Abstract
Background
Aerva javanica (Burm. f.) Juss. ex Schult. (Amaranthacea) is traditionally used for the treatment of wound healings, cough, diarrhoea, ulcer and hyperglycaemia. The current study was aimed to fractionate and isolate bioactive compounds and ultimately to evaluate their anti-ulcereogenic potential.
Results
In order to achieve these aims, the fractionation, purifications and then biological potential determination of the isolated compounds was carried out. For purification purpose, initially extraction of the plant material was done with aqueous MeOH in the order of increasing polarity by using solvent-solvent extraction method. Phytochemical analysis revealed the presence of three compounds, 3-hydroxy-4 methoxybenzaldehyde (1), ursolic acid (2) and (E)-N-(4-hydroxy-3-methoxyphenethyl)-3-(4-hydroxy-3-ethoxyphenyl) acryl amide (3). Inhibition of urease activity of various fractions revealed that ethyl acetate fraction showed significant activity (P <0.05) as compared to other fractions. (E)-N-(4-hydroxy-3-methoxyphenethyl)-3-(4-hydroxy-3-ethoxyphenyl) acryl amide (3) showed marked anti ulcer activity (P <0.05).
Conclusion
These results suggested the mild potential of A. javanica against ulcer.
Keywords: Aerva javanica, Urease activity, Ethyl acetate fraction, Solvent-solvent extraction
Background
Medicinal plants play important role in the treatment of various disorders [1,2]. The plants of genus Aerva (Amaranthaceae) are perennial herb under shrubs and are found in the North Temperate Zone, especially in the Mediterranean regions and Asia. About 20 species of genus Aerva are present in Pakistan and India; most of them are used in traditional medicinal system [3]. A. javanica (Burm.f.) Juss. ex Schult., locally known as Khar Buta, is found over a broad range of sandy sediments. Various chemical constituents including steroids, triterpenes, lipids, flavonoids, tannins, saponins, alkaloids, sulphates, carbohydrates and glycosides have been isolated from this plant [4,5]. The plant has been widely used for its therapeutic effects in relieving the swelling and pain due to kidney stones [6]. The plant decoction is used for dysentery, gonorrhea and cutaneous infections [7]. A. javanica showed antioxidant [8], antiviral [9], antiplasmodial [10] and antidiabetic activities [11].
Urease (urea amidohydrolase) is an enzyme that catalyzes the hydrolysis of urea to ammonia and carbamate, which is the final step of nitrogen metabolism in living organisms [12]. Carbamate rapidly and spontaneously decomposes, yielding a second molecule of ammonia. These reactions may cause significant increase in pH and are responsible for negative effects of urease activity in human health and agriculture [13,14]. Urease is responsible for urinary tract and gastrointestinal infections, possibly causing severe diseases such as peptic ulcers and stomach cancer as in the case of Helicobacter pylori. Ureases are also involved in the development of urolithiasis, pyelonephritis, hepatic encephalopathy, hepatic coma and urinary catheter encrustation [15,16]. Here, we investigated the antiulcer activity of different fractions as well as pure constituents of A. javanica.
Results and discussion
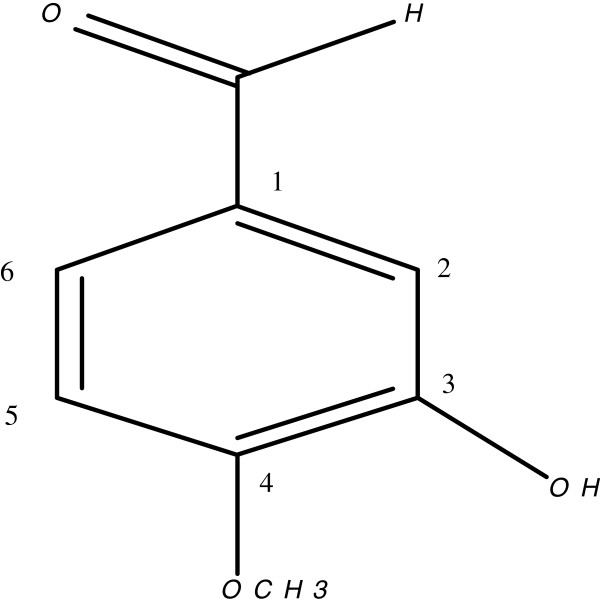
Compound 1 was isolated as white powder from the ethyl acetate fraction by CC and through elution with hexane: EtOAc (5:5). The HR-EIMS of 1 gave the molecular ion peak (m/z 152.0012) corresponding to the molecular formula C8H8O3 (calcd. for C8H8O3, 152.0054). The IR spectrum indicated hydroxyl (3600–2500 cm-1), aldehydic (2685 cm-1), carbonyl (1705 cm-1) and aromatic ring (1626 cm-1). An aldehyde was further deduced through EIMS (m/z 151, M+-1, 100%). The 1HNMR spectrum of 1 (Figure 1 & Table 1) showed aldehyde (δH 9.82), three aromatic protons (δ 7.34 dd, 7.43 d and 7.1 d), hydroxyl X(δH 8.12) and a methoxy (δH 3.93, s) functionality. 13 C-NMR signals at δc 153.8, 148.0, 131.6, 125.1, 114.5, 111.9 further supported the proposed structure. The signal at δC 56.4 indicated –OCH3 funtionality. Comparison of spectral data of 1 with literature data identified compound 1 to be 3-hydroxy-4-methoxybenzaldehyde. Ursolic acid (2) (Figure 2 & Table 2) was obtained as colorless white crystals from the ethyl acetate fraction. IR spectrum showed strong absorptions for hydroxyl (3510 cm-l), carbonyl (1697 cm-l) and double bond (1635 and 815 cm-1). The molecular ion peak was at m/z 456.3599 in HR-EI-MS, corresponding to the molecular formula C30H48O3 (calcd for, 456.3603). The base peak at m/z 248 was due to β-type triterpenes. The HR-EIMS also exhibited a prominent peak at m/z 411.3640, due to the loss of COOH group. The peak at m/z 203.1810 was attributed to the loss of COOH from fragment at m/z 248.1743 at C-17, representing retero-Diels Alder fragmentation, which is a characteristic of Δ12 ursane type triterpene. Five tertiary methyl singlets at δH 1.20, 1.11, 0.97, 0.86, 0.82, along with two doublets at δH 1.06 (3 H, d, J = 6.6 Hz) and δH 0.99 (3 H, d, J = 6.4 Hz) in 1HNMR spectrum were the indication of ursane basic skeleton. The olefinic proton was at δH 5.20 (J = 3.5 Hz). A doublet at 2.20 ppm with J value of 11.3 Hz revealed that the protons at C-18 and C-19 were trans to eachother. Compound 3 (Figure 3 & Table 3) was obtained as yellowish white amorphous powder from EtOAc fraction. Molecular formula of 3 was established as C19H21NO5 by HR-EIMS, due to an ion at m/z 343.1411 (calcd 343.1420). The IR absorption bands at 3350 cm-1and 1650 cm-1 showed hydroxyl and amide functionalities, respectively. Highly conjugated system was exhibited due to strong absorption at 319, 290 and 220 nm in UV spectrum.
Figure 1.
3-hydroxy-4 methoxybenzaldehyde.
Table 1.
1H and 13C NMR spectral data for compound 1
| Position | 13C-NMR (δC) | 1H-NMR (δH) | JHH(Hz) |
|---|---|---|---|
| 1 |
131.6 |
- |
- |
| 2 |
114.5 |
7.43 |
d, J = 2.0 |
| 3 |
148.0 |
- |
- |
| 4 |
153.8 |
- |
- |
| 5 |
111.9 |
7.12 |
d, J = 8.7 |
| 6 |
125.1 |
7.34 |
dd, J = 8.7, 2.0 |
| OMe |
56.4 |
3.93 |
s |
| CHO | - | 9.82 | s |
Figure 2.
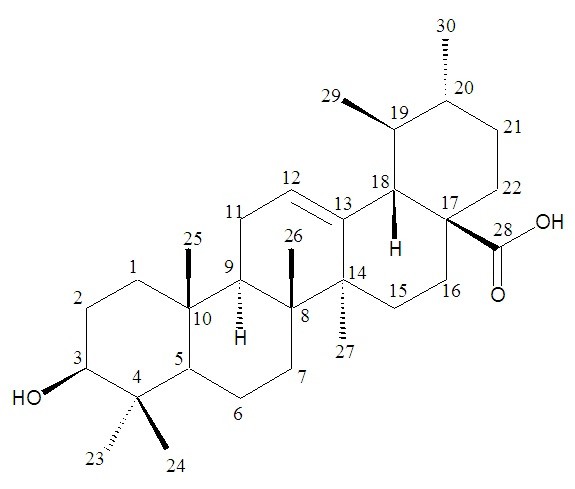
ursolic acid.
Table 2.
1H and 13CNMR spectral data for compound 2
| Position | 13C-NMR (δC) | 1H-NMR (δH) | JHH(Hz) |
|---|---|---|---|
| 1 |
38.5 |
1.56 |
m |
| 2 |
27.4 |
1.43 |
m |
| 3 |
79.1 |
3.13 |
dd, J = 10.0, 4.5 |
| 4 |
38.7 |
- |
- |
| 5 |
52.4 |
0.66 |
s |
| 6 |
18.3 |
1.47 |
m |
| 7 |
33.2 |
1.27 |
m |
| 8 |
39.6 |
- |
- |
| 9 |
47.4 |
1.58 |
s |
| 10 |
37.1 |
- |
- |
| 11 |
23.9 |
1.92 |
dd, J = 13.7, 3.5 |
| 12 |
125.8 |
5.20 |
t, J = 3.5 |
| 13 |
138.7 |
- |
- |
| 14 |
42.0 |
- |
- |
| 15 |
29.4 |
1.01 |
m |
| 16 |
23.5 |
1.53 |
m |
| 17 |
47.9 |
- |
- |
| 18 |
55.2 |
2.20 |
d, J = 11.3 |
| 19 |
30.5 |
1.31 |
m |
| 20 |
30.3 |
1.52 |
m |
| 21 |
27.5 |
1.29 |
m |
| 22 |
37.0 |
1.54 |
m |
| 23 |
24.0 |
1.11 |
s |
| 24 |
15.4 |
0.82 |
s |
| 25 |
15.9 |
0.97 |
s |
| 26 |
17.2 |
0.86 |
s |
| 27 |
24.5 |
1.20 |
s |
| 28 |
176.2 |
- |
- |
| 29 |
22.4 |
0.79 |
d, J = 6.8 |
| 30 | 24.0 | 1.06 | d, J = 6.6 |
Figure 3.
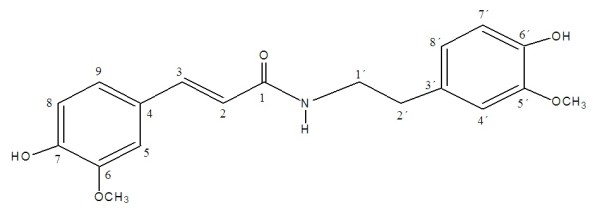
(E)-N-(4-hydroxy-3-methoxyphenethyl)-3-(4-hydroxy-3-ethoxyphenyl) acryl amide.
Table 3.
Prominent 1H and 13CNMR spectral data for compound 3
| Position | 13C-NMR (δC) | 1H-NMR (δH) | JHH(Hz) |
|---|---|---|---|
| Feruloyl moiety | |||
| 1 |
169.2 |
- |
- |
| 2 |
118.8 |
5.82 |
d, J = 12.8 |
| 3 |
142.0 |
6.61 |
d, J = 12.8 |
| 4 |
132.1 |
- |
- |
| 5 |
111.6 |
7.36 |
d, J = 2.0 |
| 6 |
149.9 |
- |
- |
| 7 |
149.0 |
- |
- |
| 8 |
116.5 |
6.73 |
d, J = 8.4 |
| 9 |
123.2 |
6.93 |
dd, J = 8.4, 2.0 |
| OMe |
56.4 |
3.82 |
s |
| Methoxytyramine moiety | |||
| 1´ |
42.5 |
3.49 |
t, J = 7.2 |
| 2´ |
36.2 |
2.77 |
t, J = 7.2 |
| 3´ |
128.3 |
- |
- |
| 4´ |
113.5 |
6.82 |
d, J = 2.0 |
| 5´ |
149.3 |
- |
- |
| 6´ |
146.1 |
- |
- |
| 7´ |
116.2 |
6.63 |
d, J = 8.0 |
| 8´ |
122.3 |
6.67 |
dd, J = 8.0, 2.0 |
| OMe´ | 56.6 | 3.88 | s |
The signals at δH 6.73 (1 H, d, J = 8.4 Hz), δH 6.93 (1 H, dd, J = s 8.4,2.0 Hz) and 7.36 (1 H, d, J = 2.0 Hz) were for H-8, H-9 and H-5 of ferulic acid moiety, respectively. The C-3 olefinic proton of the ferulic acid moiety exhibited a doublet at δH 6.61 (J = 12.8 Hz) which showed the trans-coupling with C-2 olefinic proton, having a doublet at δH 5.82 (J = 12.8 Hz). A further ABX was observed at δH 6.67 (1 H, dd, J = 8.0.2.0 Hz) for H-8´, δH 6.63 (1 H, d, J = 8.0 Hz) for H-7´ and 6.82 (1 H, d, J = 2.0 Hz) for H-4´ in the methoxytyramine moiety. Two coupled triplets of methylene protons at δH 2.77 and δH 3.49 (each 2 H, t, J = 7.2 Hz) were assigned to H-2´ and H-1´, in the methoxtyramine moiety, respectively. In 13C-NMR spectrum, the signal at 169.2 indicated an amide functionality. 13C-NMR spectrum of compound 3 showed resonances of two methyls; two methylenes, eight methines and seven quaternary carbons. The mass, UV, IR and 1 H-NMR data proposed compound 3 an amide with phenolic acid funtionality. The position of aromatic substituent was deduced by NOESY experiment. Detailed spectroscopic data established the structure of compound 3 as the (E)-N-(4-hydroxy-3-methoxyphenethyl)-3-(4-hydroxy-3-ethoxyphenyl) acryl amide. Similarly various bioactive compounds were isolated from A. javanica[17].
Various fractions of A. javanica were tested preliminary for inhibition of jack bean urease enzyme for their potential against ulcer by using 0.2 mg/ml of each fraction and thiourea (Table 4). The data of various fractions revealed that ethyl acetate fraction of A. javanica exhibited significant inhibition as compared to other fractions. Therefore further chemical investigation was focused on this fraction, which led to the isolation of compounds 1–3.
Table 4.
Effects of various fractions on Urease inhibition
| Sr.# | Name of the fraction | % Inhibition |
|---|---|---|
| 1 |
0.2 mg/ml Hexane |
15.3 ± 1.2a |
| 2 |
0.2 mg/ml Dichloromethane |
33.4 ± 3.2b |
| 3 |
0.2 mg/ml Ethyl acetate |
54.6 ± 4.1c |
| 4 |
0.2 mg/ml Water |
5.1 ± 0.9a |
| 5 | 0.2 mg/ml Thiourea | 98.2 ± 5.1d |
Each value (a,b,c and d) in the table is represented as mean ± SD (n = 3).
X3-Hydroxy-4-methoxybenzaldehyde (1), ursolic acid (2), and (E)-N-(4-hydroxy-3-methoxyphenethyl)-3-(4-hydroxy-3-ethoxyphenyl) acryl amide (3) were screened for inhibition of urease activity (Table 5). Table data revealed that (E)-N-(4-hydroxy-3-methoxyphenethyl)-3-(4-hydroxy-3-methoxyphenyl) acryl amide (3) showed maximum inhibition might be responsible for antiulcer activity.
Table 5.
Effects of isolated compounds 1–3 on Urease inhibition
| Sr.# | Name of the compounds | % Inhibition |
|---|---|---|
| 1 |
0.2 mg/ml Compound 1 |
15.3 ± 0.8a |
| 2 |
0.2 mg/ml Compound 2 |
33.4 ± 2.7b |
| 3 |
0.2 mg/ml Compound 3 |
64.6 ± 4.2c |
| 4 | 0.2 mg/ml Thiourea | 98.2 ± 4.3d |
Each value (a,b,c and d) in the table is represented as mean ± SD (n = 3).
Experimental
General
Electron Impact Mass Spectra (EI-MS) were measured on Finnigan MAT-311 mass Spectrometer. High-Resolution Electron Impact Mass spectra (HREI-MS) were obtained on Jeol HX mass spectrometer. IR spectra were obtained in chloroform by using JASCO IRA-1 and JASCO A-302 spectrophotometers. KBr discs as supporting suface and CHCl3 was used as dissolving solvent. Ultraviolet (UV) spectra of UV active compounds were recorded in methanol by using a Shimadzu UV-240 and U-3200 Hitachi spectrophotometer. The 1H and 13CNMR, COSY 45°, HMQC and HMBC spectra were recorded on Bruker AV-400 and AV-500 spectrometer.
Plant materials
The A. javanica (whole plant) was collected from Bannu Township, Bannu, Khyber Pakhtunkhwa in March 2010. The plant was identified by Professor Abdur Rehman, Department of Botany, Government Post Graduate College Bannu, Pakistan and a specimen (W-12) was submitted at Herbarium of Biotechnology and Botany University of Science and Technology Bannu KPK, Pakistan.
Extraction and isolation
The air-dried plant (20 kg) was chopped and was initially extracted with methanol/water (4:1) at room temperature. The hydromethanolic extract (700 g) was initially partitioned into n-hexane (84 g). The defatted MeOH extract was dried and then suspended in H2O (2 L), was successively partitioned with dichloromethane (110 g), and ethyl acetate (27 g). The EtOAc soluble fraction was subjected to CC by using normal silica gel. The eluent used were n-hexane, EtOAc, DCM and MeOH in gradient manner to obtain compounds 1–3.
Compound 1
White Powder; m.p 116–117°C; IR (KBr, CHCl3) vmax (cm-1): 3600–2500, 2685; 1H and 13C NMR spectral data, see Table 1; EI-MS m/z (rel. int.): [M]+, 152.0 (100), 151 (94), 137 (5), 109 (24), 95 (3), 81 (27), 43(20); HR-EI-MS: m/z [M]+ 152.0 (calcd for C8H8O3, 152.05).
Compound 2
White crystals; m.p 283–285°C; [α]D20 + 62.5° (c = 0.2, CHCl3); IR (KBr, CHCl3) vmax (cm-1): 3510, 3050, 1697, 1635, 820; 1H and 13C NMR spectral data, see Table 2; EI-MS m/z (rel. int.): [M]+, 456 (M+, 10), 411 (22), 248 (34), 203 (100), 189 (16); HR-EI-MS: m/z [M]+ 456.3599 (calcd for C30H48O3, 456.3603).
Compound 3
Yellowish white amorphous powder; m.p 111-113°C, IR (KBr, CHCl3) vmax (cm-1): 3440, 3350, 1680, 1650; 1H and 13C NMR spectral data, see Table 3; EI-MS m/z (rel. int.): [M]+, 343 (20), 193 (30), 177 (94), 151 (25), 150 (100), 145 (30), 55 (6); HR-EI-MS: m/z [M]+ 343.1411 (calcd for C19H21NO5, 343.1420).
Urease enzyme inhibition assay
Urease (Jack bean) solution (25 μl) was mixed with the 5 mg (500 μg) extracts and the mixture was incubated at 30°C. Aliquot were taken after 15 min and then was transferred to assay mixtures having urea (100 mM) in buffer (40 μl) and again incubated for 30 min in 96 well plates. Urease activity was determined by measuring ammonia production using the indophenol method as described [18]. Briefly, 50 μ1 each of phenol reagent (1% w/v phenol and 0.005% w/v sodium nitroprusside) and 70 μ1 of alkali reagent (0.5% w/v NaOH and 0.1% active chloride NaOCI) were added to each well. The increasing absorbance was measured after 50 min at wavelength of 630 nm using microtitre plate reader (Spectramax plus 384 Molecular Device, USA). All reactions were performed in triplicates in final volume of 200 μl. All the assays were performed at pH 8.2 (0.01 M K2HPO4. 3H2O, 1 mM EDTA and 0.01 M LiC12). Thiourea was used as standard and percentage inhibitions were calculated from formula, 100 - (OD test/OD control) x 100. In this study, the reductive ability was measured by investigating the Fe+3 → Fe transformation in the presence of various extracts of plants and standard antioxidant (BHA) by using the Oyaizu method [19].
Statistical analysis
The parametric data were expressed as the mean ± SEM for the 03 replicates in each group. To determine the differences between groups one-way analysis of variance (ANOVA) was carried out by using the SPSS software (version 13.0) using the least significant difference (LSD) test at P<0.01. Means not sharing the same letter are significantly different (LSD) at P < 0.01 probability level in each column.
Conclusion
The current phytochemical study provided preliminary data for the first time that the A. javanica possesses significant anti-ulcer activity. This might be contributed towards the presence of some bioactive constituents contributed towards the various biological activities including the in the treatment of gastric ulcer. The compound 3 shows mild activity which might be due to the presence of amide group. Further study on the plant and specifically on the compound 3 could provide many chemically interesting and biologically active drugs, including, some with potential anti-ulcer properties.
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
SJ has supervised all the research work carried out. AWK carried out isolation, purification and characterization of the constituents. SP and AAS facilitate in research work. RAK made a significant contribution to acquisition of data, analysis, drafting of the manuscript. AJT and AS helped in extraction. All authors read and approved the final manuscript.
Contributor Information
Abdul Wajid Khan, Email: sawajidkhan@gmail.com.
Saleem Jan, Email: saleem_jann@hotmail.com.
Shaista Parveen, Email: shaista-19@live.com.
Rahmat Ali Khan, Email: rahmatgul_81@yahoo.com.
Asma Saeed, Email: asmasaeed80@hotmail.com.
Abdul Jabbar Tanveer, Email: drjabbbar@yahoo.com.
Anwar Ali Shad, Email: anwaralishad@yahoo.com.
Acknowledgment
Provision of research facility at the premier research center, the H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi is highly acknowledged.
References
- Alvarez-Suarez JM, Dekanski D, Risti S, Radonji NV, Petronijevi ND, Giampieri F, Astolfi P, González-Paramás AM, Santos-Buelga C, Tulipani S, Quiles JL, Mezzetti B, Battino M. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One. 2011;6(10):e25878. doi: 10.1371/journal.pone.0025878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforte MT, Lanuzza F, Pergolizzi S, Mondello F, Tzakou O, Galati EM. Protective effect of Calamintha officinalis Moench leaves against alcohol-induced gastric mucosa injury in rats. Macroscopic, histologic and phytochemical analysis. Phytother Res. 2012;26(6):839–44. doi: 10.1002/ptr.3647. [DOI] [PubMed] [Google Scholar]
- Burkill HM. The useful plants of West Tropical Africa, Families A-D. UK: Royal Botanic Gardens; 1985. pp. 446–447. [Google Scholar]
- Emam SS. Phytochemical studies on the herb Aerva javanica growing in Egypt. Cairo University Faculty of Agriculture Bulletin. 1999;50:488–514. [Google Scholar]
- Khan UG, Nazir T, Ahmed VU. Chemical Constituents of Aerva javanica. Fitoterapia. 1982;53:75–77. [Google Scholar]
- Deshmukh T, Yadav BV, Badole SL, Bodhankar SL, Dhaneshwar SR. Antihyperglycaemic activity of alcoholic extract of Aerva lanata (L.) A. L. Juss. Ex J. A. Schultes leaves in alloxan induced diabetic mice. J App Biomed. 2008;6:81–87. [Google Scholar]
- Garg SP, Bhushan R, Kapoor RC. Aervanone, a new flavanone from Aerva persica. Phytochemistry. 1980;19:1265. doi: 10.1016/0031-9422(80)83110-4. [DOI] [Google Scholar]
- Singh R, Jain SC, Jain R. Antioxidant activity of some medicinally important arid zone plants. Asian J Exp Sci. 2010;23:215–221. [Google Scholar]
- Baltina LA, Flekhter OB, Nigmatullina LR, Boreko EI, Pavlova NI, Nikolaeva SN, Tolstikov GA. Lupane triterpenes and derivatives with antiviral activity. J Bioorganic Med Chem Lett. 2003;13:3549–3552. doi: 10.1016/S0960-894X(03)00714-5. [DOI] [PubMed] [Google Scholar]
- El-Hadi MA, Barki YMN, Yousif GM, Hassan SK. Antiplasmodial activity of some medicinal plants used in sudanese folk-medicine. Environ Health Insight. 2010;4:1–6. doi: 10.4137/EHI.S4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas KR, Reddy VM. Antihyperglycaemic activity of ethanol extract of Aerva javanica leaves in alloxan- induced diabetic mice. J of Pharmacy Research. 2009;2:1259–1261. [Google Scholar]
- Okwu DE. Flavouring properties of spices on cassava Fufu. Afric J Roots Tuber Crops. 1999;2:19–21. [Google Scholar]
- Rama NH, Wadood A, Obaid OR, Baber TM, Ali FI, Ahmed S, Haq Z, Khan RA, Choudhary MI. Synthesis of 1,4-diaryl-2-naphthoates based on site-selective Suzuki-Miyaura reactions. Med Chem Lett. 2010;1:145–149. doi: 10.1021/ml100068u. [DOI] [Google Scholar]
- Spiro HM. Clinical Gastroenterology. Macmillan Pub. Newyork; 1977. pp. 292–304. [Google Scholar]
- Kurata JH, Haile BM. Epidemiology of peptic ulcer disease. Clin Gastroenterol. 1984;13:289–307. [PubMed] [Google Scholar]
- Pharmaguide, Handbook of all medicines in Pakistan. Karachi, Pakistan: Pharmaguide Publishing Company; 2006. p. 25. [Google Scholar]
- Sharif A, Ahmed E, Malik A, Hassan MU, Munawar MA, Farrukh A, Nagra SA, Anwar J, Ashraf M, Mahmood Z. Antimicrobial Constituents from Aerva javanica. J Chem Soc Pak. 2011;33:3. [Google Scholar]
- Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. doi: 10.1021/ac60252a045. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]