Abstract
Objective
To compare the effectiveness of biologics, non-biologic systemic therapies, and phototherapy for psoriasis.
Design
Cross-sectional
Setting
Ten outpatient dermatology sites across the U.S.
Participants
713 plaque psoriasis patients receiving monotherapy: methotrexate, adalimumab, etanercept, ustekinumab, or narrowband ultraviolet B phototherapy.
Main Outcome Measures
Primary outcome: Clear or almost clear on the Physician Global Assessment. Secondary outcomes: Psoriasis Area and Severity Index, affected body surface area, and Dermatology Life Quality Index.
Results
The proportion of patients clear or almost clear on the Physician Global Assessment differed among treatments: methotrexate (23.8%), adalimumab (47.7%), etanercept (34.2%), ustekinumab (36.1%), NB-UVB (27.6%) (p < 0.001). In adjusted analyses, patients on adalimumab (relative response rate 2.15, 95% CI 1.60–2.90), etanercept (1.45, 95% CI 1.06–1.97) and ustekinumab (1.57, 95% CI 1.06–2.32) were more likely to have clear or almost clear skin versus patients on methotrexate, while patients receiving phototherapy showed no significant difference (1.35, 95% CI 0.93–1.96) compared to methotrexate. No response difference was observed with respect to quality of life. 36.1% of etanercept and 11.8% of adalimumab patients received double the recommended doses, while 10.6% of phototherapy patients received the recommended treatment frequency.
Conclusions
The effectiveness of psoriasis therapies in clinical practice may be lower than previously reported in trials. Although relative differences in objective response rates among therapies may exist, absolute differences are small and may not be clinically significant. Dosing of common therapies varied from trial recommendations. These results provide novel benchmarks emphasizing the critical importance of studying effectiveness in real-world practice.
Introduction
Psoriasis is a common, chronic Th1 and Th17 mediated inflammatory disease of the skin and joints1,2. It can occur at any age but onset most commonly occurs in young adulthood. The disease is felt to be incurable and long-term spontaneous remissions are rare. Psoriasis is associated with impairment in physical and emotional health even in patients with mild disease, and patients with psoriasis requiring systemic or phototherapy (i.e., those with moderate-to-severe disease) have an increased risk of major cardiovascular events and mortality independent of traditional risk factors3–9.
Moderate-to-severe psoriasis is typically defined as disease affecting ≥3–5% of body surface area (BSA) or requiring systemic treatment or phototherapy for successful management10,11. It is estimated that over 1.4 million Americans and 25 million individuals worldwide have moderate-to-severe psoriasis12. Traditional oral systemic therapies such as methotrexate, acitretin, and cyclosporine have been available for several decades but their use can be limited by patient intolerance or organ-specific toxicity with long-term use13. In the last decade, the treatment of moderate-to-severe psoriasis has undergone a revolution with the U.S. Food and Drug Administration (FDA) approval of six biologic drugs that target T-cells and cytokines critical to the pathogenesis of psoriasis14. Although these new therapies have been proven efficacious for psoriasis in short-term studies, they are associated with high costs, diminished efficacy with long-term treatment, and risks of rare but serious side effects that are still being defined15. For example, efalizumab, which targets T-cells, was voluntarily removed from the market due to a rare risk of progressive multifocal leukoencephalopathy identified in post-marketing spontaneous reports16.
Despite the growing repertoire of psoriasis treatments, insufficient data exist to determine which therapies are first-, second-, and third-line17. Only a few short-term comparative trials of oral systemic and biologic agents for psoriasis have been conducted and, to our knowledge, there are no data available to evaluate the effectiveness of these therapies under real-world conditions, which is a critical and recognized data gap in comparative effectiveness research18–20. Therefore, the purpose of this multi-center study was to describe and compare the effectiveness of commonly used systemic and phototherapy treatments for moderate-to-severe psoriasis in patients being evaluated as part of routine medical care.
Methods
Study design and human subject protections
We conducted a cross-sectional study to determine the effectiveness of commonly used systemic or phototherapy treatments for moderate-to-severe psoriasis. The study was approved by the University of Pennsylvania and University of Utah Institutional Review Boards and informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
Setting
Data were collected by 12 clinicians (10 dermatologists and 2 physician assistants) who are members of the Dermatology Clinical Effectiveness Research Network (DCERN). Developed through funding received from the American Recovery and Reinvestment Act, DCERN includes two academic medical centers (University of Pennsylvania and University of Utah, each with a hospital-based site and a separate community-based site) and six private practices in Georgia, Pennsylvania, New York, and Colorado (see www.dermcern.org for details). Data were collected from February 2010 through June 2011. Patient data were collected prospectively at a single, regularly scheduled clinic appointment and no follow-up data were collected.
Participants
To minimize bias, broad inclusion criteria were used for the enrollment of consecutive patients being seen by their dermatology provider in DCERN practices for a routine follow-up appointment. Study subjects were established patients who met at least one of the following criteria: currently receiving a biologic, oral systemic, or phototherapy prescribed by the dermatologist or PA for their psoriasis, were candidates for systemic therapy as defined by a history of ≥ 5% BSA involvement as documented in the medical record, or were previously treated with a biologic, oral systemic or phototherapy for their psoriasis. To further reduce bias, new patients to the practice became eligible for study inclusion only at their next regularly scheduled visit subsequent to the initial appointment. Patients were excluded if they did not meet the above criteria or were unable or unwilling to provide consent. Enrolled patients were compensated $10 for completing the study surveys and interviews. In the analyses presented here, we included patients if they were currently receiving a single commonly used (i.e., >5% of subjects) systemic therapy or phototherapy for a primary indication of plaque psoriasis. We excluded patients from this analysis who were not currently receiving systemic or phototherapy for their psoriasis, who were receiving more than one systemic or phototherapy at the time of their visit, and whose primary indication was another variant of psoriasis other than plaque (e.g., guttate, palmar plantar, etc).
Variables
Trained study coordinators collected data using standardized case report forms. Data were gathered via patient self-report with confirmation by the patient’s dermatology clinic record and assessments by the clinician investigators. Detailed data were collected on exposure factors including medical history, current and past psoriasis treatments, socio-demographic factors, psoriasis characteristics, height, weight, alcohol use, and tobacco use history. Current psoriasis monotherapy was the main exposure with the other variables serving as potential confounders or effect modifiers. The primary outcome variable was a Physician Global Assessment of psoriasis lesions (0=clear, 1=minimal, 2=mild, 3=moderate, 4=marked, 5=severe, individually scored for erythema, induration, and scaling and then averaged), dichotomized as clear or almost clear disease (0–1) versus mild to severe disease (2–5). which has been widely used in psoriasis clinical trials21–23. The Psoriasis Area and Severity Index (PASI) and BSA were also evaluated as objective outcomes, and the Dermatology Life Quality Index (DLQI) and patient report of current prescription topical treatment use within the last week were assessed as patient-reported outcomes. The PASI was dichotomized such that a score of 2 or less was considered to indicate no or minimal disease (based on a receiver operating characteristic analysis comparing PASI scores to PGA scores). A BSA of < 3% was considered mild disease based on National Psoriasis Foundation definitions which have been extensively used in research, and previously published banding of DLQI scores were used to determine cut points upon which to dichotomize this endpoint12,24.
Study size
The study is descriptive in nature and therefore a sample size for specific analyses was not determined a priori. We estimated that DCERN would collect data on approximately 2000 patients which would yield precise estimates, with the half-width of the 95% confidence interval around rates for dichotomous variables being approximately 0.02.
Statistical Analysis
We first conducted descriptive statistics of the patient population and evaluated univariate analyses using the Kruskal-Wallis test for grouped ordinal data, t-tests and Mann-Whitney tests for pair-wise comparisons of continuous data, and chi-square or Fisher’s exact test for dichotomous data. We then performed modified Poisson regression with robust error variance to determine which factors independently predicted optimal patient outcomes as defined above (see “Variables” section)25. Methotrexate was chosen as the base (reference) treatment as it is often considered the standard to which novel therapies are compared. To build our model, we used a purposeful selection approach in which all covariates thought to be clinically important a priori as well as any covariates with a p-value <0.10 in univariate analyses were included in the initial multivariable model26. Non-significant covariates were eliminated from the model if their removal did not change the risk ratio estimates of other covariates by more than 10%. Variables were considered for removal first if they were included in the model based on p-value and then subsequently based on their perceived clinical importance. Model fit was assessed using goodness-of-fit tests based on deviance and Pearson statistics. The modified Poisson modeling approach was used to yield the clinically relevant statistic of relative response rates (i.e., relative risk), which were then used to calculate the relative response difference and the number needed to treat. As a sensitivity analysis, we performed logistic regression and converted odds ratios (OR) to relative risks (RR) using previously published formulae27. We also performed a variety of sensitivity analyses, including varying the outcome definition by using PASI, BSA, DLQI, and more stringent cut points of PGA and examining different durations of treatment use.
Results
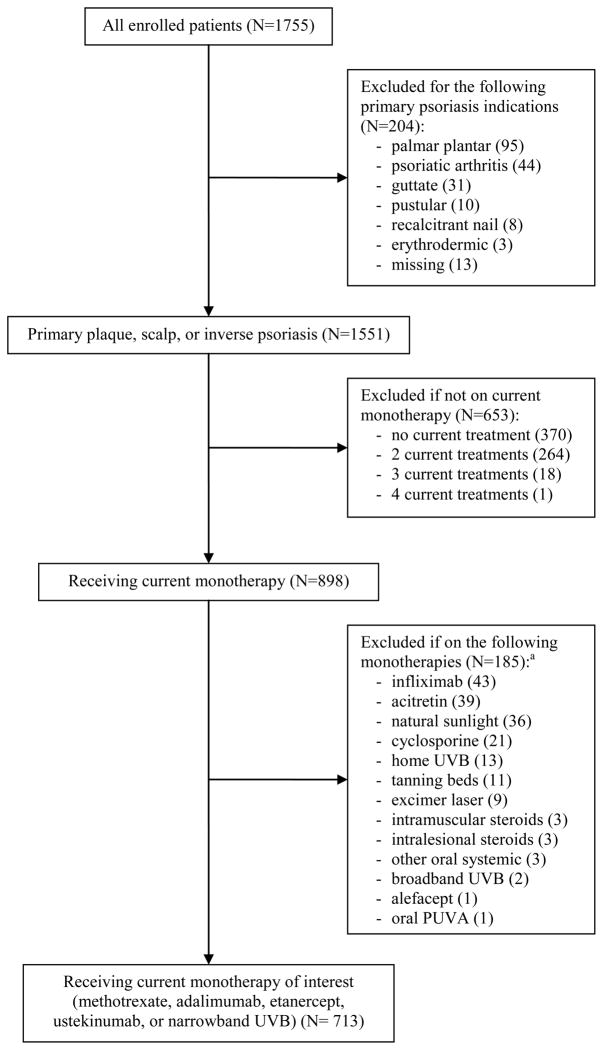
We collected data on 1755 consecutively eligible psoriasis patients (5% of patients declined to participate), which was within 12% of our projected sample size; the 713 patients who were receiving commonly used mono- systemic or phototherapy for plaque psoriasis are included in this analysis (Figure 1). Missing data did not exceed 2.8% for any of the variables analyzed. Patients were 48.6 (standard deviation (SD) 16) years old on average, had a median (interquartile range (IQR) 1–4) of 2 co-morbidities in addition to psoriasis, and were overweight on average (Table 1). The study sample consisted of nearly equal numbers of males and females; patients of higher socioeconomic groups tended to be over-represented. The patients’ median age at psoriasis onset was 25 years, with median disease duration of 19 years; forty percent of patients had a family history of psoriasis and 22.6% had a physician diagnosis of psoriatic arthritis. Patients had used a median of 1 (IQR 0–2) systemic or phototherapy treatment prior to the current therapy being evaluated at their visit.
Figure 1.
Flow diagram of patient inclusion.
aCurrent monotherapies of interest were selected if received by more than 5% of patients on current monotherapy.
Table 1.
Baseline patient and psoriasis characteristics (N=713)
| Characteristic | N (%) |
|---|---|
| Age, mean, median (SD, IQR), y | 48.6, 49 (15.5, 38–60) |
| Female sex | 352 (49.37) |
| Practice setting of dermatologist | |
| Academic | 409 (57.36) |
| Private | 304 (42.64) |
| White/Caucasian race | 606 (84.99) |
| Body mass index (BMI) (kg/m2), median (IQR) | 28.8 (25.3, 33.0) |
| Total number of comorbidities, median (IQR)a | 2 (1–4) |
| Duration of psoriasis, median (IQR), y | 19 (8–29) |
| No. of days of topical medication use in last week, Median (IQR) | 2 (0–6) |
| Psoriatic arthritis diagnosed by a physician | 161 (22.58) |
| No. of previous biologic, oral systemic, or phototherapy treatments, median (IQR) | 1 (0–2) |
| Previous type(s) of psoriasis treatment usedb | |
| Biologic | 266 (37.31) |
| Oral systemic | 314 (44.04) |
| Phototherapy | 295 (41.37) |
| None | 184 (25.81) |
IQR, interquartile range; SD, standard deviation
Note: Percentages may not total 100% due to missing data, which did not exceed 1.5% for any particular characteristic.
Including cardiovascular, lung, infection, gastrointestinal, renal, endocrine, musculoskeletal, psychiatric, neurologic, malignant or autoimmune diseases
Percentages do not total 100% because some patients may have used more than one previous treatment
The most commonly used monotherapies (and their corresponding median duration of use) were methotrexate 10.5 months (IQR 4–24), adalimumab 11.0 months (IQR 3–17), etanercept 12.0 months (IQR 6–36), ustekinumab 4.0 months (IQR 2.0–6.0), and narrowband ultraviolet B phototherapy (NB-UVB) 1.8 months (IQR 1.0–4.0) (Table 2). Further, there were differences in duration of current treatment use with patients on ustekinumab and NB-UVB having had shorter durations of use compared to methotrexate, adalimumab, and etanercept (p<0.001). Noteworthy findings regarding dosing of psoriasis therapies were observed: 36.1% of etanercept patients received 50mg twice a week and 11.8% of adalimumab patients received either 80mg every two weeks or 40mg weekly. After excluding patients with treatment duration of less than 3 months, 30.1% and 11.5% of patients on etanercept and adalimumab received these drug doses, respectively. Moreover, 10.6% of NB-UVB patients received twelve or more phototherapy treatments in the past 4 weeks.
Table 2.
Current monotherapy use with corresponding physician- and patient-reported outcomes (N=713)
| Current treatment
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methotrexate N=174 (24.4%) | Adalimumab N=152 (21.3%) | Etanercept N=191 (26.8%) | Ustekinumab N=73 (10.2%) | Narrowband UVB N=123 (17.3%) | P valuea | ||||||
| PGA, median (IQR) | 1.7 (1.3–2.0) | 1.3 (1.0–1.7) | 1.7 (1.0–2.0) | 1.7 (1.0–2.1) | 1.7 (1.0–2.0) | <0.001 | |||||
| PASI, median (IQR) | 3.8 (1.8–6.6) | 2.5 (1.2–4.8) | 2.9 (1.8–4.9) | 4.0 (1.0–7.9) | 3.5 (2.0–5.5) | 0.02 | |||||
| BSA, median (IQR) | 3.0 (1.0–6.0) | 2.0 (0.7–5.0) | 2.0 (0.5–4.5) | 3.0 (0.6–9.1) | 3.3 (1.0–6.5) | 0.01 | |||||
| DLQI, median (IQR) | 3 (1–5) | 2 (0–5) | 2 (1–5) | 3 (1–6) | 3 (1–7) | 0.15 | |||||
| Weekly dose: | Dose: | Dose: | Three-month dose: | No. of treatments in the past 4 weeks: | |||||||
| Treatment dosing/Frequency | <7.5 mg | 1.7% | 40mg q2w | 86.8% | 50mg q2w | 4.7% | 45mg/kg: | 56.2% | <3: | 5.7% | N/A |
| 7.5–15mg | 62.6% | 80mg q2w | 0.7% | 25mg qw | 3.1% | 90mg/kg: | 35.6% | 3–5: | 23.6 % | ||
| 17.5–25mg | 27.6% | 40mg qw | 11.2% | 50mg qw | 49.7% | 6–8: | 31.7% | ||||
| ≥30mg | 5.2% | 25mg 2x/wk | 3.1% | 9–11: | 28.5% | ||||||
| 50mg 2x/wk | 36.1% | ≥12: | 10.6% | ||||||||
| Other | 2.9% | Other | 1.3% | Other | 2.6% | Other | 5.5% | ||||
| Prescription topical use in past week, median (IQR), days | 2 (0–7) | 2 (0–6) | 1 (0–4) | 0 (0–4) | 4 (1–7) | <0.001 | |||||
| Duration of use without interruptions, median (IQR), months | 10.5 (4.0–24.0) | 11.0 (3.0–16.8) | 12.0 (6.0–36.0) | 4.0 (2.0–6.0) | 1.8 (1.0–4.0) | <0.001 | |||||
BSA, body surface area (%); DLQI, Dermatology Life Quality Index; IQR, interquartile range; PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; UV, ultraviolet; q2w, every two weeks; qw, weekly; 2x/wk, twice a week
Note: Percentages may not total 100% due to missing data, which did not exceed 2.8% for any particular outcome.
Kruskal-Wallis test
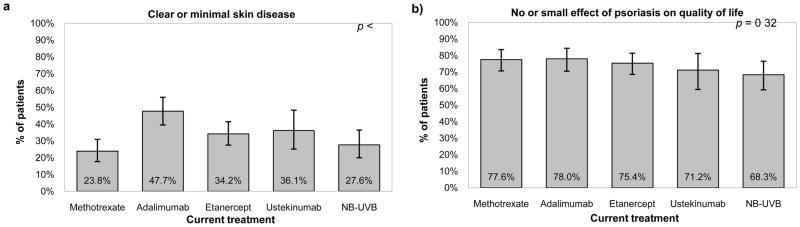
In terms of objective response measurements, we observed statistically significant differences in median PGA (p<0.001), PASI (p=0.02), and BSA (p=0.01) across these therapies, however absolute differences were small and there was no statistically significant difference in DLQI (p=0.15). There were differences in the frequency of topical prescription use within the last week with patients on NB-UVB reporting the most frequent usage (p <0.001). The crude response rate (“clear” or “almost clear” on PGA) was highest for adalimumab (47.7%, 95% confidence interval (CI) 39.5–56.0%), followed by ustekinumab (36.1%, 95% CI 25.1–48.3%), etanercept (34.2%, 95% CI 27.5–41.4%), NB-UVB (27.6%, 95% CI 20.0–36.4%), and methotrexate (23.8%, 95% CI 17.7–30.9%) (Figure 2a). Using DLQI to assess outcome provides a different profile; the response rate, defined as no or small effect (as indicated by scores of 5 or less), was higher and more closely aggregated among the treatments, ranging from 68.3% (95% CI 59.2–76.5%) with NB-UVB to 78.0% (95% CI 70.5–84.3%) with adalimumab (Figure 2b).
Figure 2.
Figure 2a. PGA clearance (PGA ≤ 1) by current psoriasis monotherapy. Figure 2b. No or small effect of psoriasis on quality of life (DLQI ≤ 5) by current psoriasis monotherapy.
PGA, Physician Global Assessment; NB-UVB, Narrowband ultraviolet B; DLQI, Dermatology Life Quality Index
Note: Error bars indicate 95% confidence interval.
Patients who were responders based on PGA were more likely to be female, normal or underweight, treated in a private practice setting, and have had longer duration of current treatment use, and less likely to have used topical prescription therapy within the last week (data not shown). The unadjusted and adjusted relative rate of PGA responses are shown in Table 3; in comparison to patients on methotrexate, those receiving adalimumab, etanercept, and ustekinumab all had significantly higher response rates. Those on NB-UVB also had a higher, although not statistically significant, response rate. Among therapies with statistically significant differences in response rates, the number needed to treat ranged from 4 to 10 (Table 3); for instance, 4 patients (rounded up from 3.6 as per convention) would need to be treated with adalimumab in order to achieve one additional treatment response over what would be expected if those same four patients were treated with methotrexate.
Table 3.
Relative rates of Physician Global Assessment clearance and risk differences by current monotherapy (N=704)
| Current Treatment | Unadjusted RR (95% CI) | Adjusted RRa (95% CI) | Risk differenceb (95% CI) | NNTc |
|---|---|---|---|---|
| Methotrexate (ref.) | 1.00 | 1.00 | - | - |
| Adalimumab | 2.00 (1.46–2.74) | 2.15 (1.60–2.90) | 0.27 (0.14–0.45) | 3.6 |
| Etanercept | 1.44 (1.03–2.00) | 1.45 (1.06–1.97) | 0.11 (0.01–0.23) | 9.4 |
| Ustekinumab | 1.51 (1.01–2.28) | 1.57 (1.06–2.32) | 0.13 (0.01–0.31) | 7.4 |
| Narrowband UVB | 1.16 (0.78–1.72) | 1.35 (0.93–1.96) | 0.08 (−0.02–0.23) | 11.9 |
CI, confidence interval; NNT, number needed to treat; RR, relative rate; UVB, ultraviolet B
Adjusted for sex, race, ethnicity, body mass index, skin type, frequency of topical use, practice setting of dermatologist, marital status, income, and insurance
Difference between adjusted and baseline risk
Number of patients needed to treat with the particular treatment to gain 1 additional patient with PGA clearance relative to the response achieved with methotrexate
In sensitivity analyses, there was no evidence of any response rate differences when using DLQI as the outcome (data not shown). When we evaluated outcomes of BSA or PASI, the differences in response rates were attenuated and occasionally lost statistical significance, particularly in the cases of etanercept and ustekinumab. When evaluating duration of current therapy use (at least 3, 6, or 12 months), estimates for adalimumab remained stable and those for ustekinumab showed evidence of increasing efficacy with longer duration of treatment while results for etanercept and NB-UVB were attenuated and lost statistical significance. The crude response rates for patients treated for three or more months were 26.4% (95% CI 19.3–34.5) for methotrexate, 50.4% (95% CI 41.2–59.6) for adalimumab, 36.4% (95% CI 29.0–44.3) for etanercept, 46% (95% CI 31.8–60.7) for ustekinumab, and 41.5% (95% CI 26.3–57.9) for NB-UVB.
Discussion
This study comprehensively details the effectiveness of commonly used systemic and phototherapy treatments for moderate-to-severe psoriasis in the real-world clinical practice setting. Based on a single assessment of PGA, only 24–48% of psoriasis patients currently receiving systemic or phototherapy treatment were clear or almost clear of their psoriasis. Of special importance, the effectiveness of systemic psoriasis therapies was lower in the real world practice setting compared to their reported efficacy in the randomized controlled trial (RCT) setting. For example, the rate of being clear or almost clear of psoriasis in our study in contrast to that in the CHAMPION trial (RCT of methotrexate vs. adalimumab vs. placebo) was 24% vs. 30%, respectively, for methotrexate and 48% vs. 73%, respectively, for adalimumab28. Similarly, the PGA response rate in our study compared to that in the ACCEPT trial (RCT of etanercept vs. ustekinumab) was 34% vs. 49%, respectively, for etanercept, and 36% vs. 65–71%, respectively, for ustekinumab29. Moreover, 36.1% of etanercept patients and 11.8% of adalimumab patients received twice the maintenance dose recommended based on clinical trial data,30,31 while only 10.6% of patients on phototherapy were receiving the frequency of treatments (i.e. at least three times per week) necessary to optimize response.32 Patients who participate in clinical trials may differ from “real world” patients in their health status, willingness to adhere to treatment regimens, and other factors which may result in discrepancies between idealized clinical trial results and real world outcomes, thus further emphasizing the need for effectiveness studies in real-world clinical practice settings. In our multivariable model, the three biologics studied – adalimumab, etanercept, and ustekinumab – were all more effective than the reference standard methotrexate based on PGA, even after comprehensively adjusting for numerous potential confounding factors. However, absolute differences in PGA were small and the relative rate of response was attenuated, and in some cases no longer statistically significant, when evaluating other physician-reported outcomes such as PASI and BSA. Importantly, although PGA has been recommended for community-based psoriasis research, there is no widely accepted gold standard for defining a psoriasis treatment response at a static point in time and our primary objective response analysis was sensitive to the type of endpoint evaluated33,34. Additionally, although to our knowledge, we used the identical PGA as reported in ACCEPT and a nearly identical PGA as used in CHAMPION other studies may use PGA’s with different ranges or a dynamic approach (i.e. relying on the investigators memory of baseline severity in comparison to current severity) and thus caution may be indicated in comparing studies that used different types of PGA’s.
In patient-reported outcomes on the DLQI, 68–78% of patients reported no or only mild effect of psoriasis on their health-related quality of life, indicating higher response to therapy on subjective, patient-reported measures than on objective, physician-reported outcomes24. Importantly, the adjusted response rate for health-related quality of life, which has been suggested to be a better metric of psoriasis severity than objective measures (i.e. BSA), was nearly identical across the therapies we evaluated. Similarly, the differences we observed in PGA response rates were not mirrored by differences in patient self-report of topical prescription treatment use. In summary, these findings suggest that although there are differences in treatment response rates based on objective measures, these differences are small and may not be of clinical significance.
Our study has important limitations to consider. Despite our inclusion of a broad range of consecutively enrolled patients and a multivariable analysis that comprehensively adjusted for covariates, treatment assignment was not randomized and therefore we cannot fully exclude confounding and selection bias as potential sources of error. Additionally, phototherapy patients tend to be purposefully evaluated at intermediate time points (i.e., it is necessary to individually fine-tune dosing prior to achieving a clinical response) so assessment patterns for NB-UVB may have systematically differed from assessment patterns of systemic medications. Similarly, ustekinumab became available in the U.S. in September 2009 resulting in differing duration of use compared to more established therapies. Moreover, study assessments were not conducted by individuals blinded to treatment status which could introduce information bias, although such error is unlikely to have systematically affected the results in any particular direction. Importantly, since this was not a longitudinal study, the phenomenon of clinical drift is likely present and thus our results may overestimate the effectiveness of therapies in clinical practice; in other words, only patients with successful response to treatment remain on the therapy. Similarly, given the cross-sectional nature of the study, we were not able to compare the relative safety of the therapies. Moreover, although we found no differences in health-related quality of life, it is possible that the DLQI was not sensitive enough to detect differences which may exist among patients taking systemic or phototherapy treatments in the real world practice setting despite its ability to distinguish between methotrexate and adalimumab in the clinical trial setting35. Additionally, we have focused only on current monotherapy in this analysis and thus cannot speak to the comparative effectiveness of combination therapies. Finally, inclusion of more practices and patients from various regions of the U.S. might further improve the generalizability of the findings.
In conclusion, we conducted a large cross-sectional study evaluating the effectiveness of commonly used systemic and phototherapy treatments for moderate to severe psoriasis in real world settings that provides important benchmarks to guide future research and policy. Our findings suggest that although differences in objective responses may exist among these treatment options, absolute differences are small and may not be clinically significant. Furthermore, the absolute response rate to therapies for moderate to severe psoriasis may be lower in the real world setting than what has been previously observed in controlled clinical trials. Future longitudinal comparative effectiveness studies in real world practice settings are necessary to confirm and extend our findings.
Acknowledgments
Funding/Support: This study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases RC1-AR058204 (J.M.G.) and the National Institute of Health Training Grant T32-AR07465 (J.W., D.B.S., H.Y.).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Attribution: Dr. Gelfand has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Gelfand, Callis Duffin, Weisman; Acquisition of data: Gelfand, Wan, Callis Duffin, Krueger, Kalb, Weisman, Sperber, Stierstorfer, Brod, Schleicher, Bebo, Shin, Steinemann, Goldfarb, Van Voorhees; Analysis and interpretation of data: Gelfand, Wan, Callis Duffin, Krueger, Kalb, Troxel, Shin, Yeung, Van Voorhees; Drafting of the manuscript: Gelfand, Krueger, Schleicher; Critical revision of the manuscript for important intellectual content: Wan, Callis Duffin, Krueger, Kalb, Weisman, Sperber, Stierstorfer, Brod, Bebo, Troxel, Shin, Steinemann, Goldfarb, Yeung, Van Voorhees; Statistical analysis: Gelfand, Wan, Troxel, Shin, Yeung; Obtained funding: Gelfand, Callis Duffin, Krueger; Administrative, technical, or material support: Kalb, Weisman, Brod, Bebo, Shin, Steinemann, Goldfarb; Study supervision: Gelfand, Krueger, Sperber, Brod, Goldfarb.
Financial Disclosures:
1) Relevant to this manuscript: Dr. Gelfand served as consultant with Abbott, Amgen, Celgene, Centocor, Novartis, and Pfizer, receiving honoraria; had grants or has pending grants from Abbott, Amgen, Genentech, Novartis, and Pfizer; and received payment for CME work related to psoriasis. He also received a donation from Amgen to the University of Pennsylvania to further develop DCERN, which were not used for the current study. Dr. Callis Duffin serves on the scientific and marketing advisory boards of Amgen; served as consultant to Amgen and Centocor, receiving honoraria; received payments for lectures from Abbott, Amgen, and Cenotcor; and served as investigator for Abbott, Amgen, Centocor, and Pfizer. Dr. Krueger served as consultant for Abbott, Amgen, and Centocor; had grants or has pending grants from Abbott and Amgen; received payment for lectures and travel-related expenses from Abbott, Amgen, and Centocor. Dr. Kalb served as consultant for Abbott, Amgen, Centocor, LEO Pharma, and Stiefel, receiving honoraria; served as investigator for Abbott, Amgen, Astellas Pharma, and Centocor, receiving honoraria, and served as speaker for Abbott, Amgen, Centocor, Galderma, and Stiefel. Dr. Weisman had grants or has pending grants from Abbott, Braintree Laboratories, Celgene, Cipher Pharmaceuticals, and Leo Pharmaceuticals; and received payments for lectures from Abbott and Amgen. Dr. Sperber is the Medical Director of Stephens & Associates; served as consultant for Amgen; and had grants or has pending grants from Abbott and Centocor. Dr. Bebo is employed by the National Psoriasis Foundation, which receives unrestricted financial support from companies that makes products used to treat psoriasis, including Amgen, Abbott, Janssen, Stielfel/GSK, Warner Chilcott, Wyeth, Pfizer, Galderma and PhotoMedix. Dr Van Voorhees served on advisory boards for Amgen, Abbott, Genentech, Warner Chilcott, Connectics, Bristol Myers Squibb, and Centocor; served as investigators for Amgen, Genentech, Warner Chilcott, Roche, Astellas, Bristol Myers Squibb, and IDEC, receiving grants; served as consultants for Amgen, Incyte, VGX, Xtrac, and Leo, receiving honoraria; served as speaker for Amgen, Abbott, Genentech, Connetics, and Centocor, receiving honoraria; and received honoraria from Synta. Ms. Wan, Dr. Stierstorfer, Dr. Brod, Dr. Schleicher, Dr. Troxel, Mr. Shin, Ms. Steinemann, Ms. Goldfarb, and Mr. Yeung have no financial disclosures to report.
2) All other relationships: Dr. Gelfand serves on the AAD task force on recent psoriasis guidelines and on the editorial boards of Pharmacoepidemiology and Drug Safety and the Journal of the American Academy of Dermatology. Dr. Kalb serves as an associate editor of the Psoriasis Forum.
Contributor Information
Joel M. Gelfand, Email: joel.gelfand@uphs.upenn.edu.
Joy Wan, Email: joywan@mail.med.upenn.edu.
Kristina Callis Duffin, Email: kristina.callis@hsc.utah.edu.
Gerald G. Krueger, Email: gerald.krueger@hsc.utah.edu.
Robert E. Kalb, Email: kalb@buffalo.edu.
Jamie D. Weisman, Email: jweisman@peachtreedermatology.com.
Brian R. Sperber, Email: sperber.derm@gmail.com.
Michael B. Stierstorfer, Email: mstierstorfer@gmail.com.
Bruce A. Brod, Email: babrod@comcast.net.
Stephen M. Schleicher, Email: sschleicher@dermdox.org.
Bruce F. Bebo, Jr., Email: bbebo@psoriasis.org.
Andrea B. Troxel, Email: atroxel@mail.med.upenn.edu.
Daniel B. Shin, Email: dbshin@mail.med.upenn.edu.
Jane M. Steinemann, Email: jane.steinemann@gmail.com.
Jennifer Goldfarb, Email: jennifer.goldfarb@uphs.upenn.edu.
Howa Yeung, Email: howa.yeung@uphs.upenn.edu.
Abby S. Van Voorhees, Email: abby.vanvoorhees@uphs.upenn.edu.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009 Jul 30;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Richarson SK, Gelfand JM. Update on the natural history and systemic treatment of psoriasis. Advances in Dermatology. 2008;24:172–196. doi: 10.1016/j.yadr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010 Sep;163(3):586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009 Oct;129(10):2411–2418. doi: 10.1038/jid.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004 Nov;51(5):704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Gelfand JM, Mehta NN, Langan SM. Psoriasis and cardiovascular risk: strength in numbers, part II. J Invest Dermatol. 2011;131:1007–1010. doi: 10.1038/jid.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. Jama. 2006 Oct 11;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 8.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010 Apr;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999 Sep;41(3 Pt 1):401–407. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 10.Pariser DM, Bagel J, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol. 2007 Feb;143(2):239–242. doi: 10.1001/archderm.143.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Stern RS, Nijsten T, Feldman SR, Margolis DJ, Rolstad T. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004 Mar;9(2):136–139. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009 Feb;60(2):218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009 Sep;61(3):451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008 May;58(5):826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 15.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: A systematic review and meta-analysis of randomized controlled trials. Journal of the American Academy of Dermatology. 2011;64(6):1035–1050. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seshadri S, Zornberg GL, Derby LE, Myers MW, Jick H, Drachman DA. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch Neurol. 2001 Mar;58(3):435–440. doi: 10.1001/archneur.58.3.435. [DOI] [PubMed] [Google Scholar]
- 17.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: Case-based presentations and evidence-based conclusions. Journal of the American Academy of Dermatology. 2011;65(1):137–174. doi: 10.1016/j.jaad.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 18.Naldi L, Svensson A, Diepgen T, et al. Randomized clinical trials for psoriasis 1977–2000: the EDEN survey. J Invest Dermatol. 2003 May;120(5):738–741. doi: 10.1046/j.1523-1747.2003.12145.x. [DOI] [PubMed] [Google Scholar]
- 19.Naldi L, Svensson A, Zenoni D, et al. Comparators, study duration, outcome measures and sponsorship in therapeutic trials of psoriasis: update of the EDEN Psoriasis Survey 2001–2006. British Journal of Dermatology. 2010;162(2):384–389. doi: 10.1111/j.1365-2133.2009.09515.x. [DOI] [PubMed] [Google Scholar]
- 20.Iglehart JK. Prioritizing Comparative-Effectiveness Research — IOM Recommendations. New England Journal of Medicine. 2009;361(4):325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 21.FDA. [Accessed December 7 2011, 2011.];Enbrel (etanercept) for the Treatment of Pediatric Plaque Psoriasis. 2011 http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4361b2-01-FDA.pdf.
- 22.Centocor [Accessed December 7 2011, 2011.];Briefing Document for Ustekinumab (CNTO 1275) 2011 http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4361b1-02-CENTOCOR.pdf.
- 23.Cook D. [Accessed December 8, 2011, 2011.];Clinical Review: BLA 125057/110. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/125057s110_MedR_P2.pdf.
- 24.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the Science of Quality of Life into Practice: What Do Dermatology Life Quality Index Scores Mean[quest] J Investig Dermatol. 2005;125(4):659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 25.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley and Sons; 2000. [Google Scholar]
- 27.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998 Nov 18;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 28.Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) British Journal of Dermatology. 2008;158(3):558–566. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of Ustekinumab and Etanercept for Moderate-to-Severe Psoriasis. New England Journal of Medicine. 2010;362(2):118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 30.Humira® (adalimumab) [package insert] North Chicago, IL: Abbott Laboratories; 2011. [Google Scholar]
- 31. [Accessed August 24 2011, 2011.];Enbrel Prescribing Information. http://pi.amgen.com/united_states/enbrel/derm/enbrel_pi.pdf.
- 32.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010 Jan;62(1):114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Robinson A, Kardos M, Kimball AB. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): Why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2011 Oct 29; doi: 10.1016/j.jaad.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Spuls PI, Lecluse LLA, Poulsen M-LNF, Bos JD, Stern RS, Nijsten T. How Good Are Clinical Severity and Outcome Measures for Psoriasis[quest]: Quantitative Evaluation in a Systematic Review. J Invest Dermatol. 2010;130(4):933–943. doi: 10.1038/jid.2009.391. [DOI] [PubMed] [Google Scholar]
- 35.Revicki D, Willian MK, Saurat JH, et al. Impact of adalimumab treatment on health-related quality of life and other patient-reported outcomes: results from a 16-week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2008 Mar;158(3):549–557. doi: 10.1111/j.1365-2133.2007.08236.x. [DOI] [PubMed] [Google Scholar]