Abstract
Synthetic biology has rapidly progressed over the past decade and is now positioned to impact important problems in health and energy. In the clinical arena, the field has thus far focused primarily on the use of bacteria and bacteriophages to overexpress therapeutic gene products. The next generation of multigene circuits will control the triggering, amplitude, and duration of therapeutic activity in vivo. This will require a host organism that is easy to genetically modify, leverages existing successful circuit designs, and has the potential for use in humans. Here, we show that gene circuits that were originally constructed and tested in Escherichia coli translate to Salmonella typhimurium, a therapeutically relevant microbe with attenuated strains that have exhibited safety in several human clinical trials. These strains are essentially nonvirulent, easy to genetically program, and specifically grow in tumor environments. Developing gene circuits on this platform could enhance our ability to bring sophisticated genetic programming to cancer therapy, setting the stage for a new generation of synthetic biology in clinically relevant microbes.
Keywords: S. typhimurium, genetic circuits, microfluidics, clinical synthetic biology
An explosion of DNA sequencing,1 synthesis,2 and manipulation3 technologies has driven the development of synthetic genetic programs of increasing complexity in living cells.4−6 Underlying this work is the hope that engineered biological systems will be used to solve important problems in energy and health over the coming years. Initially inspired by electronic circuits, researchers began by designing small transcriptional switches7 and oscillators.8 These early successes fostered a growing population of physicists, computer scientists, and engineers that aimed to apply an engineering-based methodology to the design of biological systems. In the past decade, substantial success has been achieved using this genetic circuits approach termed synthetic biology.4,5,9
Multigene logic gates capable of integrating environmental signals have been constructed in bacteria,10 yeast,11 and mammalian cells.12 Electronics-inspired networks have included counters,13 pulse generators,14 filters,15,16 and communication modules.14,17 Sophisticated circuits can now be controlled by light, yielding genetic programs readily tunable both in vitro(18) and in vivo in live animals.19 Dynamic genetic clocks have been constructed that function at the single-cell,20 colony,21 and multicolony22 level in growing bacterial populations, and even in mammalian cells.23 In a recent study, redox signaling mediated by H2O2 vapor permitted the synchronization of millions of oscillating bacteria across an LCD-like sensor array.22
Early efforts toward clinical applications have utilized bacteria24−28 and bacteriophages29,30 (viruses that infect bacteria) to perform therapeutic functions in vivo. Commensal bacteria have been engineered to fight diabetes,26 HIV,27 and cholera28 by producing and delivering therapeutic agents directly in the human microbiome. Because certain bacteria grow preferentially in hypoxic environments, a number of studies have engineered cancer-fighting bacteria to selectively attack tumors.24,25 Toward still another application, a pair of studies has engineered phages to produce foreign enzymes, making them far more potent than their unmodified counterparts at dispersing bacterial biofilms.29,30
In most of these cases, the genetic programs involved were responsible for overexpressing target genes, similar to traditional genetic engineering where genes are added, removed, or modified one at a time in a stepwise fashion. To truly achieve its clinical potential, synthetic biology must continue to do what has made it successful: engineer progressively more complex, multi-input networks in which the triggering, amplitude, and duration of therapeutic activity is controllable. This will require using hosts that are easy to genetically modify and compatible with the clinical requirements regarding safety, immunogenicity, and drug resistance. While bacteriophage and adenovirus have their advantages, viruses have smaller genomes and therefore have a narrower range of genetic modifications, frequently induce host resistance, and are highly cell-type specific.31,32
As one potential bridge between organisms such as Escherichia coli and clinically relevant microbes, Salmonella typhimurium is a bacterial anticancer platform that is closely related to E. coli, has been extensively studied in vivo for therapeutic applications,33−38 and has been shown to be safe in human clinical trials.39−41 The development of attenuated strains has utilized auxotrophy and phoPQ deletions to suppress virulence cell invasion and virulence.35 Lipid A mutations have been generated to reduce immunogenicity, stimulating a much weaker immune response than wild-type strains.38 Despite this reduced potency, systemically injected S. typhimurium cells retain their ability to target and selectively replicate within tumors, displaying a thousand-fold growth preference relative to other organs.33−38 Their motility allows them to follow chemical gradients and penetrate deep into the tumor vasculature,42,43 much further than passively diffusing small molecules.33 And many of these strains also display innate oncolytic activity, regressing tumors simply by growing in them.34,38,44,45
Perhaps the most important property of S. typhimurium for synthetic biology is the ease of genetic modification. It is a model organism whose genome is sequenced,46 has knockout collections, and the genetic tools are almost identical to E. coli. S. typhimurium is capable of stably expressing recombinant DNA from plasmid-based circuits in vivo. This approach has already been used to produce a number of therapeutic compounds directly within tumors, but most often via “always on” expression of well-established genes.35,47,48 This work has laid the foundation for more sophisticated functionality, such as programmed delivery profiles that take advantage of plasmid instability.49 Such a focus will merge the dynamic sensing, production, and delivery capabilities of genetic circuits with the native tumor seeking and penetration of S. typhimurium.
Experimental Results
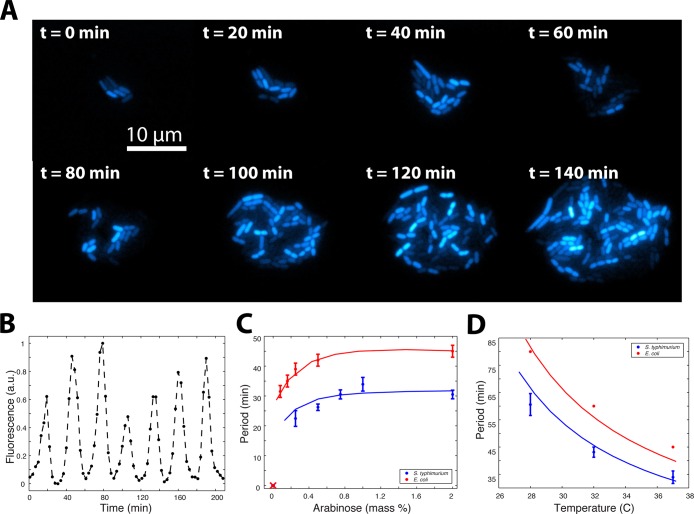
In order to test the degree to which existing synthetic circuits function in S. typhimurium, we transformed the attenuated strain ELH430 (SL1344 ΔphoPQ, gift of Elizabeth Hohmann, MGH)50 with several genetic oscillator constructs. First, we tested a single-plasmid variant of a published single-cell gene oscillator.20 Using our microfluidic platform,51,52 we observed robust oscillations for all S. typhimurium cells over many generations (Figure 1A,B). While the qualitative period-inducer relationship was similar to E. coli, the curve was shifted toward faster periods as compared to E. coli strain JS006 (MG1655 ΔaraC,lacI) (Figure 1C). In contrast, we initially expected S. typhimurium to oscillate slower since longer division times generally result in period lengthening.20 When we measured the dependence of oscillatory period on temperature in S. typhimurium, we found the trend qualitatively similar to E. coli, where lower temperatures (and therefore longer doubling times) resulted in longer oscillatory periods (Figure 1D). We therefore hypothesized that the faster oscillations in S. typhimurium are not due to growth rate differences, but rather a strain-dependent factor such as mean promoter level, transcription rate, or enzymatic degradation rate.
Figure 1.
A fast, robust, and tunable genetic oscillator in S. typhimurium. (A) Timelapse fluorescence microscopy depicting asynchronous oscillations in a growing colony of S. typhimurium. (B) A single-cell trajectory extracted from image data. (C) Period vs inducer concentration for S. typhimurium compared to original data taken in E. coli. The trends are qualitatively similar, yet S. typhimurium is shifted toward shorter periods. Points are experimental measurements fit to a line generated by computational modeling. (D) Period vs temperature for S. typhimurium compared to original data taken in E. coli with similar trends.
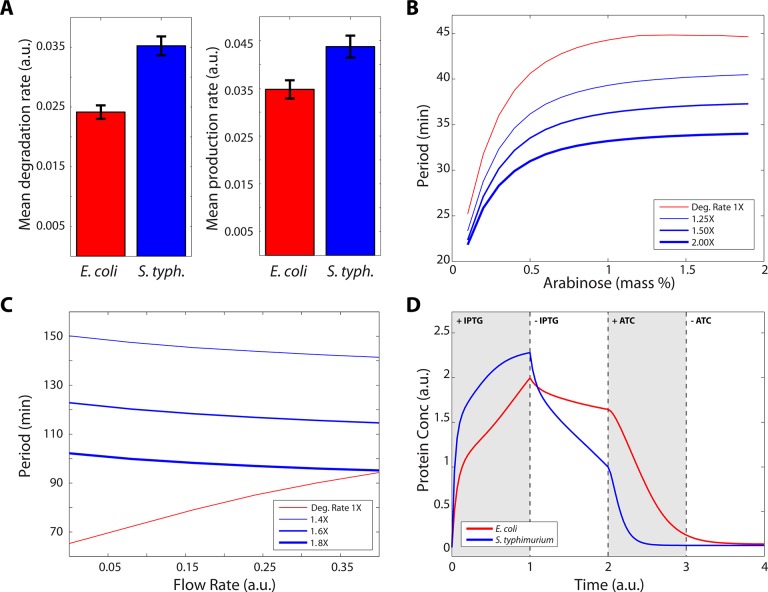
To explore this quantitatively, we used automated single-cell tracking using a previously developed algorithm53 to compare a large number of single-cell time courses from S. typhimurium and E. coli (Supporting Information). Oscillators are an ideal circuit to quantify strain-specific parameters such as transcription and degradation rates since they allow for hundreds of measurements in a single experiment. For each oscillatory period, the trough-to-peak and peak-to-trough slopes were measured. Since the ClpXP degradation machinery is likely saturated,54 the peak-to-trough slope yields an estimate for the zeroth-order enzymatic degradation rate in degrade-and-fire oscillators.55 Interestingly, we found that the apparent enzymatic degradation rate in S. typhimurium was roughly 1.5-fold that of E. coli (Figure 2A). In our computational model of the oscillator, this increase reproduced the experimentally observed period-inducer relationship (Figure 2B).
Figure 2.
Computational modeling of S. typhimurium genetic circuits. (A) Comparison of enzymatic degradation rate between S. typhimurium and E. coli generated from automated single-cell tracking. Degradation rate is approximately 1.5× higher in S. typhimurium. (B) A higher degradation rate results in the shorter periods observed experimentally for the single-cell oscillator. (C) In contrast, increased degradation rate results in longer periods for the quorum-sensing oscillator that are comparatively unchanged with flow rate. (D) Increased degradation and expression rates produce the experimentally observed behavior for the S. typhimurium toggle switch.
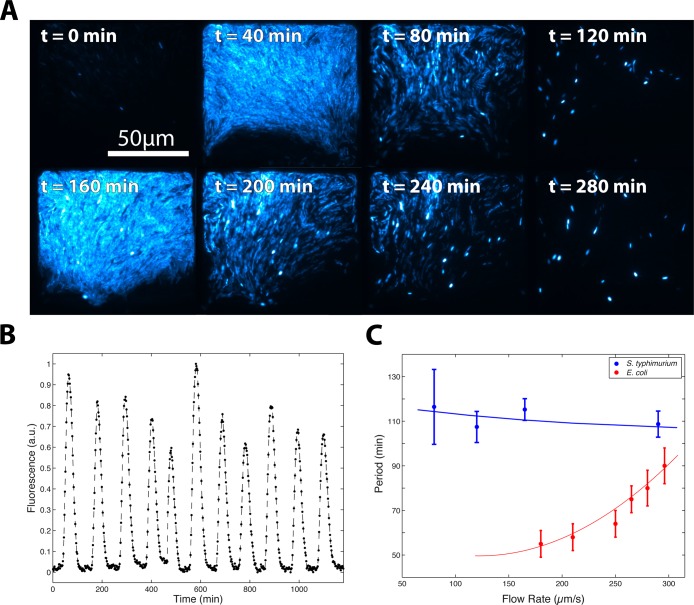
Next, we transformed S. typhimurium with a quorum-sensing oscillator that had been previously characterized in E. coli,21 and observed coherent, colony-level oscillations for more than 48 h (Figure 3A,B). Here, we found that the period-flow rate dependence was markedly different in S. typhimurium than in the original study, where oscillatory period was much longer and changed very little across a wide range of flow rates (Figure 3C). Interestingly, while increased degradation rate resulted in faster oscillations for single cells (Figure 2B), our computational model correctly predicts the opposite trend for the quorum-sensing oscillator when degradation is increased (Figure 2C).
Figure 3.
A synchronized quorum of genetic clocks in S. typhimurium. (A) Timelapse fluorescence microscopy depicting coherent oscillations at the colony-level for a growing colony of S. typhimurium. (B) A colony trajectory extracted from image data that illustrates the regularity of oscillations over time. (C) Period vs flow rate for S. typhimurium compared to original data taken in E. coli. S. typhimurium displays much higher periods that appear to be independent of flow rate.
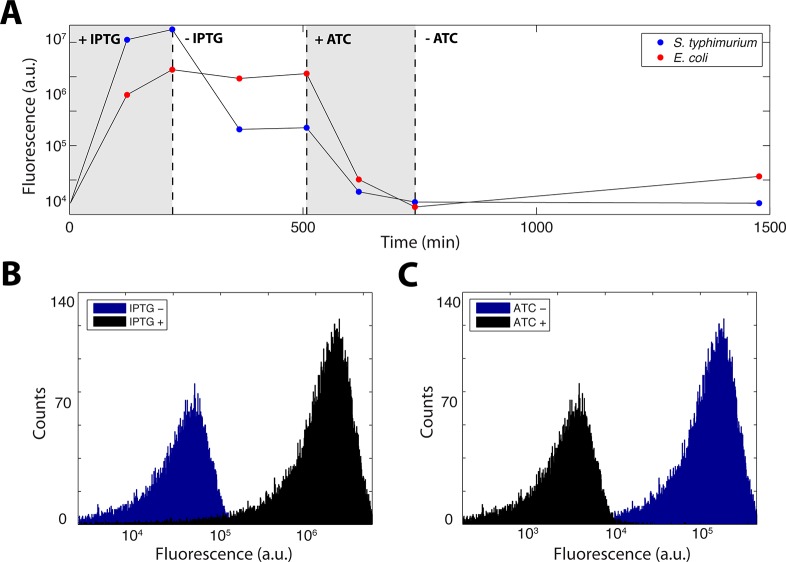
Finally, we tested the original genetic toggle switch, plasmid pIKE107.7 In this circuit, a transient pulse of IPTG inducer turns the switch ON and reporter expression is maintained at a high level. A second pulse of ATC inducer turns the switch OFF, dropping reporter expression indefinitely. In periodically diluted batch culture experiments similar to the original study, we used flow cytometry to observe robust switching and bistability when inducing with either 2 mM IPTG or 500 ng/μL dox in cultures growing at 37 °C (Figure 4A–C). Interestingly, the fluorescence level at which S. typhimurium settled after we removed IPTG was lower than the same circuit in E. coli (Figure 4A). We suspected that the differences in apparent degradation and expression rates (Figure 2A) might explain this change, since the steady-state repressor balance would be adjusted.
Figure 4.
A genetic toggle switch in S. typhimurium. (A) A time course of fluorescence output that illustrates switching by both IPTG and ATC quantified by flow cytometry in periodically diluted batch culture experiments. (B) Raw flow cytometer data illustrating switching by 2 mM IPTG and (C) 500 ng/μL of ATC.
To test this hypothesis, we used the original computational model of the toggle switch7 and quantified the steady-state expression level over time for strain parameters measured in E. coli and S. typhimurium. We found that the S. typhimurium parameters reproduced the experimentally observed curves, where expression rises to a higher level when switched ON then decays to a lower steady-state when IPTG is removed (Figure 2D). While these parameters are particularly important for dynamic circuits, they can also impact the performance of stable switches since repressors are continuously being produced and degraded.
A central issue in the design of genetic circuits is the degree to which native and engineered networks should be integrated. Synthetic biology began by fully isolating itself from the strain background, using it solely to supply energy, enzymatic machinery, and a cellular volume in which to function. In contrast, industrial applications in medicine and energy have commonly utilized a variety of microbes for their native networks.4,56,57 As our biological knowledge of native networks and our ability to engineer new circuits has improved, it has become increasingly possible to blend these two strategies.5
S. typhimurium is an ideal strain for clinical synthetic biology since it is closely related to E. coli, well studied in vivo, has safety precedence for clinical trials in humans, and displays a thousand-fold growth preference for tumor environments.33−36 Moving to other microbes for clinical and industrial purposes will require the determination of the critical strain parameters that define the space of bacteria capable of hosting genetic circuits. Next steps will involve measurement of these parameters and testing circuits in strains of interest that are further removed in the phylogenetic tree.58 One such roadmap would begin with more distantly related gamma proteobacteria like Pseudomonas aeruginosa before moving outside the phylum to alpha proteobacteria such as Calubacter crescentus. Additionally, individual components and modules can also receive a “portability” score that estimate the degree to which they translate to other hosts. For example, while lacI- and tetR-based circuits are nearly universal, more generally the function of other components are likely to be more sensitive to strain-specific parameters. This work will enable synthetic biology to move beyond E. coli into a diverse range of microbes for clinical and industrial applications.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (GM069811), the Department of Defense National Defense Science and Engineering Graduate Fellowship (A.P.), the National Science Foundation Graduate Research Fellowship (P.S.), and the Misrock Postdoctoral fellowship (T.D.). We would like to thank K. Pogliano for helpful discussions.
Supporting Information Available
Microscopy and microfluidics, degradation and production rate quantification, and modeling. This material is available free of charge via the Internet at http://pubs.acs.org/.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Jasny B. R.; Zahn L. M. (2011) A celebration of the genome, part i. Science 331(6017), 546. [DOI] [PubMed] [Google Scholar]
- Matzas M.; Stähler P. F.; Kefer N.; Siebelt N.; Boisguérin V.; Leonard J. T.; Keller A.; Stähler C. F.; Häberle P.; Gharizadeh B.; Babrzadeh2 F.; Church G. M. (2010) High-fidelity gene synthesis by retrieval of sequence-verified dna identified using high-throughput pyrosequencing. Nat. Biotechnol. 28(12), 1291–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. G.; Glass J. I.; Lartigue C.; Noskov V. N.; Chuang R. Y.; Algire M. A.; Benders G. A.; Montague M. G.; Ma L.; Moodie M. M.; Merryman C.; Vashee S.; Krishnakumar R.; Assad-Garcia N.; Andrews-Pfannkoch C.; Denisova E.; Young L.; Qi Z.-Q.; Segall-Shapiro T. H.; Calvey C. H.; Parmar P. P.; Hutchison C. III.; Smith H.; Venter J. C. (2010) Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329(5987), 52. [DOI] [PubMed] [Google Scholar]
- Weber W.; Fussenegger M. (2011) Emerging biomedical applications of synthetic biology. Nat. Rev. Genet. 13(1), 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandagopal N.; Elowitz M. B. (2011) Synthetic biology: Integrated gene circuits. Science 333(6047), 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A. S.; Collins J. J. (2010) Synthetic biology: applications come of age. Nat. Rev. Genet. 11(5), 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner T. S.; Cantor C. R.; Collins J. J. (2000) Construction of a genetic toggle switch in Escherichia coli. Nature 403(6767), 339–342. [DOI] [PubMed] [Google Scholar]
- Elowitz M. B.; Leibler S. (2000) A synthetic oscillatory network of transcriptional regulators. Nature 403(6767), 335–338. [DOI] [PubMed] [Google Scholar]
- Hasty J.; McMillen D.; Collins J. J. (2002) Engineered gene circuits. Nature 420(6912), 224–230. [DOI] [PubMed] [Google Scholar]
- Tamsir A.; Tabor J. J.; Voigt C. A. (2010) Robust multicellular computing using genetically encoded nor gates and chemical 'wires'. Nature 469(7329), 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regot S.; Macia J.; Conde N.; Furukawa K.; Kjellén J.; Peeters T.; Hohmann S.; De Nadal E.; Posas F.; Solé R. (2010) Distributed biological computation with multicellular engineered networks. Nature 469(7329), 207–211. [DOI] [PubMed] [Google Scholar]
- Guido N. J.; Wang X.; Adalsteinsson D.; McMillen D.; Hasty J.; Cantor C. R.; Elston T. C.; Collins J. J. (2006) A bottom-up approach to gene regulation. Nature 439(7078), 856–860. [DOI] [PubMed] [Google Scholar]
- Friedland A. E.; Lu T. K.; Wang X.; Shi D.; Church G.; Collins J. J. (2009) Synthetic gene networks that count. Science 324(5931), 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S.; Mehreja R.; Thiberge S.; Chen M. T.; Weiss R. (2004) Spatiotemporal control of gene expression with pulse-generating networks. Proc. Natl. Acad. Sci. U. S. A. 101(17), 6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohka T.; Heins R. A.; Phelan R. M.; Greisler J. M.; Townsend C. A.; Ostermeier M. (2009) An externally tunable bacterial band-pass filter. Proc. Natl. Acad. Sci. U. S. A. 106(25), 10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshangi S.; Thiberge S.; Weiss R. (2005) Ultrasensitivity and noise propagation in a synthetic transcriptional cascade. Proc. Natl. Acad. Sci. U. S. A. 102(10), 3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L.; Cox R. S.; Weiss R.; Arnold F. H. (2004) Programmed population control by cell-cell communication and regulated killing. Nature 428(6985), 868–871. [DOI] [PubMed] [Google Scholar]
- Tabor J. J.; Salis H. M.; Simpson Z. B.; Chevalier A. A.; Levskaya A.; Marcotte E. M.; Voigt C. A.; Ellington A. D. (2009) A synthetic genetic edge detection program. Cell 137(7), 1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H.; Daoud-El Baba M.; Peng R. W.; Fussenegger M. (2011) A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332(6037), 1565–1568. [DOI] [PubMed] [Google Scholar]
- Stricker J.; Cookson S.; Bennett M. R.; Mather W. H.; Tsimring L. S.; Hasty J. (2008) A fast, robust and tunable synthetic gene oscillator. Nature 456(7221), 516–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danino T.; Mondragon-Palomino O.; Tsimring L.; Hasty J. (2010) A synchronized quorum of genetic clocks. Nature 463, 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prindle A.; Samayoa P.; Razinkov I.; Danino T.; Tsimring L.S.; Hasty J. (2012) A sensing array of radically coupled genetic 'biopixels'. Nature 481, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M.; Marquez-Lago T. T.; Stelling J.; Fussenegger M. (2009) A tunable synthetic mammalian oscillator. Nature 457(7227), 309–312. [DOI] [PubMed] [Google Scholar]
- Anderson C.; Clarke E.; Arkin A.; Voigt C. (2006) Environmentally controlled invasion of cancer cells by engineered bacteria. J. Mol. Biol. 355(4), 619–627. [DOI] [PubMed] [Google Scholar]
- Xiang S.; Fruehauf J.; Li C. J. (2006) Short hairpin rna-expressing bacteria elicit rna interference in mammals. Nat. Biotechnol. 24(6), 697–702. [DOI] [PubMed] [Google Scholar]
- Duan F.; Curtis K. L.; March J. C. (2008) Secretion of insulinotropic proteins by commensal bacteria: Rewiring the gut to treat diabetes. Appl. Environ. Microbiol. 74(23), 7437–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.; Hu S.; McHugh L.; Lueders K.; Henry K.; Zhao Q.; Fekete R. A.; Kar S.; Adhya S.; Hamer D. H. (2005) Toward a live microbial microbicide for hiv: commensal bacteria secreting an hiv fusion inhibitor peptide. Proc. Natl. Acad. Sci. U. S. A. 102(34), 11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan F.; March J. C. (2010) Engineered bacterial communication prevents vibrio cholerae virulence in an infant mouse model. Sci. Signaling 107(25), 11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. K.; Collins J. J. (2007) Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 104(27), 11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T. K.; Collins J. J. (2009) Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc. Natl. Acad. Sci. U. S. A. 106(12), 4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R.; Scholl D.; Adhya S. L. (2003) The prospect for bacteriophage therapy in western medicine. Nat. Rev. Drug Discovery 2(6), 489–497. [DOI] [PubMed] [Google Scholar]
- Projan S. (2004) Phage-inspired antibiotics?. Nat. Biotechnol. 22(2), 167–168. [DOI] [PubMed] [Google Scholar]
- Forbes N. S. (2010) Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 10(11), 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek J. M.; Low K. B.; Bermudes D. (1997) Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 57(20), 4537. [PubMed] [Google Scholar]
- Hoffman R. M. (2011) Tumor-seeking Salmonella amino acid auxotrophs. Curr. Opin. Biotechnol. 22(6), 917–923. [DOI] [PubMed] [Google Scholar]
- Zheng L. M.; Luo X.; Feng M.; Li Z.; Le T.; Ittensohn M.; Trailsmith M.; Bermudes D.; Lin S. L.; King I. C. (2001) Tumor amplified protein expression therapy: Salmonella as a tumor-selective protein delivery vector. Oncol. Res. 12(3), 127–135. [DOI] [PubMed] [Google Scholar]
- Forbes N. S.; Munn L. L.; Fukumura D.; Jain R. K. (2003) Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 63(17), 5188. [PubMed] [Google Scholar]
- Low K. B.; Ittensohn M.; Le T.; Platt J.; Sodi S.; Amoss M.; Ash O.; Carmichael E.; Chakraborty A.; Fischer J.; Lin S. L.; Luo X.; Miller S. I.; Zheng L.-m.; King I.; Pawelek J. M.; Bermudes D. (1999) Lipid a mutant Salmonella with suppressed virulence and tnfα induction retain tumor-targeting in vivo. Nat. Biotechnol. 17(1), 37–41. [DOI] [PubMed] [Google Scholar]
- Heimann D. M.; Rosenberg S. A. (2003) Continuous intravenous administration of live genetically modified Salmonella typhimurium in patients with metastatic melanoma. J. Immunother. 26(2), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso J. F.; Gill V. J.; Hwu P.; Marincola F. M.; Restifo N. P.; Schwartzentruber D. J.; Sherry R. M.; Topalian S. L.; Yang J. C.; Stock F.; Freezer L. J.; Morton K. E.; Seipp C.; Haworth L.; MavrouKakis S.; White D.; MacDonald S.; Mao J.; Sznol M.; Rosenberg S. A. (2002) Phase i study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. 20(1), 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J.; Cunningham C.; Senzer N.; Kuhn J.; Cramm J.; Litz C.; Cavagnolo R.; Cahill A.; Clairmont C.; Sznol M. (2003) Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 10(10), 737–744. [DOI] [PubMed] [Google Scholar]
- Kasinskas R. W.; Forbes N. S. (2006) Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 94(4), 710–721. [DOI] [PubMed] [Google Scholar]
- Kasinskas R. W.; Forbes N. S. (2007) Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 67(7), 3201–3209. [DOI] [PubMed] [Google Scholar]
- Nagakura C.; Hayashi K.; Zhao M.; Yamauchi K.; Yamamoto N.; Tsuchiya H.; Tomita K.; Bouvet M.; Hoffman R. M. (2009) Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 29(6), 1873–1878. [PubMed] [Google Scholar]
- Jia L. J.; Wei D. P.; Sun Q. M.; Huang Y.; Wu Q.; Hua Z. C. (2007) Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Sci. 98(7), 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M.; Sanderson K. E.; Spieth J.; Clifton S. W.; Latreille P.; Courtney L.; Porwollik S.; Ali J.; Dante M.; Du F.; Hou S.; Layman D.; Leonard S.; Nguyen C.; Scott K.; Holmes A.; Grewal N.; Mulvaney E.; Ryan E.; Sun H.; Florea L.; Miller W.; Stoneking T.; Nhan M.; Waterston R.; Wilson R. K. (2001) Complete genome sequence of Salmonella enterica serovar typhimurium lt2. Nature 413(6858), 852–856. [DOI] [PubMed] [Google Scholar]
- Guo H.; Zhang J.; Inal C.; Nguyen T.; Fruehauf J. H.; Keates A. C.; Li C. J. (2011) Targeting tumor gene by shrna-expressing Salmonella-mediated rnai. Gene Ther. 18(1), 95–105. [DOI] [PubMed] [Google Scholar]
- Nguyen V. H.; Kim H. S.; Ha J. M.; Hong Y.; Choy H. E.; Min J. J. (2010) Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 70(1), 18–23. [DOI] [PubMed] [Google Scholar]
- Danino T., Lo J., Prindle A., Hasty J., Bhatia S. (2012) In vivo gene expression dynamics of tumor-targeted bacteria. ACS Synth. Biol., submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann E. L.; Oletta C. A.; Miller S. I. (1996) Evaluation of a phop/phoq-deleted, aroa-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine 14(1), 19–24. [DOI] [PubMed] [Google Scholar]
- Ferry M. S.; Razinkov I. A.; Hasty J. (2011) Microfluidics for synthetic biology from design to execution. Methods Enzymol. 497, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson S.; Ostroff N.; Pang W. L.; Volfson D.; Hasty J. (2005) Monitoring dynamics of single-cell gene expression over multiple cell cycles. Mol. Syst. Biol. 10.1038/msb4100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondragon-Palomino O.; Danino T.; Selimkhanov J.; Tsimring L.; Hasty J. (2011) Entrainment of a population of synthetic genetic oscillators. Science 333(6047), 1315–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson N. A.; Mather W. H.; Danino T.; Mondragon-Palomino O.; Williams R. J.; Tsimring L. S. (2011) Queueing up for enzymatic processing: Correlated signaling through coupled degradation. Mol. Syst. Biol. 10.1038/msb.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather W.; Bennett M. R.; Hasty J.; Tsimring. L. S. (2009) Delay-induced degrade-and-fire oscillations in small genetic circuits. Phys. Rev. Lett. 102, 068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder W. C.; Lu T.; James J. (2011) Synthetic biology moving into the clinic. Science 333(6047), 1248–1252. [DOI] [PubMed] [Google Scholar]
- Alper H.; Stephanopoulos G. (2009) Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential?. Nat. Rev. Microbiol. 7(10), 715–723. [DOI] [PubMed] [Google Scholar]
- Wu D.; Hugenholtz P.; Mavromatis K.; Pukall R.; Dalin E.; Ivanova N. N.; Kunin V.; Goodwin L.; Wu M.; Tindall B. J.; Hooper S. D.; Pati A.; Lykidis A.; Spring S.; Anderson I. J.; D’haeseleer P.; Zemla A.; Singer M.; Lapidus A.; Nolan M.; Copeland A.; Han C.; Chen F.; Cheng J.-F.; Lucas S.; Kerfeld C.; Lang E.; Gronow S.; Chain P.; Bruce D.; Rubin E. M.; Kyrpides N. C.; Klenk H.-P.; Eisen J. A. (2009) A phylogeny-driven genomic encyclopaedia of bacteria and archaea. Nature 462(7276), 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.