include data from studies that did not report detailed findings. Authors were requested to provide raw data or to provide adjusted estimates of the association between breast-feeding and type 1 diabetes after conducting specified additional analyses.
Details of each study were extracted by one reviewer (C.R.C.) and agreed with the study author.
Statistical analysis
A two-stage technique was used to calculate pooled estimates of the association between breast-feeding and diabetes, before and after adjustment for potential confounders (7). Odds ratios (ORs) and SEs were calculated for the association between diabetes and breast-feeding within each study. Unconditional and conditional logistic regression was used to calculate the ORs and SEs for the unmatched and matched case-control studies, respectively. In cohort studies with varying length of participant follow-up, rate ratios and their SEs were used instead of ORs, which were not directly calculable. As type 1 diabetes is a rare disease, these measures should be approximately equal. In one study (8) with a zero cell count for an exposure category, a correction was added to obtain an estimate and SE for use in the meta-analysis. In another study, an additional adjustment was always made for the perinatal cohort entry score to reflect the study design (9). Finally, in studies for which no author could be contacted or data were not available, if possible, required estimates were extracted from published reports. Meta-analysis techniques were then applied to these estimates. Tests for heterogeneity between studies were conducted and random-effects models used to calculate pooled ORs. Random-effects models were used, instead of fixed-effects models, because between-study heterogeneity was anticipated from these observational studies. The I2 statistic was calculated to quantify the degree of heterogeneity between studies. Small study effects (possibly due to publication bias) were investigated by checking for asymmetry in funnel plots of the study ORs against one over the SE of the logarithm of the ORs.
Similar methods were conducted to pool adjusted estimates. First, adjusted estimates and SEs were calculated within each study using regression models appropriate to the study design including diabetes as the outcome variable and breast-feeding and the potential confounder(s) of interest as explanatory variable(s). Meta-analysis techniques were then applied to these adjusted estimates.
The analysis was initially conducted based upon a priori categorizations of nonexclusive and exclusive breast-feeding ≥2 weeks versus breast-feeding <2 weeks and was repeated for nonexclusive breast-feeding ≥3 months versus breast-feeding <3 months in which these data or similar were available. Subgroup analyses were also conducted including only studies with a low risk of bias based upon high response rates (>70%) in both cases and controls and the use of randomly selected population-based controls. Subgroup analysis was also performed by age at diagnosis of diabetes, geographic region, and incidence rate based upon a published summary of worldwide incidence (10).
All statistical analyses were performed using STATA 11.0 (Stata, College Station, TX).
RESULTS
Search results
The results from the literature search are shown in Supplementary Fig. 1. Briefly, initial searches identified 238 articles from MEDLINE, 393 from Web of Science, and 609 from EMBASE. Of these, 40 articles contained relevant data from 47 studies, as information from 7 centers was taken from 1 article (11) and information from 2 centers was taken from another (12). An investigator from each of the 47 studies was invited to provide raw data (or estimates from prespecified analyses). Estimates were obtained from 43 of the 47 identified studies covering 95% (9,874 of 10,390) of potential patients with type 1 diabetes. Individual patient data were obtained from 27 of these studies, 8 supplied prespecified estimates, in 8 studies data could be extracted from published reports, and 4 studies could not be included.
Characteristics of included studies
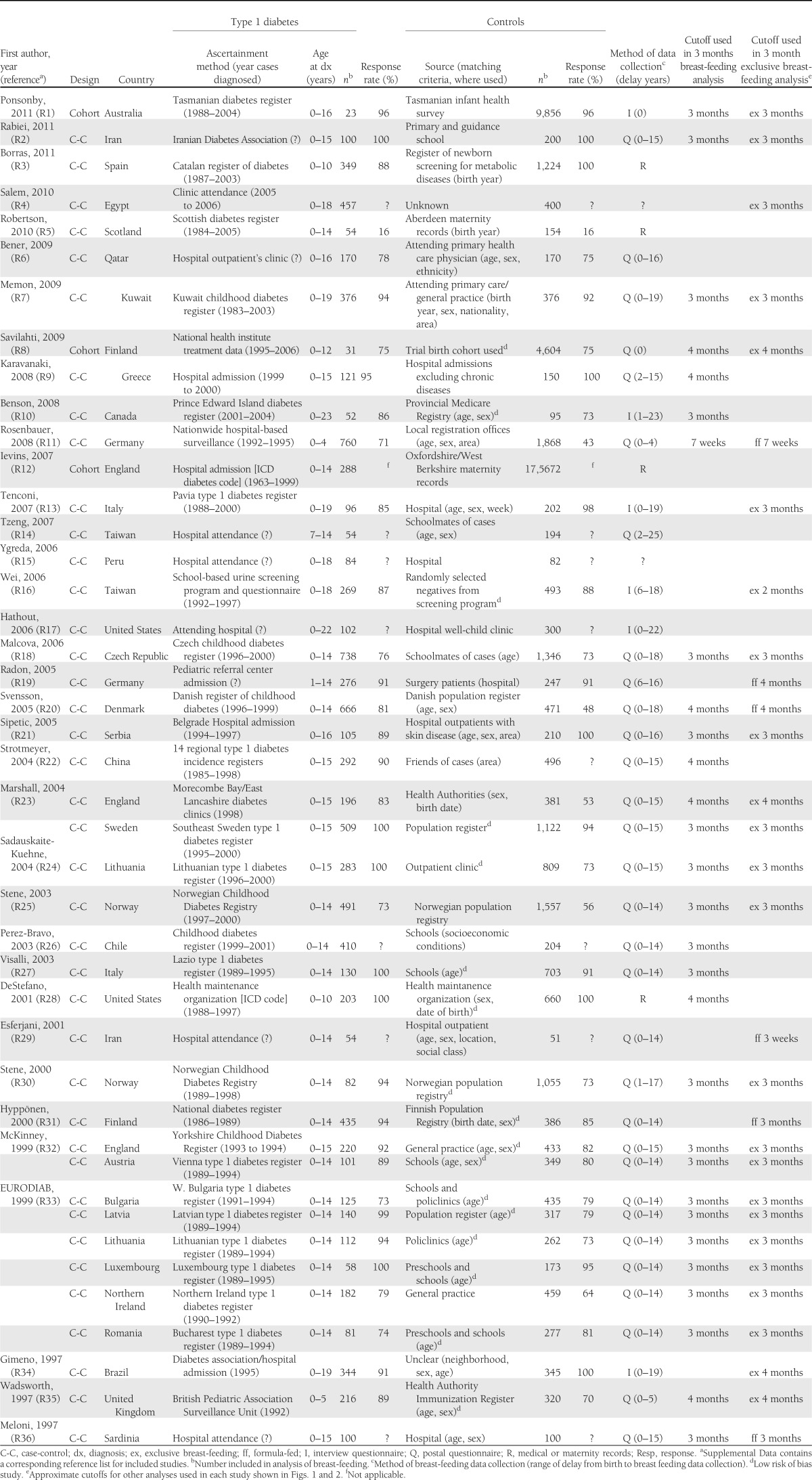
Table 1 contains the characteristics of the 43 studies from which relevant data were obtained (see Supplemental Data for full list of included articles). Of these, 40 were case-control studies, and 3 were cohort studies. Twenty-eight studies were from Europe, and in 24 studies, cases were identified using a national or local diabetes register. Thirty-seven studies ascertained breast-feeding data using questionnaires or interviews, and in the majority, breast-feeding data were recalled many years after the birth of the child.
Table 1.
Characteristics of included studies published 2004–2011 investigating the association between breast-feeding and childhood-onset type 1 diabetes, ordered by publication date
Overall findings for any (nonexclusive) breast-feeding
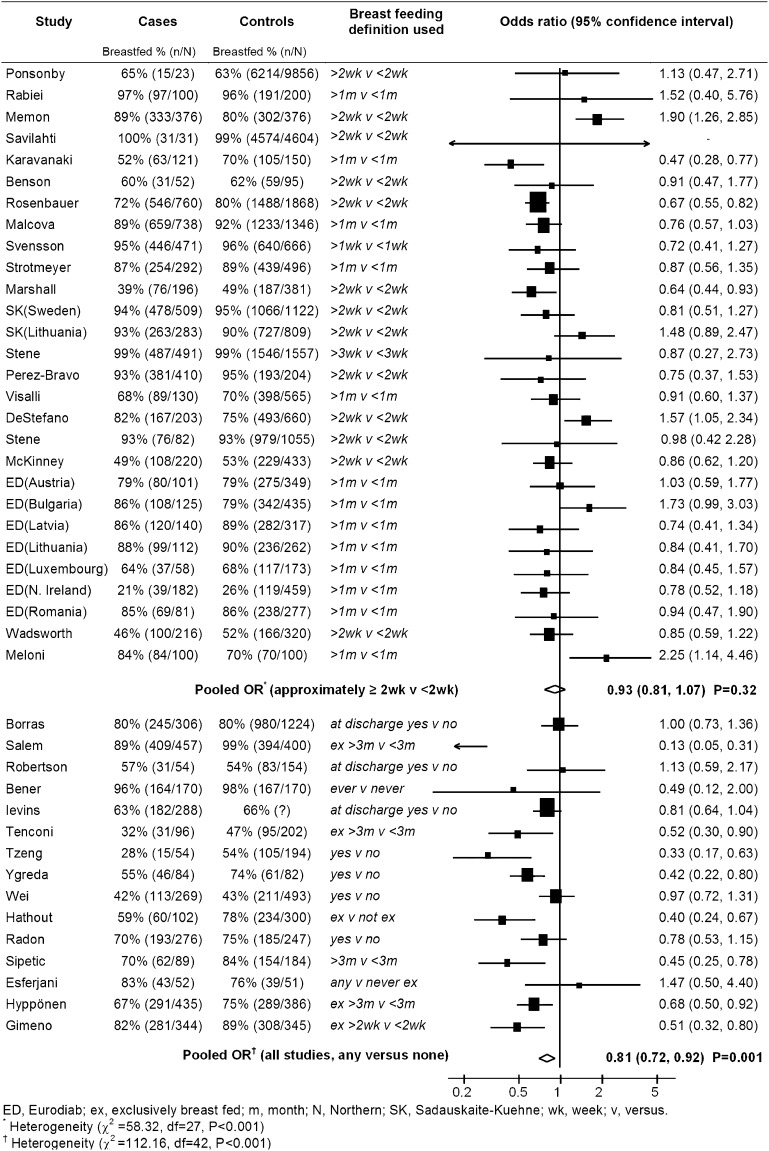
The associations between any (nonexclusive) breast-feeding and type 1 diabetes from the 43 studies (including 9,874 patients with type 1 diabetes) are shown in Fig. 1 and Table 2. Initially, an analysis was conducted investigating any recorded duration of breast-feeding (the breast-feeding duration used in this analysis for each study is shown in Fig. 1). Overall, in studies which investigated any measure of breast-feeding, there was a reduction in the risk of type 1 diabetes in breast-fed children of 18% (OR = 0.81, 95% CI 0.72–0.92; P < 0.001). There was, however, marked heterogeneity between studies (I2 = 63%; heterogeneity P < 0.001). Restricting this analysis to studies with a low risk of bias (due to high response rates and randomly selected population-based controls, as shown in Table 1), there was no evidence of an association (OR = 1.00, 95% CI 0.89–1.11; P = 0.93) and little evidence of heterogeneity (I2 = 1%; heterogeneity P = 0.51).
Figure 1.
Pooled analysis of association between (nonexclusive) breast-feeding and childhood-onset type 1 diabetes in studies investigating ∼2 weeks (nonexclusive) breast-feeding and studies investigating any measure of breast-feeding.
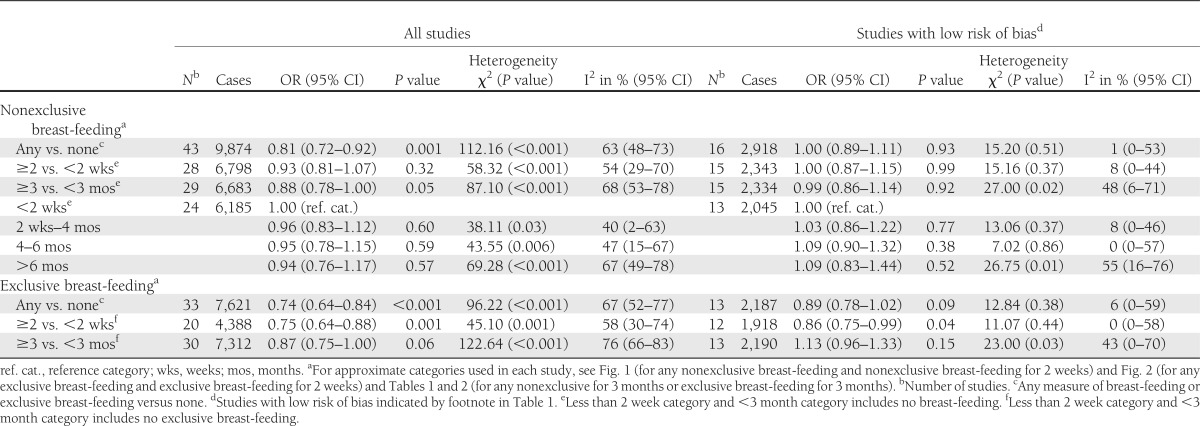
Table 2.
Pooled analyses of the association between breast-feeding and childhood-onset type 1 diabetes in all studies and in studies with a lower risk of bias
Analyses were also conducted in subgroups of studies recording breast-feeding of ∼2 weeks’ duration or more (see Fig. 1 for exact duration of breast-feeding used in each study) and ∼3 months duration or more (see Table 1 for exact duration of breast-feeding used in each study). In 28 studies, there was little evidence of any difference in the risk of type 1 diabetes in children breast-fed for ∼2 weeks or more compared with <2 weeks (OR = 0.93, 95% CI 0.81–1.07; P = 0.32), but again there was heterogeneity between studies (I2 = 54%; heterogeneity P < 0.001). This heterogeneity was reduced in 15 studies with a low risk of bias (I2 = 8%; heterogeneity P = 0.37), but the overall finding was little altered (OR = 1.00, 95% CI 0.87–1.15; P = 0.99). In 29 studies, there was also little evidence of a reduction in the risk of type 1 diabetes in children breast-fed for ∼ 3 months or more compared with children breast-fed for <3 months (OR = 0.88, 95% CI 0.78–1.00; P = 0.05), but the heterogeneity between studies persisted (I2 = 68%; heterogeneity P < 0.001). Repeating this analysis in 15 studies with a low risk of bias showed no evidence of an association (OR = 0.99, 95% CI 0.86–1.14; P = 0.92) and slightly less, though still significant, heterogeneity (I2 = 48%; heterogeneity P = 0.02). Funnel plots (not shown) provided little evidence of asymmetry (potentially caused by publication bias).
Finally, an analysis was conducted in the following approximate categories of (nonexclusive) breast-feeding: <2 weeks, 2 weeks to 4 months, 4–6 months, and >6 months. In 24 studies with available data, when compared with children breast-fed for <2 weeks children breast-fed for 2 weeks to 4 months had a 4% reduction in diabetes risk (OR = 0.96, 95% CI 0.83–1.12), children breast-fed for 4–6 months had a 5% reduction in diabetes risk (OR = 0.95, 95% CI 0.78–1.15), and children breast-fed for >6 months had a 6% reduction in diabetes risk (OR = 0.94, 95% CI 0.76–1.17), though none of these differences were significant. These findings were similar when restricted to 13 studies with a low risk of bias.
Additional analyses (shown in Supplementary Table 2) in subgroups of studies defined by geographic region (European, non-European) and low and high incidence rate countries (<15 cases of type 1 diabetes per 100,000 person-years and >15 per 100,000 person-years, respectively) did not reveal any marked differences in association.
There was little evidence of a difference in the association between childhood type 1 diabetes and (nonexclusive) breast-feeding in early diagnosed diabetes (i.e., <5 years old) and later diagnosed diabetes (i.e., between 5 and 15 years old) in studies in which both age groups were available (shown in Supplementary Table 3). Specifically, in 22 studies, the OR for breast-feeding for ∼2 weeks or more was 0.84 (95% CI 0.65–1.08) in early diagnosed disease and 0.95 (95% CI 0.82–1.11) for later diagnosed disease. Similarly, in 23 studies, the OR for breast-feeding for ∼3 months or more was 0.86 (95% CI 0.69–1.08) in early diagnosed disease and 0.90 (95% CI 0.79–1.02) for later diagnosed disease.
Supplementary Table 1 shows the findings for (nonexclusive) breast-feeding for ∼2 weeks or more after adjustment for potential confounders. The association between type 1 diabetes and breast-feeding was little altered after individual adjustment for maternal diabetes, birth weight, gestational age, maternal age, birth order, Caesarean section delivery, or socioeconomic status, where possible. There was also little change in the estimate when adjustment for all available variables simultaneously was conducted.
Overall findings for exclusive breast-feeding
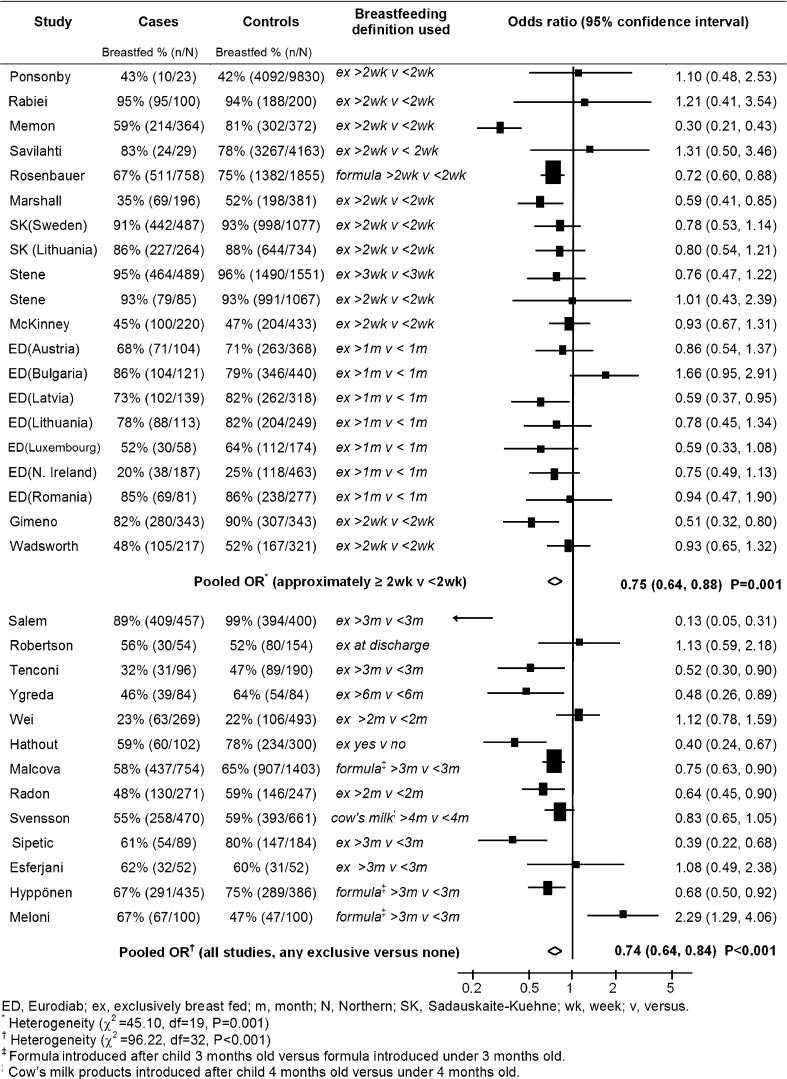
Similar analyses were conducted for the associations between exclusive breast-feeding and type 1 diabetes (investigated by 33 studies including 7,621 patients with type 1 diabetes) that are shown in Fig. 2 (which contains the duration of exclusive breast-feeding used in each study) and Table 1. Overall, in studies which investigated any measure of exclusive breast-feeding, there was a reduction in the risk of type 1 diabetes in exclusively breast-fed children of 26% (OR = 0.74, 95% CI 0.64–0.84; P < 0.001), but there was marked heterogeneity between studies (I2 = 67%; heterogeneity P < 0.001). When restricting this analysis to 13 studies with a low risk of bias, the association was no longer significant (OR = 0.89, 95% CI 0.78–1.02; P = 0.09), and there was less heterogeneity (I2 = 6%; heterogeneity P = 0.38).
Figure 2.
Pooled analysis of association between exclusive breast-feeding and childhood-onset type 1 diabetes in studies investigating ∼2 weeks exclusive breast-feeding and studies investigating any measure of exclusive breast-feeding.
As before, analysis was conducted in subgroups of studies recording ∼2 weeks or more (see Fig. 1 for definition) and ∼3 months or more of exclusive breast-feeding (see Table 1 for definition). In 20 studies, children exclusively breast-fed for ∼2 weeks or more had a 25% reduction in their risk of type 1 diabetes (OR = 0.75, 95% CI 0.64–0.88; P = 0.001) compared with children exclusively breast-fed for <2 weeks, but there was marked heterogeneity between studies (I2 = 58%; heterogeneity P < 0.001). This association was slightly attenuated but remained significant in 12 studies with a low risk of bias (OR = 0.86, 95% CI 0.75–0.99; P = 0.04), but in these studies, there was less heterogeneity (I2 = 0%; heterogeneity P = 0.44). There was less evidence of a reduction in diabetes risk (in 30 studies) in children exclusively breast-fed for ∼3 months or more (OR = 0.87, 95% CI 0.75–1.00; P = 0.06) compared with children exclusively breast-fed for <3 months but there was marked heterogeneity (I2 = 76%; heterogeneity P < 0.001). In 13 studies with a low risk of bias, the heterogeneity remained (I2 = 43%; heterogeneity P = 0.03), and there was little evidence of an association (OR = 1.13, 95% CI 0.96–1.33; P = 0.15). Insufficient data were available to conduct an analysis of exclusive breast-feeding in finer duration categories. Funnel plots for these associations (not shown) provided little evidence of asymmetry (potentially caused by publication bias).
Additional analyses (shown in Supplementary Table 2) in subgroups of studies defined by geographic region (European, non-European) and incidence rates of type 1 diabetes (low incidence, high incidence) did not reveal any marked differences in association.
As before, a comparison of the associations with exclusive breast-feeding by early diagnosed diabetes (i.e., <5 years old) and later diagnosed diabetes (i.e., between 5 and 15 years old) showed little evidence of a difference in studies in which both age groups were available (shown in Supplementary Table 3). Specifically, in 18 studies, the OR for exclusive breast-feeding for ∼2 weeks or more was 0.73 (95% CI 0.58–0.91) in early diagnosed disease and 0.75 (95% CI 0.60–0.95) for later diagnosed disease. Similarly, in 24 studies, the OR for exclusive breast-feeding for ∼3 months or more was 0.86 (95% CI 0.71–1.03) in early diagnosed disease and 0.90 (95% CI 0.76–1.05) for later diagnosed disease.
Supplementary Table 1 shows the findings for exclusive breast-feeding for ∼2 weeks or more after adjustment for potential confounders. The association between type 1 diabetes and exclusive breast-feeding was little altered after adjustment for any of these confounders individually, where possible, or when adjustments were conducted for all available confounders simultaneously.
CONCLUSIONS
This pooled analysis suggests that breast-feeding exclusively in the early weeks of life could reduce the risk of childhood onset type 1 diabetes by 15%, based upon the highest quality studies. This pooled analysis provides little evidence that longer exclusive or nonexclusive breast-feeding has a protective effect. However, firm conclusions are difficult to reach because of the marked heterogeneity in the observed associations between studies and the weaknesses inherent in many of the included studies. The observed association between exclusive breast-feeding and type 1 diabetes could not be explained by confounding from established risk factors for diabetes including maternal diabetes, birth weight, gestational age, maternal age, birth order, or Caesarean section delivery.
This is, to our knowledge, the first pooled analysis of individual participant data on breast-feeding and diabetes. The main strengths of using individual participant data in this study was that it allowed analysis to be conducted in similar categories of breast-feeding duration between studies and enabled adjustment of associations for various confounders. This analysis contains data from 43 studies and includes 9,874 patients with type 1 diabetes allowing the power to identify associations of relatively small magnitude. Also, the analyses benefited from coverage of 95% of eligible cases from studies identified by the comprehensive search strategies. However, it remains possible that studies with relevant data were not identified by the searches. A weakness of this pooled analysis was that the majority of studies were questionnaire-based case-control studies, and their findings are based upon breast-feeding information usually recalled over many years, potentially resulting in recall and other biases. There were only two cohort studies (8,9) that prospectively recorded breast-feeding at 2 weeks and 3 months, but these contained relatively few patients with type 1 diabetes, 31 and 24 cases, respectively. Consequently, there is a need for further studies prospectively or routinely recording breast-feeding in early life. Although attempts were made to identify studies with lower risk of bias (with higher response rates and randomly selected population-based controls), even in these studies, response rates varied, and in many, recall bias is possible. As with all observational studies, it is impossible to rule out the effect of unmeasured confounding, for example, by other infant food intake.
The association between breast-feeding and type 1 diabetes differed markedly between studies. This could reflect real differences in the association in different populations or biases specific to each study. In studies with a low risk of bias, less heterogeneity was observed, allowing more confidence to be placed on the resulting pooled estimates.
The findings of our pooled analysis of individual participant data differ from two published literature-based meta-analyses (3,4). The first meta-analysis (3) (containing studies conducted prior to 1994) demonstrated a reduction in diabetes risk of 30% with breast-feeding >3 months, and a 35% reduction with exclusive breast-feeding for >3 months. The second meta-analysis (containing studies conducted prior to 1996, many of which were in the first meta-analysis) demonstrated a reduction in diabetes risk of 20% with breast-feeding for 3 months and 40% for exclusive breast-feeding for >3 months (4). In our pooled analysis, there was little evidence of any reduction in the risk of diabetes after 3 months of breast-feeding (exclusive or nonexclusive). Unfortunately, neither of the two earlier meta-analyses presented estimates for breast-feeding in the first 2 weeks of life to allow comparison with our results. Both these meta-analyses were largely based upon questionnaire-based case-control studies, and the second meta-analysis (4) highlighted that the observed association could reflect methodological weaknesses, particularly recall bias. The authors suggested that mothers of children with diabetes may more critically remember occasions when they deviated from the breast-feeding recommendations of health care personnel (4). As previously discussed, the majority of studies included in this pooled analysis were questionnaire-based case-control studies and would also be subject to this bias.
In 2007, the Agency for Healthcare Research and Quality reviewed the evidence of the effect of breast-feeding on type 1 diabetes (13). However, the report did not contain a meta-analysis because their searches identified only 6 studies after 1996 (up to 2007) when the previous meta-analyses were conducted, in contrast to the 43 studies included in our pooled analysis between 1996 and 2011. The estimated OR of 0.75 for any breast-feeding for 3 months from the Agency for Healthcare Research and Quality report (13) (based solely on the two previous meta-analyses) has since been used to estimate the cost-effectiveness of breast-feeding in the U.S. (14). Our results would suggest that this OR of 0.75 may overestimate any protective effect against type 1 diabetes and that consequently the cost-effectiveness of breast-feeding with respect to type 1 diabetes could also be exaggerated. A recent narrative review (15) commenting on the effects of breast-feeding and complementary feeding noted that the associations were controversial and that contradictory outcomes had been observed. Studies have also been conducted into β-cell autoimmunity and breast-feeding, but the results have been mixed, with some studies observing protective effects of breast-feeding, whereas others have observed no association [see Knip et al. (15) for references].
This pooled analysis suggests that initial exclusive breast-feeding may reduce diabetes risk, indicating that the introduction of formula feeding may explain any association. The responsible mechanism is unclear, but researchers have speculated (15) that breast-feeding could protect against type 1 diabetes risk by increasing gut permeability; previous studies have shown that gut permeability decreases faster in breastfed children (16) or by reducing the risk of enterovirus infections (17), which have been associated with increased diabetes risk (18). Alternatively, any increased risk of diabetes associated with formula feeding could reflect early exposure to a variety of cow’s milk proteins [studies have shown children with type 1 diabetes have increased concentrations of antibodies to dietary antigens (19)], modification of the gut microflora (15), and increased weight gain in early life [although one study suggested that any effect of breast-feeding was independent of weight gain (20)]. In our analysis, an association was only observed for initial exclusive breast-feeding (for ≥2 weeks) but not for exclusive breast-feeding for ≥3 months. This distinction may be real or could reflect the relatively accurate maternal recall of initial breast-feeding but less accurate recall of the duration of feeding, as previously suggested (3). If early introduction of formula feeding is responsible then ongoing efforts to investigate alternative formula, such as the Trial to Reduce Insulin-dependent Diabetes Mellitus in the Genetically at Risk (21), may be productive.
In conclusion, our pooled analysis suggests that children who are initially exclusively breast-fed have a small reduction in their risk of type 1 diabetes. This finding is difficult to interpret because the associations varied markedly between studies and because the results are based primarily upon questionnaire-based case-control studies, which could have been affected by recall and other biases.
Acknowledgments
This work was supported by National Institutes of Health grants R01-DK-46498 and R01-DK-42316, the Chinese Foundation of Health, a grant (DOH91-TD1167) from the Department of Health, Executive Yuan, Republic of China, the Ministry for Science and Technology of the Republic of Serbia through Contract 175042 (2011–2014), a grant from the German Research Foundation (HE 234/1-1), and the Research Council of Norway (148359/330).
No potential conflicts of interest relevant to this article were reported.
C.R.C., L.C.S., J.L., J.R., O.C., J.S., F.P.-B., A.M., S.G.G., E.J.K.W., E.S.S., M.J.G., K.R., L.-M.C., R.C.P., A.C., K.K., G.B., P.P., B.U., E.S., G.D., S.S., G.J., C.I.-T., C.E.d.B., K.H., V.B., E.S., A.-L.P., M.S., S.R., and C.C.P. researched data. C.R.C., L.C.S., J.R., K.R., A.C., S.S., E.S., and M.S. were responsible for statistical analysis. C.R.C. wrote the manuscript. C.R.C., L.C.S., J.L., J.R., O.C., J.S., F.P.-B., A.M., S.G.G., E.J.K.W., E.S.S., M.J.G., K.R., L.-M.C., R.C.P., A.C., K.K., G.B., P.P., B.U., E.S., G.D., S.S., G.J., C.I.-T., C.E.d.B., K.H., V.B., E.S., A.-L.P., M.S., S.R., and C.C.P. reviewed and edited the manuscript. C.R.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank A. Pezic (Murdoch Children’s Research Institute, Parkville, Victoria, Australia), R. Portuesi (Department of Endocrinology and Diabetes, University Campus Bio-Medico, Rome, Italy), E. Weintraub (Centers for Disease Control and Prevention, Atlanta, GA), R. Rastmanesh (Clinical Nutrition and Dietetics Department, Shahid Beheshti University of Medical Sciences, Tehran, Iran), J. Dorman (Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA), and M. Yang (Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA) for support. The authors also thank G. Soltész (University of Pecs, Pecs, Hungary) and G. Dahlquist (Umea University, Umea, Sweden), coordinators of the EURODIAB Substudy.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0438/-/DC1.
References
- 1.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 2.Hirschhorn JN. Genetic epidemiology of type 1 diabetes. Pediatr Diabetes 2003;4:87–100 [DOI] [PubMed] [Google Scholar]
- 3.Gerstein HC. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care 1994;17:13–19 [DOI] [PubMed] [Google Scholar]
- 4.Norris JM, Scott FW. A meta-analysis of infant diet and insulin-dependent diabetes mellitus: do biases play a role? Epidemiology 1996;7:87–92 [DOI] [PubMed] [Google Scholar]
- 5.Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof 2002;25:76–97 [DOI] [PubMed] [Google Scholar]
- 6.Owen CG, Whincup PH, Kaye SJ, et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am J Clin Nutr 2008;88:305–314 [DOI] [PubMed] [Google Scholar]
- 7.Stukel TA, Demidenko E, Dykes J, Karagas MR. Two-stage methods for the analysis of pooled data. Stat Med 2001;20:2115–2130 [DOI] [PubMed] [Google Scholar]
- 8.Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. Eur J Nutr 2009;48:243–249 [DOI] [PubMed] [Google Scholar]
- 9.Ponsonby AL, Pezic A, Cochrane J, et al. Infant anthropometry, early life infection, and subsequent risk of type 1 diabetes mellitus: a prospective birth cohort study. Pediatr Diabetes 2011;12:313–321 [DOI] [PubMed] [Google Scholar]
- 10.DIAMOND Project Group Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 11.Dahlquist GG, Patterson C, Soltesz G. Perinatal risk factors for childhood type 1 diabetes in Europe. The EURODIAB Substudy 2 Study Group. Diabetes Care 1999;22:1698–1702 [DOI] [PubMed] [Google Scholar]
- 12.Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Z, Jasinskiene E, Samuelsson U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev 2004;20:150–157 [DOI] [PubMed] [Google Scholar]
- 13.Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Evidence Report/Technology Assessment No. 153. Rockville, MD, Agency for Healthcare Research and Quality, 2007 [PMC free article] [PubMed] [Google Scholar]
- 14.Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics 2010;125:e1048–e1056 [DOI] [PubMed] [Google Scholar]
- 15.Knip M, Virtanen SM, Akerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr 2010;91:1506S–1513S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr 1995;21:383–386 [DOI] [PubMed] [Google Scholar]
- 17.Sadeharju K, Knip M, Virtanen SM, et al. Finnish TRIGR Study Group Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics 2007;119:941–946 [DOI] [PubMed] [Google Scholar]
- 18.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savilahti E, Akerblom HK, Tainio VM, Koskimies S. Children with newly diagnosed insulin dependent diabetes mellitus have increased levels of cow’s milk antibodies. Diabetes Res 1988;7:137–140 [PubMed] [Google Scholar]
- 20.Hyppönen E, Kenward MG, Virtanen SM, et al. Infant feeding, early weight gain, and risk of type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care 1999;22:1961–1965 [DOI] [PubMed] [Google Scholar]
- 21.Knip M, Virtanen SM, Becker D, Dupré J, Krischer JP, Åkerblom HK, TRIGR Study Group Early feeding and risk of type 1 diabetes: experiences from the Trial to Reduce Insulin-dependent diabetes mellitus in the Genetically at Risk (TRIGR). Am J Clin Nutr 2011;94(Suppl.):1814S–1820S [DOI] [PMC free article] [PubMed] [Google Scholar]